Indole-3-Carbonitriles as DYRK1A Inhibitors by Fragment-Based Drug Design
Abstract
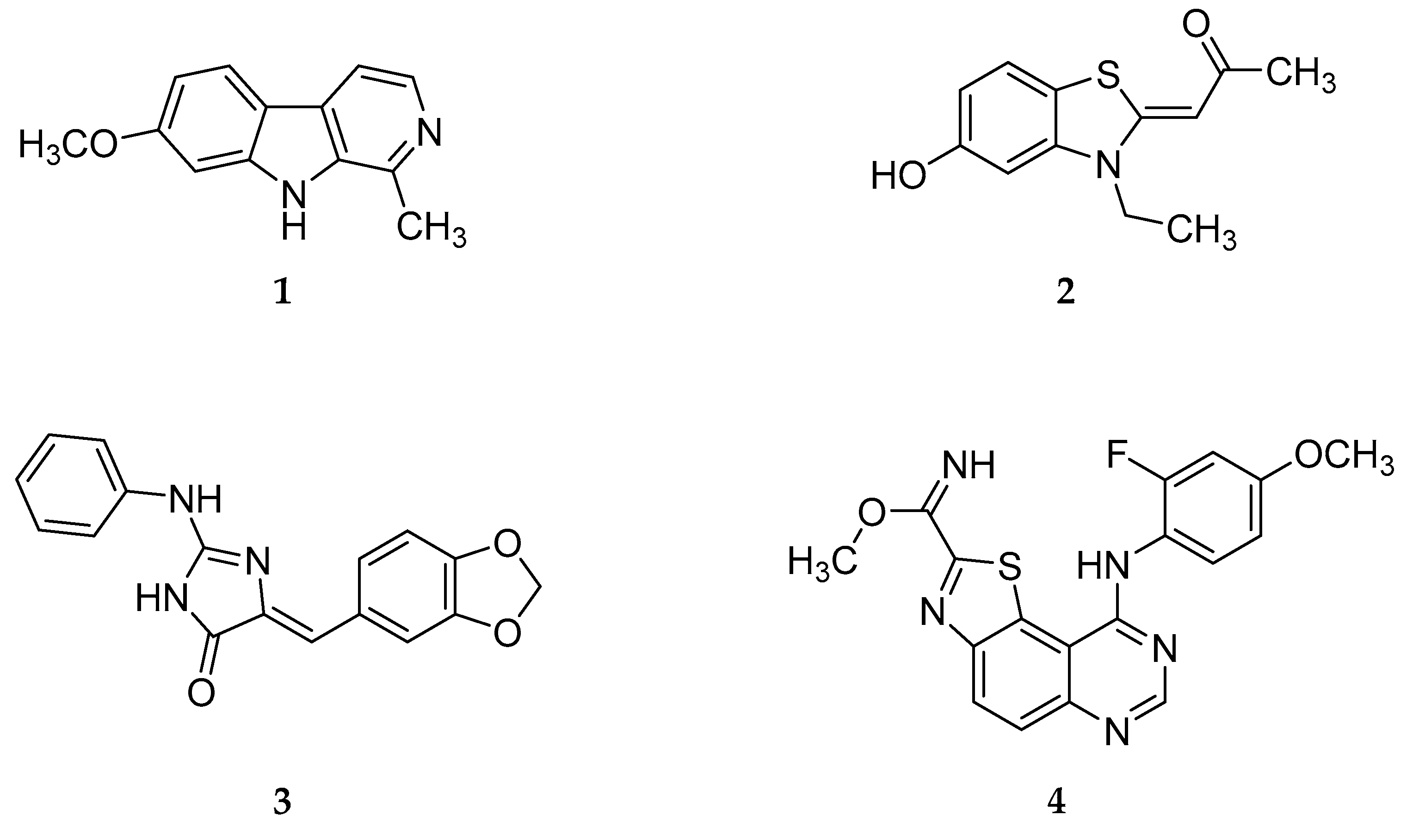
:1. Introduction
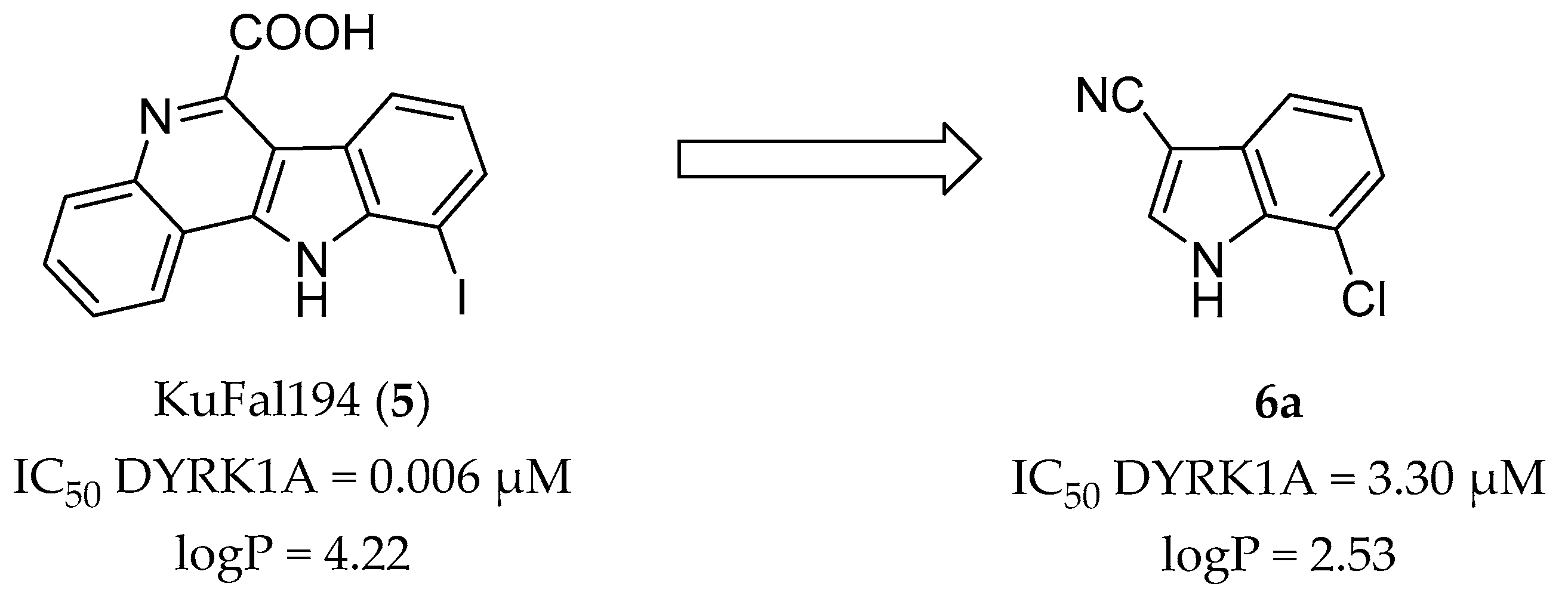
2. Results and Discussion
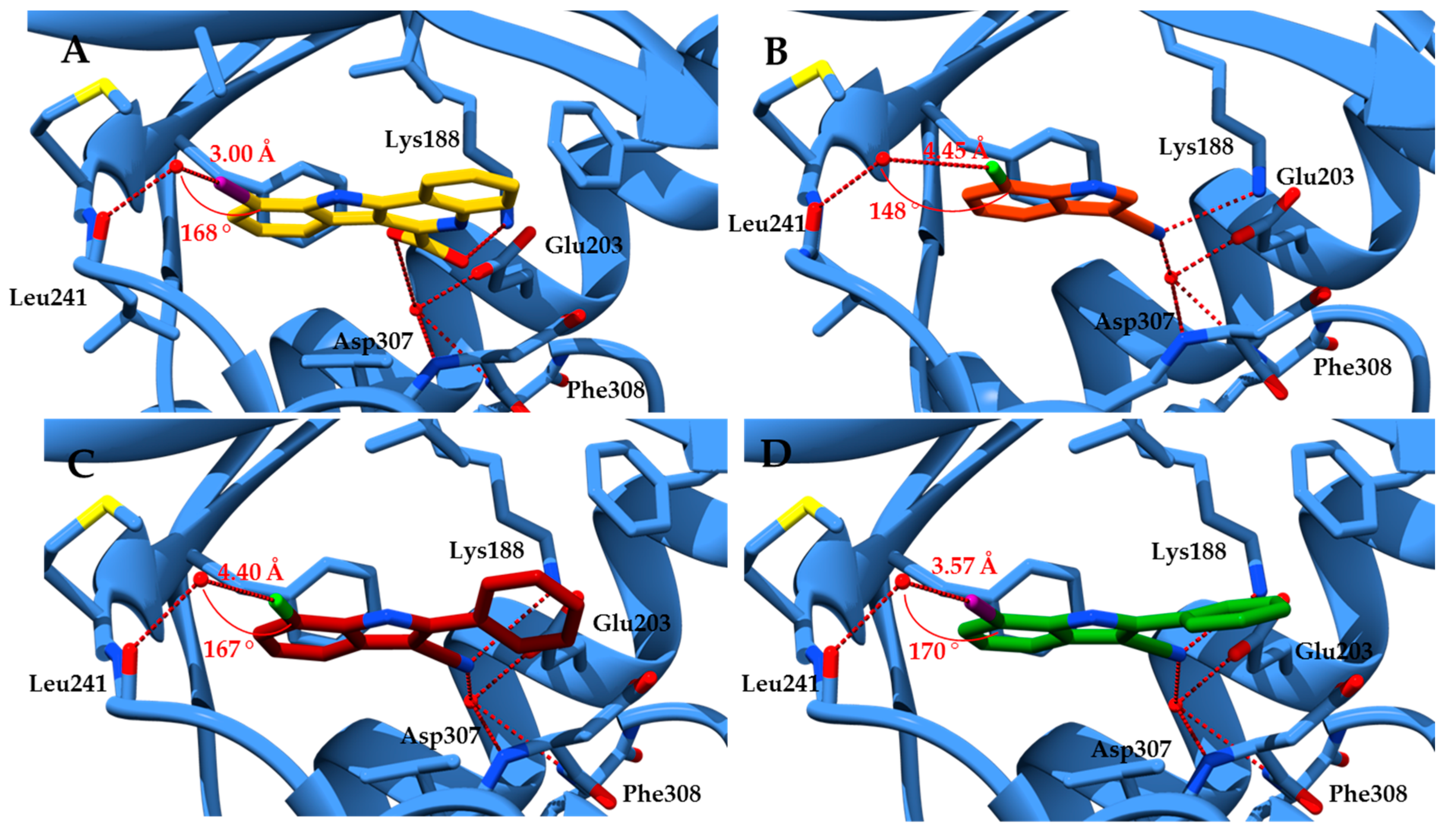
2.1. Molecular Docking Studies
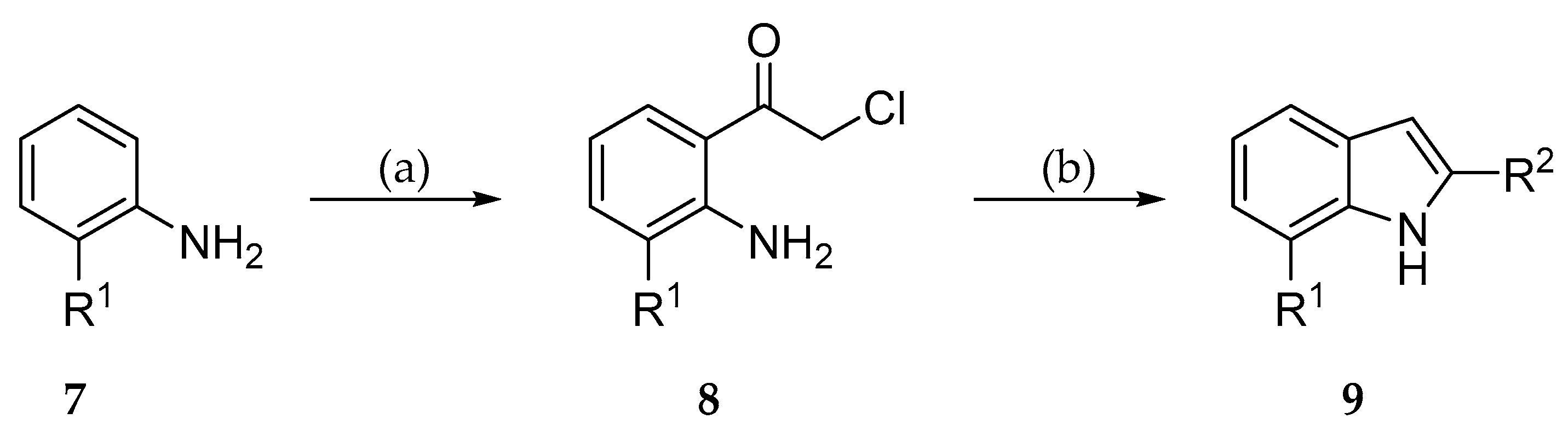
2.2. Syntheses
2.3. Kinase Inhibitory Activity
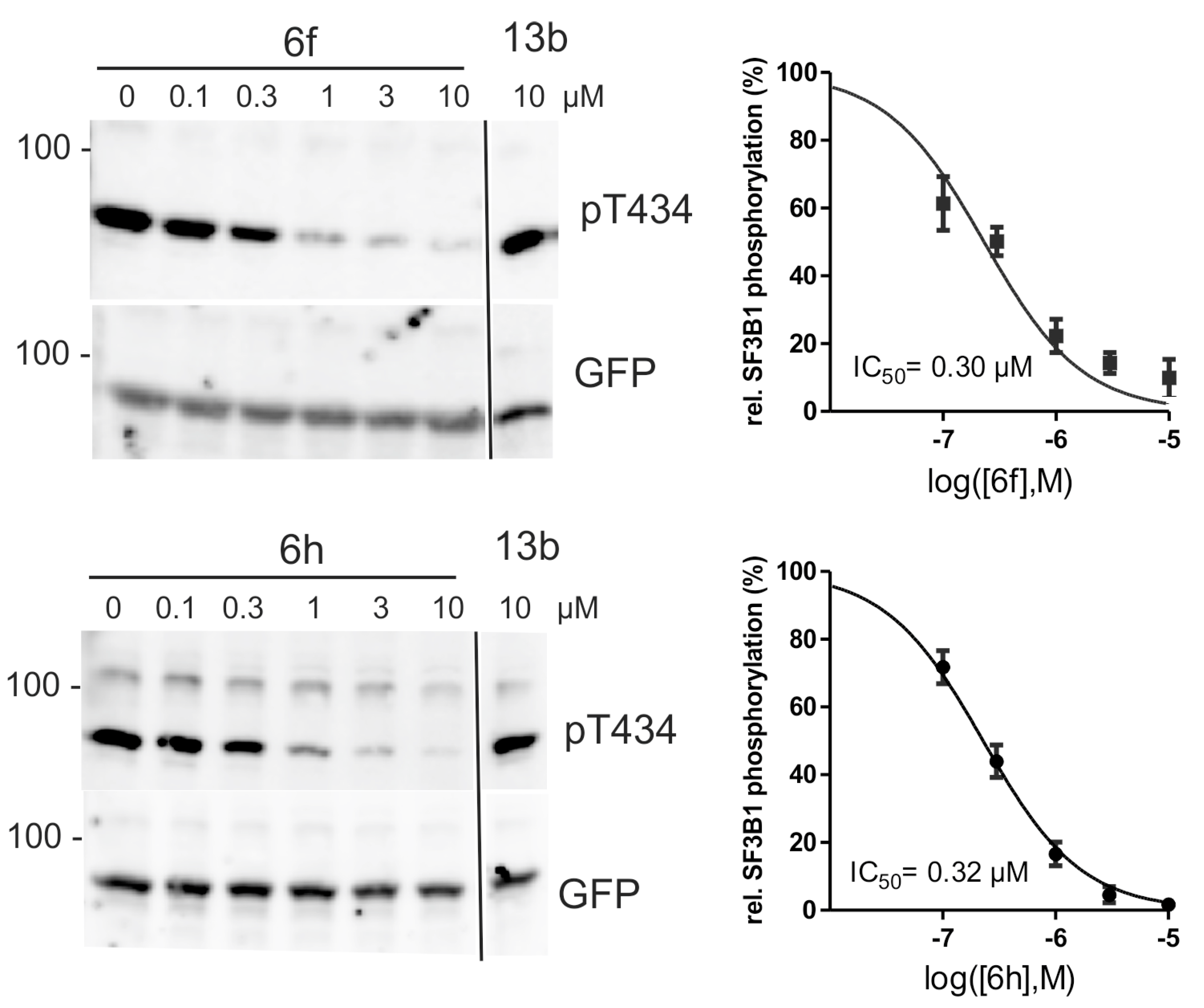
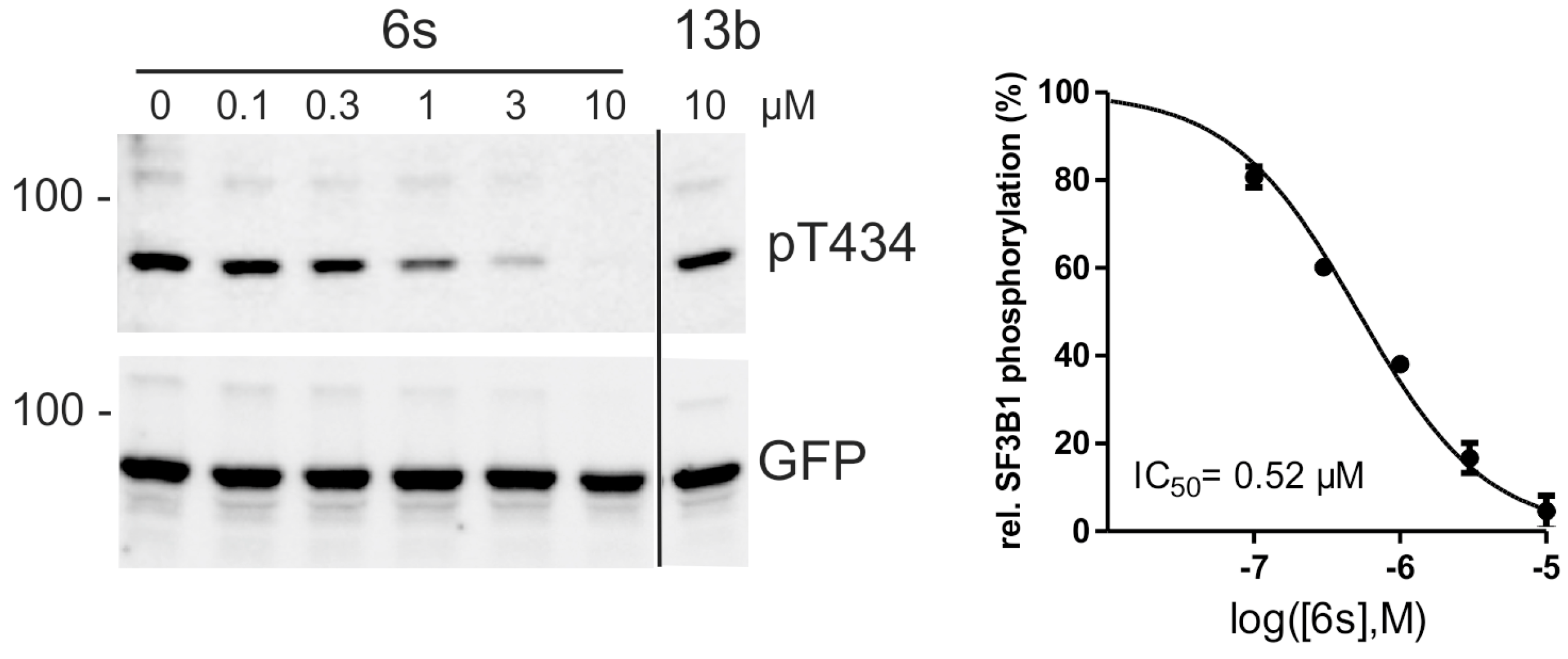
2.4. Inhibition of DYRK1A Activity in Cell Culture
2.5. Physicochemical Properties and Solubility Assays
3. Materials and Methods
3.1. General Information
3.2. Synthesis and Characterization of 6, 13 and 14
3.2.1. General Procedure for the Synthesis of Indole-3-Carbonitriles (Procedure A)
3.2.2. General procedure for the synthesis of indole-3-carbonitriles (Procedure B)
3.2.3. General procedure for the synthesis of indole-3-carbonitriles (Procedure C)
3.3. Molecular Docking
3.4. Protein Kinase Assays
3.5. Cell-Based Assays
3.6. Calculation of Physicochemical Properties
3.7. Determination of Thermodynamic Solubility
3.8. Determination of Kinetic Solubility
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fabbro, D. 25 years of small molecular weight kinase inhibitors: Potentials and limitations. Mol. Pharmacol. 2015, 87, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Becker, W.; Joost, H.G. Structural and functional characteristics of Dyrk, a novel subfamily of protein kinases with dual specificity. Prog. Nucleic Acid Res. Mol. Biol. 1999, 62, 1–17. [Google Scholar] [PubMed]
- Becker, W.; Soppa, U.; Tejedor, F.J. DYRK1A: A potential drug target for multiple Down syndrome neuropathologies. CNS Neurol. Disord. 2014, 13, 26–33. [Google Scholar] [CrossRef]
- Stotani, S.; Giordanetto, F.; Medda, F. DYRK1A inhibition as potential treatment for Alzheimer’s disease. Future Med. Chem. 2016, 8, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Duchon, A.; Herault, Y. DYRK1A, a dosage-sensitive gene involved in neurodevelopmental disorders, is a target for drug development in down syndrome. Front. Behav. Neurosci. 2016, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Song, W.-J.; Chung, K.C. Function and regulation of Dyrk1A: Towards understanding Down syndrome. Cell. Mol. Life Sci. 2009, 66, 3235–3240. [Google Scholar] [CrossRef] [PubMed]
- Yabut, O.; Domogauer, J.; D’Arcangelo, G. Dyrk1A overexpression inhibits proliferation and induces premature neuronal differentiation of neural progenitor cells. J. Neurosci. 2010, 30, 4004–4014. [Google Scholar] [CrossRef] [PubMed]
- Tejedor, F.J.; Hämmerle, B. MNB/DYRK1A as a multiple regulator of neuronal development. FEBS J. 2011, 278, 223–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbassi, R.; Johns, T.G.; Kassiou, M.; Munoz, L. DYRK1A in neurodegeneration and cancer: Molecular basis and clinical implications. Pharmacol. Ther. 2015, 151, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Wegiel, J.; Gong, C.-X.; Hwang, Y.-W. The role of DYRK1A in neurodegenerative diseases. FEBS J. 2011, 278, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, T.; Zhou, C.; Chohan, M.O.; Gu, X.; Wegiel, J.; Zhou, J.; Hwang, Y.-W.; Iqbal, K.; Grundke-Iqbal, I.; et al. Increased dosage of Dyrk1A alters alternative splicing factor (ASF)-regulated alternative splicing of tau in Down syndrome. J. Biol. Chem. 2008, 283, 28660–28669. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.S.; Park, S.Y.; Jung, M.-S.; Yoon, S.-H.; Kwen, M.-Y.; Lee, S.-Y.; Choi, S.-H.; Radnaabazar, C.; Kim, M.-K.; Kim, H.; et al. Dyrk1A-mediated phosphorylation of Presenilin 1: A functional link between Down syndrome and Alzheimer’s disease. J. Neurochem. 2010, 115, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, S.-R.; Jeong, H.K.; Radnaabazar, C.; Yoo, J.-J.; Cho, H.-J.; Lee, H.-W.; Kim, I.-S.; Cheon, Y.-H.; Ahn, Y.S.; Chung, S.-H.; et al. DYRK1A-mediated hyperphosphorylation of Tau. A functional link between Down syndrome and Alzheimer disease. J. Biol. Chem. 2007, 282, 34850–34857. [Google Scholar] [CrossRef] [PubMed]
- Wegiel, J.; Dowjat, K.; Kaczmarski, W.; Kuchna, I.; Nowicki, K.; Frackowiak, J.; Mazur Kolecka, B.; Wegiel, J.; Silverman, W.P.; Reisberg, B.; et al. The role of overexpressed DYRK1A protein in the early onset of neurofibrillary degeneration in Down syndrome. Acta Neuropathol. 2008, 116, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Esvan, Y.J.; Zeinyeh, W.; Boibessot, T.; Nauton, L.; Thery, V.; Knapp, S.; Chaikuad, A.; Loaec, N.; Meijer, L.; Anizon, F.; et al. Discovery of pyrido3,4-gquinazoline derivatives as CMGC family protein kinase inhibitors: Design, synthesis, inhibitory potency and X-ray co-crystal structure. Eur. J. Med. Chem. 2016, 118, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.; Medda, F.; Gokhale, V.; Dunckley, T.; Hulme, C. Recent advances in the design, synthesis, and biological evaluation of selective DYRK1A inhibitors: A new avenue for a disease modifying treatment of Alzheimer’s? ACS Chem. Neurosci. 2012, 3, 857–872. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.; Fruit, C.; Hérault, Y.; Meijer, L.; Besson, T. Dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A) inhibitors: A survey of recent patent literature. Expert Opin. Ther. Pat. 2017, 27, 1183–1199. [Google Scholar] [CrossRef] [PubMed]
- Bain, J.; Plater, L.; Elliott, M.; Shpiro, N.; Hastie, C.J.; McLauchlan, H.; Klevernic, I.; Arthur, J.; Simon, C.; Alessi, D.R.; et al. The selectivity of protein kinase inhibitors: a further update. Biochem. J. 2007, 408, 297–315. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Nonaka, Y.; Goto, T.; Ohnishi, E.; Hiramatsu, T.; Kii, I.; Yoshida, M.; Ikura, T.; Onogi, H.; Shibuya, H.; et al. Development of a novel selective inhibitor of the Down syndrome-related kinase Dyrk1A. Nat. Commun. 2010, 1, 86. [Google Scholar] [CrossRef] [PubMed]
- Tahtouh, T.; Elkins, J.M.; Filippakopoulos, P.; Soundararajan, M.; Burgy, G.; Durieu, E.; Cochet, C.; Schmid, R.S.; Lo, D.C.; Delhommel, F.; et al. Selectivity, cocrystal structures, and neuroprotective properties of leucettines, a family of protein kinase inhibitors derived from the marine sponge alkaloid leucettamine B. J. Med. Chem. 2012, 55, 9312–9330. [Google Scholar] [CrossRef] [PubMed]
- Göckler, N.; Jofre, G.; Papadopoulos, C.; Soppa, U.; Tejedor, F.J.; Becker, W. Harmine specifically inhibits protein kinase DYRK1A and interferes with neurite formation. FEBS J. 2009, 276, 6324–6337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Alvarez-Perez, J.-C.; Felsenfeld, D.P.; Liu, H.; Sivendran, S.; Bender, A.; Kumar, A.; Sanchez, R.; Scott, D.K.; Garcia-Ocana, A.; et al. A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication. Nat. Med. 2015, 21, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Frost, D.; Meechoovet, B.; Wang, T.; Gately, S.; Giorgetti, M.; Shcherbakova, I.; Dunckley, T. β-carboline compounds, including harmine, inhibit DYRK1A and tau phosphorylation at multiple Alzheimer’s disease-related sites. PLoS ONE 2011, 6, e19264. [Google Scholar] [CrossRef] [PubMed]
- Debdab, M.; Carreaux, F.; Renault, S.; Soundararajan, M.; Fedorov, O.; Filippakopoulos, P.; Lozach, O.; Babault, L.; Tahtouh, T.; Baratte, B.; et al. Leucettines, a class of potent inhibitors of cdc2-like kinases and dual specificity, tyrosine phosphorylation regulated kinases derived from the marine sponge leucettamine B: Modulation of alternative pre-RNA splicing. J. Med. Chem. 2011, 54, 4172–4186. [Google Scholar] [CrossRef] [PubMed]
- Naert, G.; Ferré, V.; Meunier, J.; Keller, E.; Malmström, S.; Givalois, L.; Carreaux, F.; Bazureau, J.-P.; Maurice, T. Leucettine L41, a DYRK1A-preferential DYRKs/CLKs inhibitor, prevents memory impairments and neurotoxicity induced by oligomeric Aβ25-35 peptide administration in mice. Eur. Neuropsychopharmacol. 2015, 25, 2170–2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, W.; Sippl, W. Activation, regulation, and inhibition of DYRK1A. FEBS J. 2011, 278, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Chaikuad, A.; Diharce, J.; Schroder, M.; Foucourt, A.; Leblond, B.; Casagrande, A.-S.; Desire, L.; Bonnet, P.; Knapp, S.; Besson, T. An unusual binding model of the methyl 9-anilinothiazolo[5,4-f]quinazoline-2-carbimidates (EHT 1610 and EHT 5372) confers high selectivity for dual-specificity tyrosine phosphorylation-regulated kinases. J. Med. Chem. 2016, 59, 10315–10321. [Google Scholar] [CrossRef] [PubMed]
- Foucourt, A.; Hédou, D.; Dubouilh-Benard, C.; Désiré, L.; Casagrande, A.-S.; Leblond, B.; Loäec, N.; Meijer, L.; Besson, T. Design and synthesis of thiazolo[5,4-f]quinazolines as DYRK1A Inhibitors, Part I. Molecules 2014, 19, 15546–15571. [Google Scholar] [CrossRef] [PubMed]
- Foucourt, A.; Hédou, D.; Dubouilh-Benard, C.; Girard, A.; Taverne, T.; Casagrande, A.-S.; Désiré, L.; Leblond, B.; Besson, T. Design and synthesis of thiazolo[5,4-f]quinazolines as DYRK1A inhibitors, part II. Molecules 2014, 19, 15411–15439. [Google Scholar] [CrossRef] [PubMed]
- Coutadeur, S.; Benyamine, H.; Delalonde, L.; Oliveira, C.; Leblond, B.; Foucourt, A.; Besson, T.; Casagrande, A.-S.; Taverne, T.; Girard, A.; et al. A novel DYRK1A (Dual specificity tyrosine phosphorylation-regulated kinase 1A) inhibitor for the treatment of Alzheimer’s disease: Effect on tau and amyloid pathologies in vitro. J. Neurochem. 2015, 133, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Falke, H.; Chaikuad, A.; Becker, A.; Loaëc, N.; Lozach, O.; Abu Jhaisha, S.; Becker, W.; Jones, P.G.; Preu, L.; Baumann, K.; et al. 10-Iodo-11H-indolo[3,2-c]quinoline-6-carboxylic acids are selective inhibitors of DYRK1A. J. Med. Chem. 2015, 58, 3131–3143. [Google Scholar] [CrossRef] [PubMed]
- Wilcken, R.; Zimmermann, M.O.; Lange, A.; Joerger, A.C.; Boeckler, F.M. Principles and applications of halogen bonding in medicinal chemistry and chemical biology. J. Med. Chem. 2013, 56, 1363–1388. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yang, Z.; Liu, Y.; Lu, Y.; Chen, K.; Zhu, W. Halogen bond: its role beyond drug-target binding affinity for drug discovery and development. J. Chem. Inf. Model. 2014, 54, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Pei, T.; Chen, C.-Y.; Dormer, P.G.; Davies, I.W. Expanding the [1,2]-aryl migration to the synthesis of substituted indoles. Angew. Chem. Int. Ed. 2008, 47, 4231–4233. [Google Scholar] [CrossRef] [PubMed]
- Pei, T.; Tellers, D.M.; Streckfuss, E.C.; Chen, C.-Y.; Davies, I.W. [1,2]-Aryl migration in the synthesis of substituted indoles: Scope, mechanism, and high throughput experimentation. Tetrahedron 2009, 65, 3285–3291. [Google Scholar] [CrossRef]
- Acheson, R.M.; Hunt, P.G.; Littlewood, D.M.; Murrer, B.A.; Rosenberg, H.E. The synthesis, reactions, and spectra of 1-acetoxy-, 1-hydroxy-, and 1-methoxy-indoles. J. Chem. Soc. 1978, 1117–1125. [Google Scholar] [CrossRef]
- Mehta, G.; Dhar, D.N.; Suri, S.C. Reaction of indoles with chlorosulphonyl isocyanate; a versatile route to 3-substituted indoles. Synthesis 1978, 374–376. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Wang, J. Lewis acid catalyzed direct cyanation of indoles and pyrroles with N-cyano-N-phenyl-p-toluenesulfonamide (NCTS). Org. Lett. 2011, 13, 5608–5611. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Larrosa, I. “On water”, phosphine-free palladium-catalyzed room temperature C-H arylation of indoles. Chemistry 2013, 19, 15093–15096. [Google Scholar] [CrossRef] [PubMed]
- Huchet, Q.A.; Kuhn, B.; Wagner, B.; Kratochwil, N.A.; Fischer, H.; Kansy, M.; Zimmerli, D.; Carreira, E.M.; Müller, K. Fluorination patterning: A study of structural motifs that impact physicochemical properties of relevance to drug discovery. J. Med. Chem. 2015, 58, 9041–9060. [Google Scholar] [CrossRef] [PubMed]
- Lovering, F.; Bikker, J.; Humblet, C. Escape from flatland: Increasing saturation as an approach to improving clinical success. J. Med. Chem. 2009, 52, 6752–6756. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, K.; Czajkowska, H.; Rottmann, S.; Packman, L.C.; Lilischkis, R.; Lüscher, B.; Becker, W. The protein kinase DYRK1A phosphorylates the splicing factor SF3b1/SAP155 at Thr434, a novel in vivo phosphorylation site. BMC Biochem. 2006, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Rüben, K.; Wurzlbauer, A.; Walte, A.; Sippl, W.; Bracher, F.; Becker, W. Selectivity profiling and biological activity of novel β-carbolines as potent and selective DYRK1 kinase inhibitors. PLoS ONE 2015, 10, e0132453. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Leeson, P.D.; Springthorpe, B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nat. Rev. Drug Discov. 2007, 6, 881–890. [Google Scholar] [CrossRef] [PubMed]
- MarvinSketch, 17.13.0, 2017, ChemAxon. Available online: http://www.chemaxon.com (accessed on 3 October 2016).
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 6th ed.; Elsevier/BH: Oxford, UK, 2009. [Google Scholar]
- Falke, H. Neue selektive Hemmstoffe der Proteinkinase DYRK1A. Doctoral Dissertation, Technische Universität Braunschweig, Shaker Verlag, Aachen, Germany, 2014. [Google Scholar]
- Molecular Operating Environment (MOE), 2013.08, 2015. Chemical Computing Group Inc., 1010 Sherbooke St. West, Suite #910, Montreal, QC, Canada, H3A 2R7 2018. Available online: https://www.chemcomp.com/Research-Citing_MOE.htm (accessed on 23 January 2018).
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Primot, A.; Baratte, B.; Gompel, M.; Borgne, A.; Liabeuf, S.; Romette, J.L.; Jho, E.H.; Costantini, F.; Meijer, L. Purification of GSK-3 by affinity chromatography on immobilized axin. Protein Expr. Purif. 2000, 20, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Klopman, G.; Li, J.-Y.; Wang, S.; Dimayuga, M. Computer automated logP calculations based on an extended group contribution approach. J. Chem. Inf. Model. 1994, 34, 752–781. [Google Scholar] [CrossRef]
- Viswanadhan, V.N.; Ghose, A.K.; Revankar, G.R.; Robins, R.K. Atomic physicochemical parameters for three dimensional structure directed quantitative structure-activity relationships. 4. Additional parameters for hydrophobic and dispersive interactions and their application for an automated superposition of certain naturally occurring nucleoside antibiotics. J. Chem. Inf. Model. 1989, 29, 163–172. [Google Scholar]
- Scientific Databases | SRC, Inc. Available online: https://www.srcinc.com/what-we-do/environmental/scientific-databases.html (accessed on 21 July 2017).
- Hou, T.J.; Xia, K.; Zhang, W.; Xu, X.J. ADME evaluation in drug discovery. 4. Prediction of aqueous solubility based on atom contribution approach. J. Chem. Inf. Model. 2004, 44, 266–275. [Google Scholar]
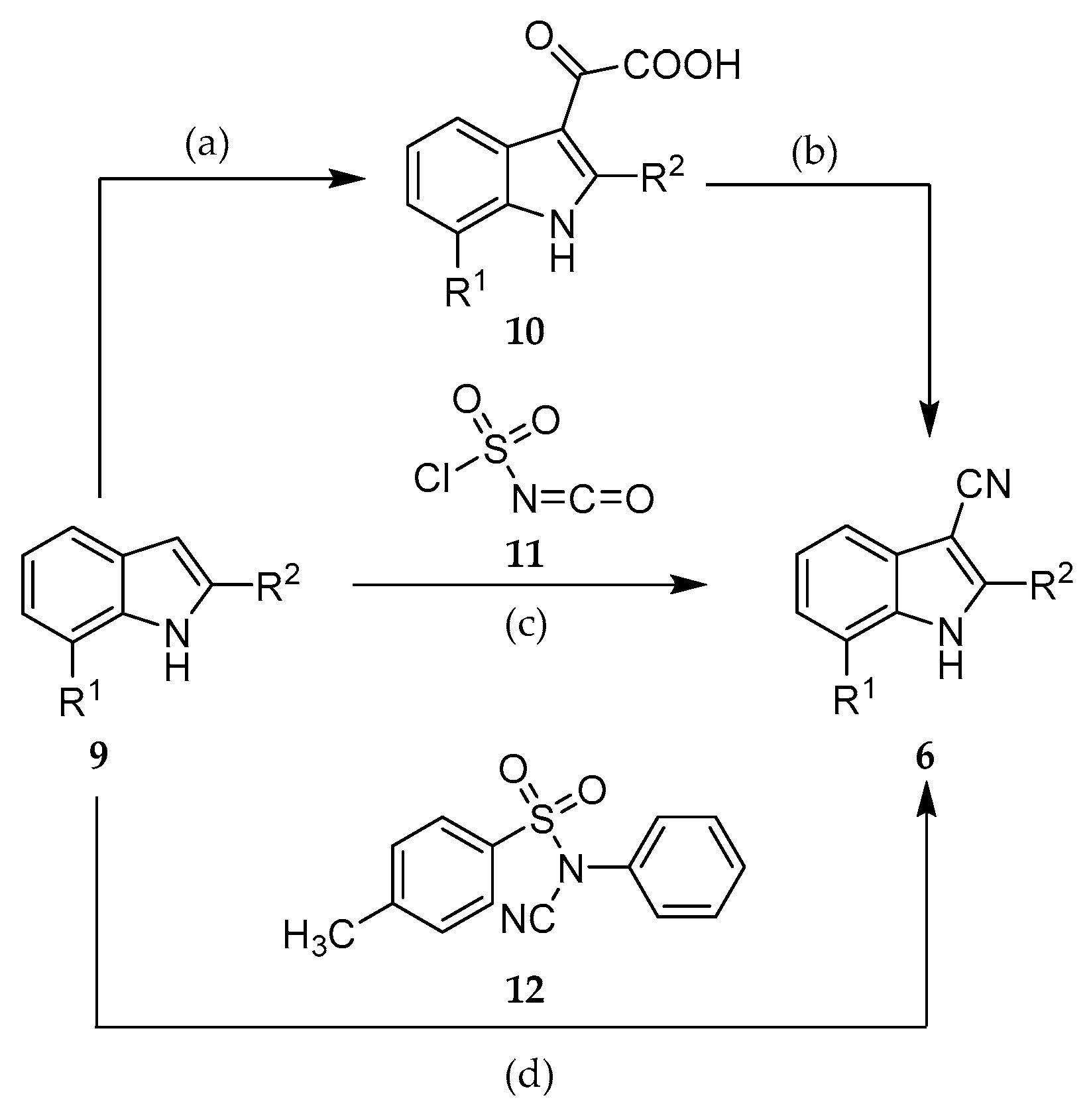
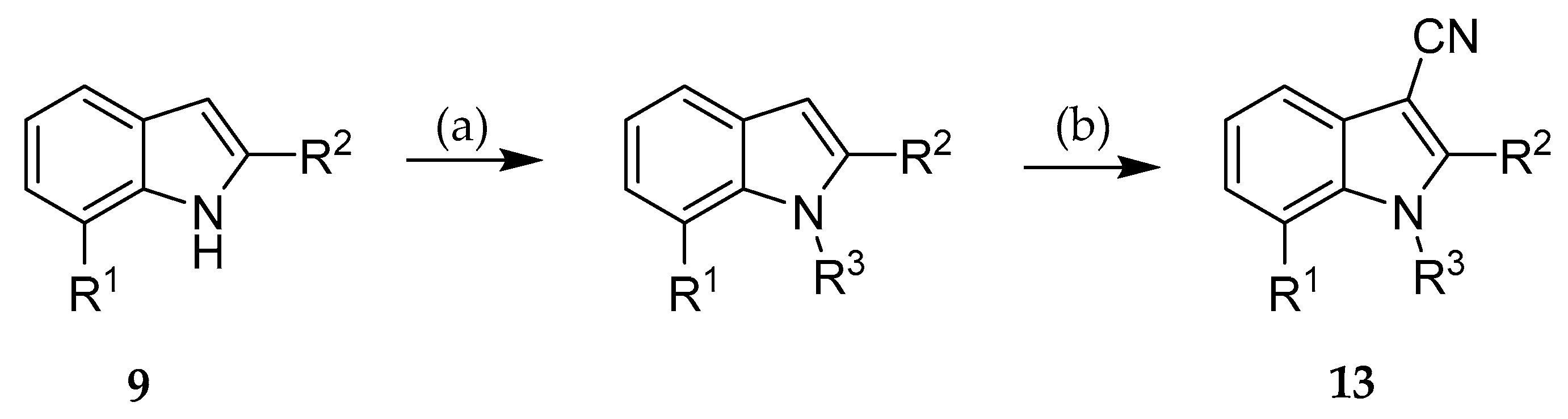
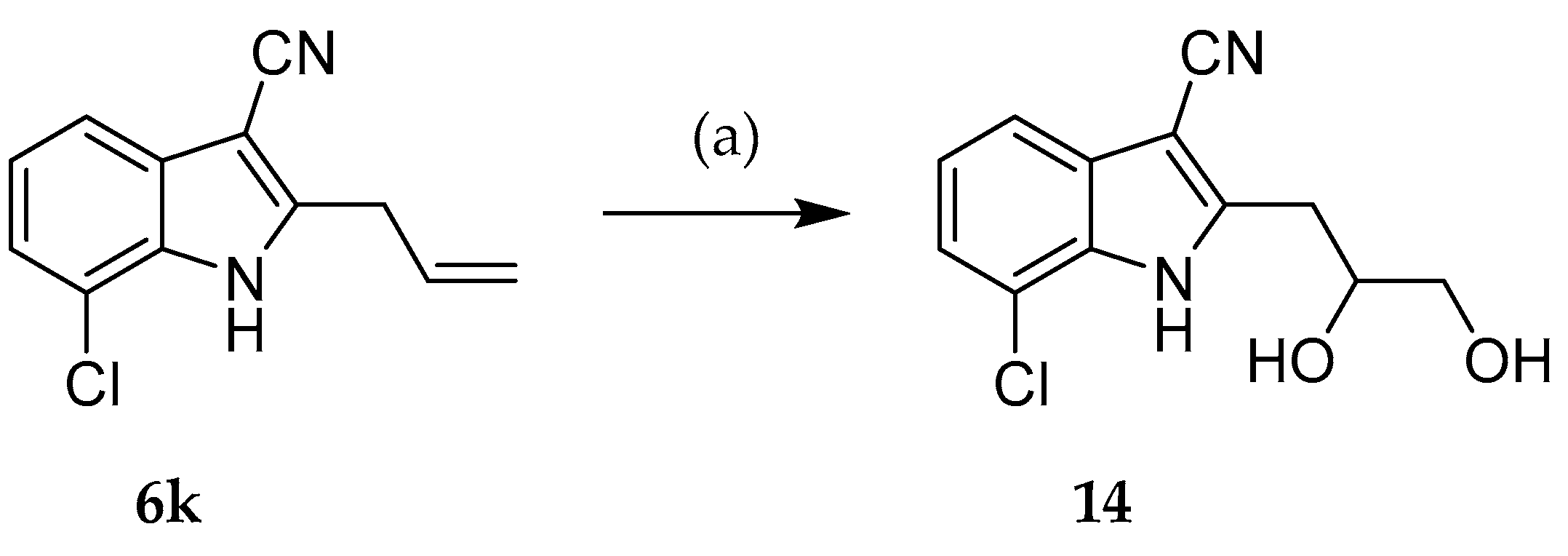
| Compound | R1 | R2 |
|---|---|---|
| 6a | Cl | H |
| 6b | Br | H |
| 6c | I | H |
| 6d | H | Phenyl |
| 6e | CH3 | Phenyl |
| 6f | Cl | Phenyl |
| 6g | Br | Phenyl |
| 6h | I | Phenyl |
| 6i | Cl | 4-Cl-Phenyl |
| 6j | Cl | 4-OCH3-Phenyl |
| 6k | Cl | Allyl |
| 6l | Cl | 2-(1,3-Dioxan-2-yl)ethyl |
| 6m | I | 4-Cl-Phenyl |
| 6n | I | 4-OCH3-Phenyl |
| 6o | I | 3-OCH3-Phenyl |
| 6p | I | Pyridin-3-yl |
| 6q | I | Isopropyl |
| 6r | I | Cyclopropyl |
| 6s | I | Cyclopentyl |
| Compound | R1 | R2 | R3 |
|---|---|---|---|
| 13a | Cl | H | Benzyl |
| 13b | Br | H | Benzyl |
| 13c | Cl | H | CH3 |
| 13d | Br | H | CH3 |
| 13e | Br | Phenyl | CH3 |
| 13f | I | Phenyl | CH3 |
| Compound | IC50 [µM]/Residual Activity at 10 µM [%] | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| DYRK 1A | DYRK 1B | DYRK2 | DYRK3 | CLK1 | CLK2 | CLK3 | CLK4 | GSK3 | |
| 6a | 3.30 | >10 | >10 | >10 | 3.33 | >10 | >10 | >10 | >10 |
| 6b | 1.10 | 5.9 | >10 | >10 | 2.9 | >10 | >10 | 4 | >10 |
| 6c | 0.410 | 1.8 | 8 | >10 | 1.9 | 8 | >10 | 1.8 | >10 |
| 6d | 0.4 | 6 | - | - | 0.800 | >10 | - | 2 | - |
| 6e | 35% | 74% | 75% | 105% | 56% | 75% | 124% | 43% | n.t. |
| 6f | 0.040 | >10 | >10 | >10 | 0.070 | >10 | >10 | 0.120 | >10 |
| 6g | 0.025 | 0.120 | >10 | >10 | 0.067 | 0.433 | >10 | 0.033 | n.t. |
| 6h | 0.010 | 0.570 | >10 | >10 | 0.080 | 2 | >10 | 0.050 | >10 |
| 6i | 60% | n.t. | n.t. | n.t. | 68% | n.t. | n.t. | n.t. | 112% |
| 6j | 60% | n.t. | n.t. | n.t. | 47% | n.t. | n.t. | n.t. | 118% |
| 6k | 0.690 | 1.3 | >10 | >10 | 0.533 | 1.3 | >10 | 0.533 | n.t. |
| 6l | 3.9 | >10 | >10 | >10 | 4.9 | >10 | >10 | 4 | n.t. |
| 6m | 42% | 79% | 116% | 101% | 55% | 92% | 130% | 36% | n.t. |
| 6n | 0.233 | >10 | >10 | >10 | 0.333 | >10 | >10 | 0.220 | n.t. |
| 6o | 0.210 | 0.633 | >10 | >10 | 0.633 | 3.3 | >10 | 0.290 | n.t. |
| 6p | 0.08 | 0.3 | - | - | 0.200 | >10 | - | >10 | n.t. |
| 6q | 0.28 | 0.53 | >10 | >10 | 0.1 | 0.7 | >10 | 0.1 | 1.3 |
| 6r | 0.14 | 0.5 | 8 | >10 | 0.110 | 1 | - | 0.1 | - |
| 6s | 0.07 | 0.6 | - | - | 0.12 | 1 | - | 0.1 | >10 |
| 13a | 97% | 78% | 144% | 95% | 102% | 95% | 127% | 84% | n.t. |
| 13b | 81% | n.t. | n.t. | n.t. | 53% | n.t. | n.t. | n.t. | 114% |
| 13c | 32% | 47% | 117% | 95% | 56% | 70% | 97% | 30% | n.t. |
| 13d | 1.9 | >10 | >10 | >10 | 3.33 | >10 | >10 | >10 | >10 |
| 13e | 2.3 | 10 | - | - | >10 | - | - | 1 | - |
| 13f | 0.65 | 2.1 | - | - | 0.6 | >10 | - | 1 | - |
| 14 | 2 | 4.2 | - | - | 2 | 10 | - | 2 | 2 |
| Compound | 1 µM | 3 µM | 10 µM |
|---|---|---|---|
| 6f | 94 | 98 | 85 |
| 6h | 97 | 97 | 87 |
| 6s | 99 | 100 | 82 |
| 13b | 94 | 94 | 66 |
| KuFal194 (5) | 78 | 75 | 45 |
| Staurosporine b | 20 |
| Compound | logP | LLE | Scalc, pH 7.4 [µM] | Sexp., therm., pH 7.4 [µM] b | Sexp., kin., pH 7.4 [µM] d |
|---|---|---|---|---|---|
| KuFal194 (5) | 4.22 | 4.00 | 13183 | < 0.5 | 5.28 (4.99–5.57) |
| 6a | 2.53 | 2.95 | 525 | 143 ± 6.3c | 116 ± 14 |
| 6b | 2.70 | 3.26 | 224 | 136 ± 11 | 142 ± 14 |
| 6c | 2.86 | 3.53 | 263 | 45.2 (44.8–45.6) | 261 (254–267) |
| 6f | 4.10 | 3.30 | 2.63 | <0.5 | 14.7 ± 4.5 |
| 6g | 4.26 | 3.34 | 1.26 | <0.5 | 14.8 ± 0.26 |
| 6h | 4.42 | 3.58 | 1.66 | <0.5 | 4.57 (4.11–5.03) |
| 6p | 3.21 | 3.89 | 25.1 | n.d. | 10.0 (7.21–12.9) |
| 6r | 3.48 | 3.37 | 37.2 | <0.5 | 38.1 (37.6–38.6) |
| 6s | 4.37 | 2.78 | 3.80 | <0.5 | 16.2 (15.2–17.3) |
| 13f | 4.65 | 1.54 | 12.0 | <0.5 | 37.5 (36.6–38.4) |
| 14 | 1.11 | 4.59 | 912 | 3447 ± 182 c | n.d. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meine, R.; Becker, W.; Falke, H.; Preu, L.; Loaëc, N.; Meijer, L.; Kunick, C. Indole-3-Carbonitriles as DYRK1A Inhibitors by Fragment-Based Drug Design. Molecules 2018, 23, 64. https://doi.org/10.3390/molecules23020064
Meine R, Becker W, Falke H, Preu L, Loaëc N, Meijer L, Kunick C. Indole-3-Carbonitriles as DYRK1A Inhibitors by Fragment-Based Drug Design. Molecules. 2018; 23(2):64. https://doi.org/10.3390/molecules23020064
Chicago/Turabian StyleMeine, Rosanna, Walter Becker, Hannes Falke, Lutz Preu, Nadège Loaëc, Laurent Meijer, and Conrad Kunick. 2018. "Indole-3-Carbonitriles as DYRK1A Inhibitors by Fragment-Based Drug Design" Molecules 23, no. 2: 64. https://doi.org/10.3390/molecules23020064





