Plants of the Genus Zingiber as a Source of Bioactive Phytochemicals: From Tradition to Pharmacy
Abstract
:1. Introduction
2. Genus Zingiber Plant Cultivation
3. Chemical Composition of Essential Oils Obtained from Genus Zingiber Plants
4. The Genus Zingiber in Traditional Medicine
4.1. Medicinal Uses of Ginger
4.1.1. Ginger in the Indian System of Medicine
4.1.2. Ginger in the Chinese and Japanese Systems of Medicine
4.1.3. Ginger in the Unani System of Medicine
4.2. Examples of Ginger Species and Their Uses in Traditional Medicine
4.2.1. Zingiber officinale Roscoe
4.2.2. Zingiber montanum (J. Koenig) Link ex A. Dietr
4.2.3. Zingiber mioga (Thunb.) Roscoe
4.2.4. Zingiber spectabile Griff.
4.2.5. Zingiber zerumbet (L.) Sm.
4.2.6. Zingiber ottensii Valeton
5. Essential Oil Obtained from Genus Zingiber Plants as a Food Preservative
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Iriti, M.; Varoni, E.M.; Vitalini, S. Melatonin in traditional Mediterranean diets. J. Pineal Res. 2010, 49, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Varoni, E.M. Melatonin in Mediterranean diet, a new perspective. J. Sci. Food Agric. 2015, 95, 2355–2359. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Varoni, E.M. Chemopreventive potential of flavonoids in oral squamous cell carcinoma in human studies. Nutrients 2013, 5, 2564–2576. [Google Scholar] [CrossRef] [PubMed]
- Varoni, E.M.; Lo Faro, A.F.; Sharifi-Rad, J.; Iriti, M. Anticancer Molecular Mechanisms of Resveratrol. Front. Nutr. 2016, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Varoni, E.M.; Vitalini, S.; Contino, D.; Lodi, G.; Simonetti, P.; Gardana, C.; Sardella, A.; Carrassi, A.; Iriti, M. Effects of red wine intake on human salivary antiradical capacity and total polyphenol content. Food Chem. Toxicol. 2013, 58, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Sahraie-Rad, M.; Izadyari, A.; Rakizadeh, S.; Sharifi-Rad, J. Preparation of strong antidandruff shampoo using medicinal plant extracts: A clinical trial and chronic dandruff treatment. J. Nat. Pharm. Prod. 2015, 10, e21517. [Google Scholar] [CrossRef]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Salehi, B.; Varoni, E.M.; Sharopov, F.; Yousaf, Z.; Ayatollahi, S.A.; Kobarfard, F.; Sharifi-Rad, M.; Afdjei, M.H.; Sharifi-Rad, M.; et al. Plants of the Melaleuca Genus as Antimicrobial Agents: From Farm to Pharmacy. Phytother. Res. 2017, 31, 1475–1494. [Google Scholar]
- Sharifi-Rad, J.; Soufi, L.; Ayatollahi, S.A.; Iriti, M.; Sharifi-Rad, M.; Varoni, E.M.; Shahri, F.; Esposito, S.; Kuhestani, K.; Sharifi-Rad, M. Anti-bacterial effect of essential oil from Xanthium strumarium against shiga toxin-producing Escherichia coli. Cell. Mol. Biol. (Noisy-Le-Grand) 2016, 62, 69–74. [Google Scholar]
- Azzimonti, B.; Cochis, A.; Beyrouthy, M.E.; Iriti, M.; Uberti, F.; Sorrentino, R.; Landini, M.M.; Rimondini, L.; Varoni, E.M. Essential Oil from Berries of Lebanese Juniperus excelsa M. Bieb Displays Similar Antibacterial Activity to Chlorhexidine but Higher Cytocompatibility with Human Oral Primary Cells. Molecules 2015, 20, 9344–9357. [Google Scholar] [PubMed]
- Sharifi-Rad, M.; Tayeboon, G.S.; Sharifi-Rad, J.; Iriti, M.; Varoni, E.M.; Razazi, S. Inhibitory activity on type 2 diabetes and hypertension key-enzymes, and antioxidant capacity of Veronica persica phenolic-rich extracts. Cell. Mol. Biol. (Noisy-Le-Grand) 2016, 62, 80–85. [Google Scholar]
- Bor, T.; Aljaloud, S.O.; Gyawali, R.; Ibrahim, S.A. Antimicrobials from Herbs, Spices, and Plants. In Encapsulations: Nanotechnology in the Agri-Food Industry, Vol. 2; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 269–288. [Google Scholar]
- El-Ghorab, A.H.; Nauman, M.; Anjum, F.M.; Hussain, S.; Nadeem, M.A. Comparative study on chemical composition and antioxidant activity of ginger (Zingiber officinale) and cumin (Cuminum cyminum). J. Agric. Food Chem. 2010, 58, 8231–8237. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, R.; Hayek, S.A.; Ibrahim, S.A. Plant extracts as antimicrobials in food products: Mechanisms of action, extraction methods, and applications. In Handbook of Natural Antimicrobials for Food Safety and Quality; Taylor, T.M., Ed.; Woodhead Publishing: Sawston, UK, 2014. [Google Scholar]
- Hayek, S.A.; Gyawali, R.; Ibrahim, S.A. Antimicrobial natural products. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Mendez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2013; Volume 2, pp. 910–921. [Google Scholar]
- Sharifi-Rad, J.; Salehi, B.; Stojanović-Radić, Z.Z.; Fokou, P.V.T.; Sharifi-Rad, M.; Mahady, G.B.; Sharifi-Rad, M.; Masjedi, M.R.; Lawal, T.O.; Ayatollahi, S.A.; et al. Medicinal plants used in the treatment of tuberculosis-Ethnobotanical and ethnopharmacological approaches. Biotechnol. Adv. 2017. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Sabulal, B.; Dan, M.; Kurup, R.; Pradeep, N.S.; Valsamma, R.K.; George, V. Caryophyllene-rich rhizome oil of Zingiber nimmonii from South India: Chemical characterization and antimicrobial activity. Phytochemistry 2006, 67, 2469–2473. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Majumder, P.B.; Mandi, S.S. Species-specific AFLP markers for identification of Zingiber officinale, Z. montanumand, Z. zerumbet (Zingiberaceae). Genet. Mol. Res. 2011, 10, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Rahmat, A. Antioxidant Activities, Total Phenolics and Flavonoids Content in Two Varieties of Malaysia Young Ginger (Zingiber officinale Roscoe). Molecules 2010, 15, 4324–4333. [Google Scholar] [CrossRef] [PubMed]
- Sivasothy, Y.; Chong, W.K.; Hamid, A.; Eldeen, I.M.; Sulaiman, S.F.; Awang, K. Essential oils of Zingiber officinale var. rubrum Theilade and their antibacterial activities. Food Chem. 2011, 124, 514–517. [Google Scholar]
- Sharma, P.K.; Singh, V.; Ali, M. Chemical composition and antimicrobial activity of fresh rhizome essential oil of Zingiber officinale Roscoe. Pharmacogn. J. 2016, 8, 185–190. [Google Scholar] [CrossRef]
- Bellik, Y. Total antioxidant activity and antimicrobial potency of the essential oil and oleoresin of Zingiber officinale Roscoe. Asian Pac. J. Trop. Dis. 2014, 4, 40–44. [Google Scholar] [CrossRef]
- Jiang, H.; Xie, Z.; Koo, H.J.; McLaughlin, S.P.; Timmermann, B.N.; Gang, D.R. Metabolic profiling and phylogenetic analysis of medicinal Zingiber species: Tools for authentication of ginger (Zingiber officinale Rosc.). Phytochemistry 2006, 67, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Shukla, Y.; Singh, M. Cancer preventive properties of ginger: A brief review. Food Chem. Toxicol. 2007, 45, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Kapoor, I.P.S.; Singh, P.; de Heluani, C.S.; de Lampasona, M.P.; Catalan, C.A.N. Chemistry, antioxidant and antimicrobial investigations on essential oil and oleoresins of Zingiber officinale. Food Chem. Toxicol. 2008, 46, 3295–3302. [Google Scholar] [CrossRef] [PubMed]
- El-Baroty, G.S.; El-Baky, H.A.; Farag, R.S.; Saleh, M.A. Characterization of antioxidant and antimicrobial compounds of cinnamon and ginger essential oils. Afr. J. Biochem. Res. 2010, 4, 167–174. [Google Scholar]
- Kumar, G.; Karthik, L.; Rao, K.B. A review on pharmacological and phytochemical properties of Zingiber officinale Roscoe (Zingiberaceae). J. Pharm. Res. 2011, 4, 2963–2966. [Google Scholar]
- Jayashree, E.; Kandiannan, K.; Prasath, D.; Rashid, P.; Sasikumar, B.; Senthil Kumar, C.M.; Srinivasan, V.; Suseela Bhai, R.; Thankamani, C.K. Ginger. ICAR-Indian Institute of Spices Research Kozhikode-673 012, Kerala; ICAR-Indian Institute of Spices Research: Kerala, India, 2015. [Google Scholar]
- Govindarajan, V.S. Ginger-chemistry, technology, and quality evaluation: Part 1. Crit. Rev. Food Sci. Nutr. 1982, 17, 1–96. [Google Scholar] [PubMed]
- Sutarno, H.; Hadad, E.A.; Brink, M. Zingiber officinale Roscoe. In Plant Resources of South-East Asia No 13. Spices; de Guzman, C.C., Siemonsma, J.S., Eds.; Backhuys Publishers: Leiden, The Netherlands, 1999; pp. 239–244. [Google Scholar]
- Nwaogu, E.N. Soil fertility changes and their effects on ginger (Zingiber officinale Rosc.) yield response in an ultisol under different pigeon pea hedgerow alley management in South Eastern Nigeria. Afr. J. Agric. Res. 2014, 9, 2158–2166. [Google Scholar] [CrossRef]
- Davidson, A. The Oxford Companion to Food, 2nd ed.; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Hayden, A.L.; Brigham, L.A.; Giacomelli, G.A. Aeroponic Cultivation of Ginger (Zingiber officinale) Rhizomes. In Proceedings of the International Symposium on Protected Cultivation in Mild Winter Climates: Production, Pest Management and Global Competition, Kissimmee, FL, USA, 23–27 March 2004. [Google Scholar]
- Archana, D.; Vigya, K.; Latha, R. Micropropagation and cytogenetic assessment of Zingiber species of Northeast India. 3 Biotech 2013, 3, 471–479. [Google Scholar]
- Mesomo, M.C.; Corazza, M.L.; Ndiaye, P.M.; Dalla Santa, O.R.; Cardozo, L.; de Paula Scheer, A. Supercritical CO2 extracts and essential oil of ginger (Zingiber officinale R.): Chemical composition and antibacterial activity. J. Supercrit. Fluid 2013, 80, 44–49. [Google Scholar] [CrossRef]
- An, K.; Zhao, D.; Wang, Z.; Wu, J.; Xu, Y.; Xiao, G. Comparison of different drying methods on Chinese ginger (Zingiber officinale Roscoe): Changes in volatiles, chemical profile, antioxidant properties, and microstructure. Food Chem. 2016, 197, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Nogueira de Melo, G.A.; Grespan, R.; Fonseca, J.P.; Farinha, T.O.; da Silva, E.L.; Romero, A.L.; Cuman, R.K.N. Inhibitory effects of ginger (Zingiber officinale Roscoe) essential oil on leukocyte migration in vivo and in vitro. J. Nat. Med. 2011, 65, 241–246. [Google Scholar] [CrossRef] [PubMed]
- De Barros Fernandes, R.V.; Borges, S.V.; Silva, E.K.; da Silva, Y.F.; de Souza, H.J.B.; do Carmo, E.L.; Botrel, D.A. Study of ultrasound-assisted emulsions on microencapsulation of ginger essential oil by spray drying. Ind. Crop Prod. 2016, 94, 413–423. [Google Scholar] [CrossRef]
- Babarinde, S.A.; Sunnie-Ododo, M.O.; Akanbi, W.B.; Oyegoke, O.O.; Tijani, R.; Olaobaju, S.F. Comparative susceptibility of two developmental stages of hide beetle (Dermestes maculatus Degeer, 1774) to ginger (Zingiber officinale Roscoe) essential oil. J. Saudi Soc. Agric. Sci. 2016. [Google Scholar] [CrossRef]
- Pushpanathan, T.; Jebanesan, A.; Govindarajan, M. The essential oil of Zingiber officinalis Linn (Zingiberaceae) as a mosquito larvicidal and repellent agent against the filarial vector Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol. Res. 2008, 102, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.; Mohamad, R.; Rahim, R.A.; Moghaddam, A.B.; Moniri, M.; Ariff, A.; Namvab, F. ZnO-Ag core shell nanocomposite formed by green method using essential oil of wild ginger and their bactericidal and cytotoxic effects. Appl. Surf. Sci. 2016, 384, 517–524. [Google Scholar] [CrossRef]
- Nampoothiri, S.V.; Venugopalan, V.V.; Joy, B.; Sreekumar, M.M.; Menon, A.N. Comparison of essential oil composition of three ginger cultivars from sub Himalayan region. Asian Pac. J. Trop. Biomed. 2012, 2, S1347–S1350. [Google Scholar] [CrossRef]
- Singh, G.; Maurya, S.; Catalan, C.; de Lampasona, M.P. Studies on essential oils, Part 42: Chemical, antifungal, antioxidant and sprout suppressant studies on ginger essential oil and its oleoresin. Flavour Fragr. J. 2005, 20, 1–6. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Li, T.; Zhou, X.; Ding, L.; Yu, Y.; Zhang, H. Rapid analysis of the essential oils from dried Illicium verum Hook. f. and Zingiber officinale Rosc. by improved solvent-free microwave extraction with three types of microwave-absorption medium. Anal. Bioanal. Chem. 2006, 386, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-J.; Chen, C.-Y.; Lu, C.-M.; Ma, Y.-H.; Chung, L.-Y.; Wang, J.-J.; Yen, C.-M. Anthelmintic constituents from ginger (Zingiber officinale) against Hymenolepis nana. Acta Trop. 2014, 140, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Stoyanova, A.; Konakchiev, A.; Damyanova, S.; Stoilova, I.; Suu, P.T. Composition and antimicrobial activity of ginger essential oil from Vietnam. J. Essent. Oil Bear. Plants 2006, 9, 93–98. [Google Scholar] [CrossRef]
- Sukatta, U.; Rugthaworn, P.; Punjee, P.; Chidchenchey, S.; Keeratinijakal, V. Chemical composition and physical properties of oil from Plai (Zingiber cassumunar Roxb.) obtained by hydrodistillation and hexane extraction. Kasetsart J. (Nat. Sci.) 2009, 43, 212–217. [Google Scholar]
- Onyenekwe, P.C.; Hashimoto, S. The composition of the essential oil of dried Nigerian ginger (Zingiber officinale Roscoe). Eur. Food Res. Technol. 1999, 209, 407–410. [Google Scholar] [CrossRef]
- Sasidharan, I.; Nirmala, M. Comparative chemical composition and antimicrobial activity fresh & dry ginger oils (Zingiber officinale Roscoe). Int. J. Curr. Pharm. Res. 2010, 2, 40–43. [Google Scholar]
- Ravi Kiran, C.; Chakka, A.K.; Padmakumari Amma, K.P.; Nirmala Menon, A.; Sree Kumar, M.M.; Venugopalan, V.V. Essential oil composition of fresh ginger cultivars from North-East India. J. Essent. Oil Res. 2013, 25, 380–387. [Google Scholar] [CrossRef]
- Nerilo, S.B.; Rocha, G.H.O.; Tomoike, C.; Mossini, S.A.G.; Grespan, R.; Mikcha, J.M.G.; Machinski, M. Antifungal properties and inhibitory effects upon aflatoxin production by Zingiber officinale essential oil in Aspergillus flavus. Int. J. Food Sci. Technol. 2016, 51, 286–292. [Google Scholar] [CrossRef]
- Das, A.; Kasoju, N.; Bora, U.; Rangan, L. Chemico-biological investigation of rhizome essential oil of Zingiber moran—Native to Northeast India. Med. Chem. Res. 2013, 22, 4308–4315. [Google Scholar] [CrossRef]
- Chaiyana, W.; Anuchapreeda, S.; Leelapornpisid, P.; Phongpradist, R.; Viernstein, H.; Mueller, M. Development of microemulsion delivery system of essential oil from Zingiber cassumunar Roxb. rhizome for improvement of stability and anti-inflammatory activity. AAPS PharmSciTech 2017, 18, 1332–1342. [Google Scholar] [CrossRef] [PubMed]
- Boonyanugomol, W.; Kraisriwattana, K.; Rukseree, K.; Boonsam, K.; Narachai, P. In vitro synergistic antibacterial activity of the essential oil from Zingiber cassumunar Roxb against extensively drug-resistant Acinetobacter baumannii strains. J. Infect. Public Health 2017, 10, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Kamazeri, T.S.A.T.; Samah, O.A.; Taher, M.; Susanti, D.; Qaralleh, H. Antimicrobial activity and essential oils of Curcuma aeruginosa, Curcuma mangga, and Zingiber cassumunar from Malaysia. Asian Pac. J. Trop. Med. 2012, 5, 202–209. [Google Scholar] [CrossRef]
- Manochai, B.; Paisooksantivatana, Y.; Choi, H.; Hong, J.H. Variation in DPPH scavenging activity and major volatile oil components of cassumunar ginger, Zingiber montanum (Koenig), in response to water deficit and light intensity. Sci. Hortic. 2010, 126, 462–466. [Google Scholar] [CrossRef]
- Zhannan, Y.; Shiqiong, L.; Quancai, P.; Chao, Z.; Zhengwen, Y. GC-MS analysis of the essential oil of Coral Ginger (Zingiber corallinum Hance) rhizome obtained by supercritical fluid extraction and steam distillation extraction. Chromatographia 2009, 69, 785–790. [Google Scholar] [CrossRef]
- Rana, V.; Verdeguer, M.; Blasquez, M. Chemical composition of the essential oil of Zingiber zerumbet var. darcyi. Nat. Prod. Commun. 2012, 7, 1369–1370. [Google Scholar] [PubMed]
- Govindarajan, M.; Rajeswary, M.; Arivoli, S.; Tennyson, S.; Benelli, G. Larvicidal and repellent potential of Zingiber nimmonii (J. Graham) Dalzell (Zingiberaceae) essential oil: An eco-friendly tool against malaria, dengue, and lymphatic filariasis mosquito vectors. Parasitol. Res. 2016, 115, 1807–1816. [Google Scholar] [PubMed]
- Chairgulprasert, V.; Prasertsongskun, S.; Wichaporn, W. Chemical constituents of the essential oil and antibacterial activity of Zingiber wrayi var. halabala. Songklanakarin J. Sci. Technol. 2005, 27, 813–818. [Google Scholar]
- Kubra, I.R.; Rao, L.J. An impression on current developments in the technology, chemistry, and biological activities of ginger (Zingiber officinale Roscoe). Crit. Rev. Food Sci. Nutr. 2012, 52, 651–688. [Google Scholar] [CrossRef] [PubMed]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A.M. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari-Khosravi, H.; Naderi, Z.; Dehghan, A.; Nadjarzadeh, A.; Fallah Huseini, H. Effect of Ginger Supplementation on Proinflammatory Cytokines in Older Patients with Osteoarthritis: Outcomes of a Randomized Controlled Clinical Trial. J. Nutr. Gerontol. Geriatr. 2016, 35, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Stoilova, I.; Krastanov, A.; Stoyanova, A.; Denev, P.; Gargova, S. Antioxidant activity of a ginger extract (Zingiber officinale). Food Chem. 2007, 102, 764–770. [Google Scholar] [CrossRef]
- Mashhadi, N.S.; Ghiasvand, R.; Askari, G.; Hariri, M.; Darvishi, L.; Mofid, M.R. Anti-Oxidative and Anti-Inflammatory Effects of Ginger in Health and Physical Activity: Review of Current Evidence. Int. J. Prev. Med. 2013, 4, S36–S42. [Google Scholar] [PubMed]
- Si, W.; Chen, Y.P.; Zhang, J.; Chen, Z.Y.; Chung, H.Y. Antioxidant activities of ginger extract and its constituents toward lipids. Food Chem. 2018, 239, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Höferl, M.; Stoilova, I.; Wanner, J.; Schmidt, E.; Jirovetz, L.; Trifonova, D.; Stanchev, V.; Krastanov, A. Composition and Comprehensive Antioxidant Activity of Ginger (Zingiber officinale) Essential Oil from Ecuador. Nat. Prod. Commun. 2015, 10, 1085–1090. [Google Scholar] [PubMed]
- Ahmad, B.; Rehman, M.U.; Amin, I.; Arif, A.; Rasool, S.; Bhat, S.A.; Afzal, I.; Hussain, I.; Bilal, S.; Mir, M.U.R. A Review on Pharmacological Properties of Zingerone (4-(4-Hydroxy-3-methoxyphenyl)-2-butanone). Sci. World J. 2015, 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Yusof, Y.A.M. Gingerol and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 929, 177–207. [Google Scholar]
- Jiang, Y.; Turgeon, D.K.; Wright, B.D.; Sidahmed, E.; Ruffin, M.T.; Brenner, D.E.; Sen, A.; Zick, S.M. Effect of ginger root on cyclooxygenase-1 and 15-hydroxyprostaglandin dehydrogenase expression in colonic mucosa of humans at normal and increased risk for colorectal cancer. Eur. J. Cancer Prev. 2013, 22, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Zick, S.M.; Turgeon, D.K.; Ren, J.; Ruffin, M.T.; Wright, B.D.; Sen, A.; Djuric, Z.; Brenner, D.E. Pilot Clinical Study of the Effects of Ginger Root Extract on Eicosanoids in Colonic Mucosa of Subjects at Increased Risk for Colorectal Cancer. Mol. Carcinog. 2015, 54, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Mansourm, M.S.; Ni, Y.M.; Roberts, A.L.; Kelleman, M.; Roychoudhury, A.; St-Onge, M.P. Ginger consumption enhances the thermic effect of food and promotes feelings of satiety without affecting metabolic and hormonal parameters in overweight men: A pilot study. Metabolism 2012, 61, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Shidfar, F.; Rajab, A.; Rahideh, T.; Khandouzim, N.; Hosseini, S.; Shidfar, S. The effect of ginger (Zingiber officinale) on glycemic markers in patients with type 2 diabetes. J. Complement. Integr. Med. 2015, 12, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Arablou, T.; Aryaeian, N.; Valizadeh, M.; Sharifi, F.; Hosseini, A.; Djalali, M. The effect of ginger consumption on glycemic status, lipid profile and some inflammatory markers in patients with type 2 diabetes mellitus. Int. J. Food Sci. Nutr. 2014, 65, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari-Khosravi, H.; Talaei, B.; Jalali, B.A.; Najarzadeh, A.; Mozayan, M.R. The effect of ginger powder supplementation on insulin resistance and glycemic indices in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Complement. Ther. Med. 2014, 22, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Tabibi, H.; Imani, H.; Atabak, S.; Najafi, I.; Hedayati, M.; Rahmani, L. Effects of Ginger on Serum Lipids and Lipoproteins in Peritoneal Dialysis Patients: A Randomized Controlled Trial. Perit. Dial. Int. 2016, 36, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh, M.; Bialvaei, A.Z.; Aghazadeh, M.; Kabiri, F.; Saliani, N.; Yousef, M.; Eslami, H.; Kafl, H.S. Survey of the Antibioflm and Antimicrobial Effects of Zingiber ofcinale (in Vitro Study). Jundishapur J. Microbiol. 2016, 9, e30167. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ng, T.B. An antifungal protein from ginger rhizomes. Biochem. Biophys. Res. Commun. 2005, 336, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Nguefack, J.; Leth, V.; Amvam Zollo, P.H.; Mathur, S.B. Evaluation of fve essential oils from aromatic plants of Cameroon for controlling food spoilage and mycotoxin producing fungi. Int. J. Food Microbiol. 2004, 94, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Ficker, C.E.; Arnason, J.T.; Vindas, P.S.; Alvarez, L.P.; Akpagana, K.; Gbeassor, M.; De Souza, C.; Smith, M.L. Inhibition of human pathogenic fungi by ethnobotanically selected plant extracts. Mycoses 2003, 46, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; Al shabrmi, F.M.; Aly, S.M. Active ingredients of ginger as potential candidates in the prevention and treatment of diseases via modulation of biological activities. Int. J. Physiol. Pathophysiol. Pharmacol. 2014, 6, 125–136. [Google Scholar] [PubMed]
- Mahyari, S.; Mahyari, B.; Emami, S.A.; Malaekeh-Nikouei, B.; Jahanbakhsh, S.P.; Sahebkar, A.; Mohammadpour, A.H. Evaluation of the efficacy of a polyherbal mouthwash containing Zingiber officinale, Rosmarinus officinalis and Calendula officinalis extracts in patients with gingivitis: A randomized double-blind placebo-controlled trial. Complement. Ther. Clin. Pract. 2016, 22, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, E.; Visser, J.; Koen, N.; Musekiwa, A. A systematic review and meta-analysis of the effect and safety of ginger in the treatment of pregnancy-associated nausea and vomiting. Nutr. J. 2014, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- McParlin, C.; O’Donnell, A.; Robson, S.C.; Beyer, F.; Moloney, E.; Bryant, A.; Bradley, J.; Muirhead, C.R.; Nelson-Piercy, C.; Newbury-Birch, D.; et al. Treatments for Hyperemesis Gravidarum and Nausea and Vomiting in Pregnancy: A Systematic Review. JAMA 2016, 316, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; Ried, K.; McCarthy, A.L.; Vitetta, L.; Sali, A.; McKavanagh, D.; Isenring, L. Ginger-Mechanism of action in chemotherapy-induced nausea and vomiting: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 141–146. [Google Scholar] [PubMed]
- Bone, M.E.; Wilkinson, D.J.; Young, J.R.; McNeil, J.; Charlton, S. Ginger root—A new antiemetic. The effect of ginger root on postoperative nausea and vomiting after major gynaecological surgery. Anaesthesia 1990, 45, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Emrani, Z.; Shojaei, E.; Khalili, H. Ginger for Prevention of Antituberculosis-induced Gastrointestinal Adverse Reactions Including Hepatotoxicity: A Randomized Pilot Clinical Trial. Phytother. Res. 2016, 30, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Shirvani, M.A.; Motahari-Tabari, N.; Alipour, A. The effect of mefenamic acid and ginger on pain relief in primary dysmenorrhea: A randomized clinical trial. Arch. Gynecol. Obstet. 2015, 291, 1277–1281. [Google Scholar] [CrossRef] [PubMed]
- Ozgoli, G.; Goli, M.; Moattar, F. Comparison of effects of ginger, mefenamic acid, and ibuprofen on pain in women with primary dysmenorrhea. J. Altern. Complement. Med. 2009, 15, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Bartels, E.M.; Folmer, V.N.; Bliddal, H.; Altman, R.D.; Juhl, C.; Tarp, S.; Zhang, W.; Christensen, R. Efficacy and safety of ginger in osteoarthritis patients: A meta-analysis of randomized placebo-controlled trials. Osteoarthr. Cartil. 2015, 23, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Bliddal, H.; Rosetzsky, A.; Schlichting, P.; Weidner, M.S.; Andersen, L.A.; Ibfelt, H.H.; Christensen, K.; Jensen, O.N.; Barslev, J. A randomized, placebo-controlled, cross-over study of ginger extracts and ibuprofen in osteoarthritis. Osteoarthr. Cartil. 2000, 8, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Maghbooli, M.; Golipour, F.; Moghimi Esfandabadi, A.; Yousefi, M. Comparison between the efficacy of ginger and sumatriptan in the ablative treatment of the common migraine. Phytother. Res. 2014, 28, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Cady, R.K.; Schreiber, C.P.; Beach, M.E.; Hart, C.C. Gelstat Migraine (sublingually administered feverfew and ginger compound) for acute treatment of migraine when administered during the mild pain phase. Med. Sci. Monit. 2005, 11, PI65–P169. [Google Scholar] [PubMed]
- Kumar, S.; Singh, U.N.; Saxena, K.; Saxena, R. Supplementation of ginger with anti-tuberculosis treatment (ATT): A better approach to treat anemic pulmonary tuberculosis patients. Int. J. Herb. Med. 2013, 1, 17–20. [Google Scholar]
- Drozdov, V.N.; Kim, V.A.; Tkachenko, E.V.; Varvanina, G.G. Influence of a specific ginger combination on gastropathy conditions in patients with osteoarthritis of the knee or hip. J. Altern. Complement. Med. 2012, 18, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Remadevi, R.; Surendran, E.; Ravindran, P.N. Properties and medicinal uses of ginger. In Ginger: The Genus Zingiber; Babu, K.N., Ravindran, P.N., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 489–508. [Google Scholar]
- Ginger: The Genus Zingiber; Nirmal Babu, K.; Ravindran, P.N. (Eds.) CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Kumar, K.M.P.; Asish, G.R.; Sabu, M.; Balachandran, I. Significance of gingers (Zingiberaceae) in Indian System of Medicine—Ayurveda: An overview. Anc. Sci. Life 2013, 32, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Aiyer, K.N.; Kolammal, M. Pharmacognosy of Ayurvedic Drugs of Kerala; Department of Pharmacognosy, University of Kerala: Kerala, India, 1996; Volume 9. [Google Scholar]
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants (Vol. 1–4), 2nd ed.; Bishen Singh Mahendrapal Singh: Delhi, India, 1991; p. 2971. [Google Scholar]
- Nadkarni, K.M. Indian Medicinal Plants and Drugs—Their Medicinal Properties and Uses; Asiatic Publishing House: New Delhi, India, 1998; p. 450. [Google Scholar]
- Pruthy, J.S. Spices and Condiments; National Book Trust of India: New Delhi, India, 1979. [Google Scholar]
- Benskey, D.; Gamble, A. (Eds.) Chinese Herbal Medicine: Materia Medica; Eastland Press: Seattle, DC, USA, 1986. [Google Scholar]
- Blumenthal, M. (Ed.) The complete German Commission E Monographs. In Therapeutic Guide to Herbal Medicine; American Botany Council: Austin, TX, USA, 1999. [Google Scholar]
- Pakrashi, S.C.; Pakrashi, A. Ginger; Vedams: New Delhi, India, 2003. [Google Scholar]
- O’Hara, M.; Keifer, D.; Farrel, K.; Kemper, K. A review of 12 commonly used medicinal herbs. Arch. Fam. Med. 1998, 7, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Awang, D.V.C. Ginger. Can. Pharm. J. 1992, 125, 309–311. [Google Scholar]
- Gupta, S.K.; Sharma, A. Medicinal properties of Zingiber officinale Roscoe—A Review. IOSR J. Pharm. Biol. Sci. 2014, 9, 124–129. [Google Scholar]
- Honolulu Advertiser. Canoe Plants of Ancient Hawai`i. 2005. Available online: https://www.canoeplants.com/awapuhi.html (accessed on 18 February 2017).
- Wolff, X.Y.; Astuti, I.P.; Brink, M. Zingiber G.R. Boehmer. In Plant Resources of South-East Asia (PROSEA) No. 13: Spices; Backhuys Publishers: Leiden, The Netherlands, 1999; pp. 233–238. [Google Scholar]
- Datiles, M.J.; Acevedo-Rodríguez, P. Zingiber montanum (cassumunar ginger). Centre for Agriculture and Biosciences International (CABI) Website. Available online: http://www.cabi.org/isc/datasheet/57536 (accessed on 2 March 2017).
- Anasamy, T.; Abdul, A.B.; Sukari, M.A.; Abdelwahab, S.I.; Mohan, S.; Kamalidehghan, B.; Azid, M.Z. A Phenylbutenoid Dimer, cis-3-(3′,4′-Dimethoxyphenyl)-4-[(E)-3′′′,4′′′-Dimethoxystyryl] Cyclohex-1-ene, Exhibits Apoptogenic Properties in T-Acute Lymphoblastic Leukemia Cells via Induction of p53-Independent Mitochondrial Signalling Pathway. Evid Based Complement. Altern. Med. 2013, 2013, 14. [Google Scholar] [CrossRef] [PubMed]
- Mihashi, H.; Okada, M. Illustrated Medicinal Plants of the World in Colour; Hokuryūkan Co., Ltd.: Tokyo, Japan, 1988; p. 666. (In Japanese) [Google Scholar]
- Wiart, C. Medicinal Plants of China, Korea, and Japan: Bioresources for Tomorrow’s Drugs and Cosmetics; CRC Press: Boca Raton, FL, USA, 2012; p. 67. [Google Scholar]
- Farifah Hanum, I.; Hamzah, N. The Use of Medicinal Plant Species by the Temuan Tribe of Ayer Hitam Forest, Selangor, Peninsular Malaysia. Pertanika J. Trap. Agric. Sci. 1999, 22, 85–94. [Google Scholar]
- Weiss, E.A. Spice Crops; CABI (Centre for Agriculture and Bioscience International): Wallingford, UK, 2002; p. 338. [Google Scholar]
- Chee, B.J. The Spectacular Ginger: Zingiber spectabile; Malaysian Naturalist: Kuala Lumpur, Malaysia, 2010; pp. 12–13. [Google Scholar]
- Koga, A.Y.; Beltrame, F.L.; Pereira, A.V. Several aspects of Zingiber zerumbet: A review. Rev. Bras. Farmacogn. 2016, 26, 385–391. [Google Scholar] [CrossRef]
- Mahmood, N.D.; Nasir, N.L.; Rofiee, M.S.; Tohid, S.F.; Ching, S.M.; The, L.K.; Salleh, M.Z.; Zakaria, Z.A. Muntingia calabura L.: A Review on Its Traditional Uses, Chemical Properties and Pharmacological Observations. Pharm. Biol. 2014, 52, 1598–1623. [Google Scholar] [CrossRef] [PubMed]
- Burkill, I.H. Dictionary of Economic Products of the Malay Peninsula; Ministry of Agriculture and Cooperatives Malaysia: Kuala Lumpur, Malaysia, 1966; Volume 2, pp. 2344–2345.
- Jansen, P.C.M. Zingiber Ottensii (PROSEA). Plant Resources of Southeast Asia. Available online: http://uses.plantnet-project.org/en/Zingiber_ottensii_(PROSEA) (accessed on 29 March 2017).
- Medicinal Properties of Zingiber officinale Roscoe—A Review. Available online: www.iosrjournals.org (accessed on 15 October 2017).
- Riazi, S.; Matthews, K.R. Failure of foodborne pathogens to develop resistance to sanitizers following repeated exposure to common sanitizers. Int. Biodeterior. Biodegr. 2011, 65, 374–378. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Baipai, V.K. Essential oils: Sources of antimicrobials and food preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.; Rowsni, A.A.; Khan, Md. M.; Kabir, M.S. Antimicrobial activity of ginger (Zingiber officinale) extracts against food-borne pathogenic bacteria. Int. J. Sci. Technol. 2014, 3, 867–871. [Google Scholar]
- Prasad, M.M.; Seenayya, G. Effect of spices on the growth of red halophilic cocci isolated from salt cured fish and solar salt. Food Res. Int. 2000, 33, 793–798. [Google Scholar] [CrossRef]
- Sa-nguanpuag, K.; Srilaong, V.; Kanlayanarat, S.; Techavuthiporng, C. Ginger (Zingiber officinale) Oil as an antimicrobial agent for minimally processed produce: A case study in shredded green papaya. Int. J. Agric. Biol. 2011, 13, 895–901. [Google Scholar]
- Ortiz, M. Antimicrobial activity of onion and ginger against two foodborne pathogens Escherichia coli and Staphylococcus aureus. MOJ Food Proc. Technol. 2015. [Google Scholar] [CrossRef]
- Debbarma, J.; Kishore, P.; Nayak, B.B.; Kannuchamy, N.; Gudipati, V. Antibacterial activity of ginger, eucalyptus and sweet orange peel essential oils on fish-borne bacteria. J. Food Proc. Preserv. 2013, 37, 1022–1030. [Google Scholar]
- Jeevani Osadee Wijekoon, M.M.; Bhat, R.; Karim, A.A.; Fazilah, A. Chemical composition and antimicrobial activity of essential oil and solvent extracts of torch ginger inflorescence (Etlingera elatior Jack.). Int. J. Food Prop. 2013, 16, 1200–1210. [Google Scholar] [CrossRef]
- Mohamed, H.G.; Gaafar, A.M.; Soliman, A. Antimicrobial activities of essential oil of eight plant species from different families against some pathogenic microorganisms. Res. J. Microbiol. 2016, 11, 28–34. [Google Scholar] [CrossRef]
- Gottardi, D.; Bukvicki, D.; Prasad, S.; Tyagi, A.K. Beneficial Effects of Spices in Food Preservation and Safety. Front. Microbiol. 2016, 7, 1394. [Google Scholar] [CrossRef] [PubMed]
- Zick, S.M.; Djuric, Z.; Ruffin, M.T.; Litzinger, A.J.; Normolle, D.P.; Alrawi, S.; Feng, M.R.; Brenner, D.E. Pharmacokinetics of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol and conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1930–1936. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, S.; Pittler, M.H.; Roufogalis, B.D. Zingiberis rhizoma: A comprehensive review on the ginger effect and efficacy profiles. Phytomedicine 2005, 12, 684–701. [Google Scholar] [CrossRef] [PubMed]
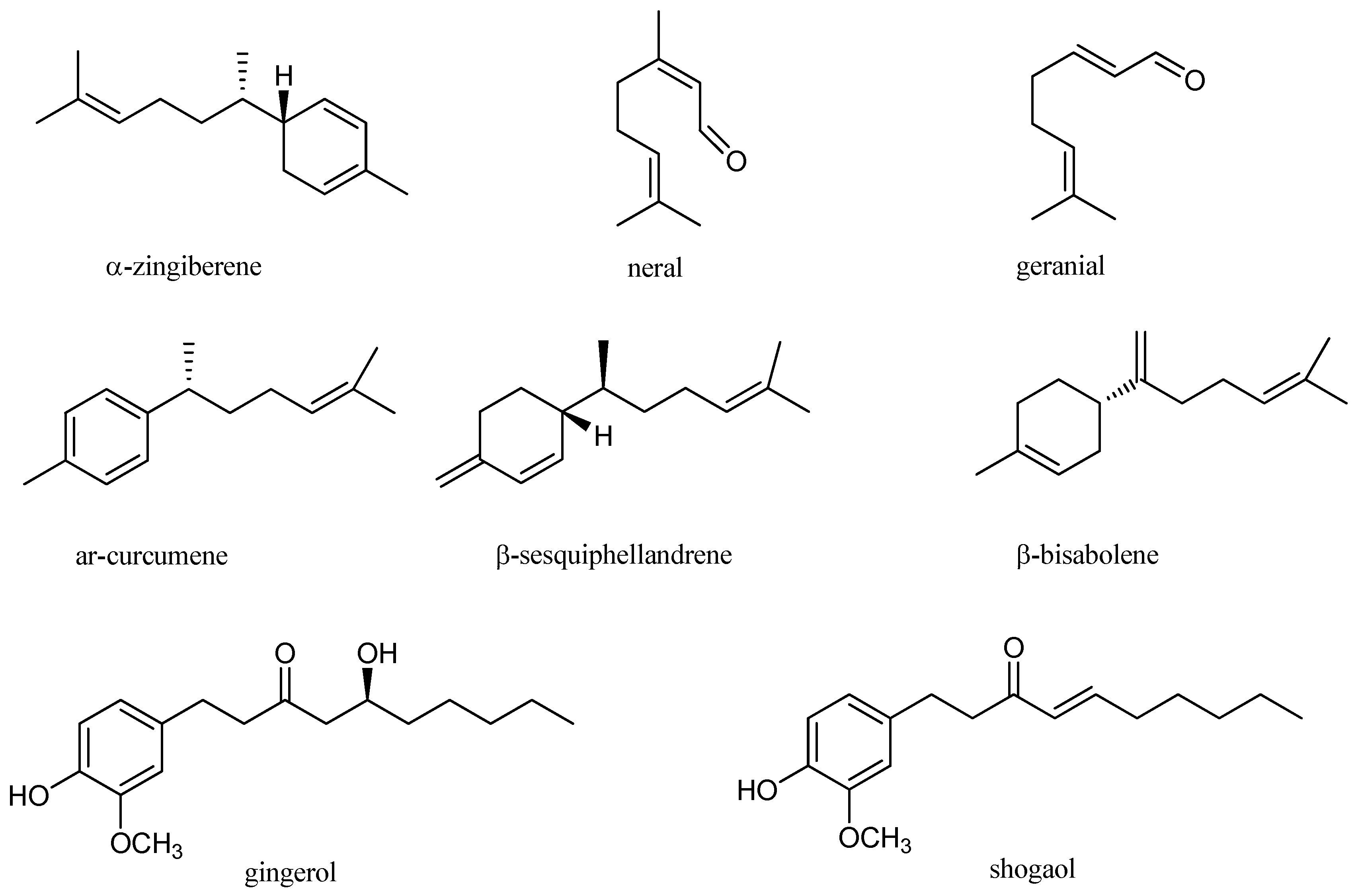
| Plant | Extraction Methods | Major Compounds | Biological Activities | References |
|---|---|---|---|---|
| Z. officinale | Hydrodistillation | ar-curcumene (11.32%), geranial (10.66%), camphene (4.88%), eucalyptol (3.14%), isobornyl formate (1.95%), α-zingiberene (1.64%) | Antibacterial | [37] |
| Z. officinale | Hydrodistillation, microwave assisted hydrodistillation, solvent-free microwave hydrodistillation, improved solvent-free microwave extraction | α-zingiberene (17.4–25.4%), ar-curcumene (14.1–16.4%), β-bisabolene (9.9–12.5%), β-sesquiphellandrene (9.7–13.4%) | NR * | [46] |
| Z. officinale | Hydrodistillation | Geranial (25.9%), α-zingiberene (9.5%), (E,E)-α-farnesene (7.6%), neral (7.4%), ar-curcumene (6.6%) | Antibacterial, antifungal, antioxidant | [28] |
| Three sub-Himalayan Z. officinale cultivars (Gorubathane, Shingboi Thingria | Hydrodistillation | Gorubathane: α-zingiberene (32.2%), β-sesquiphellandrene (10.9%); Thingria: α-zingibirene (12.58%), ar-curcumene (9.89%); Shingboi: geranial (20.07%), neral (9.44%) | NR | [44] |
| Fresh and dry Z. officinale var. Nedumangadu | Hydrodistillation | Fresh ginger: α-zingiberene (28.6%), geranial (8.5%) ar-curcumene (5.6%), β-bisabolene (5.8%); Dry ginger: α-zingiberene (30.9%), ar-urcumene (11%), β-bisabolene (7.2%), β-sesquiphellandrene (6.6%), germacrene-D (4.2%) | Antibacterial, antifungal | [51] |
| Z. officinale | Hydrodistillation | α-zingiberene (28.62%), camphene (9.32%), ar-curcumene (9.09%), β-phellandrene (7.97%) | Antifungal, antioxidant | [45] |
| Z. officinale | Hydrodistillation | β-sesquiphellandrene (27.16%), caryophyllene (15.29%), zingiberene (13.97%), α-farnesene (10.52%), ar-curcumin (6.62%) | Antibacterial, antioxidant | [28] |
| Z. montanum | Hydrodistillation | Sabinene (52.64–56.34%), terpinen-4-ol (7.1–10.17%), (E)-1-(3-4-dimethoxyphenyl) butadiene (10.8–14.7%) | NR | [58] |
| Z. cassumunar (three native cultivars) | Hydrodistillation | Sabinene (36.71–53.50%), γ-terpinene (5.27–7.25%), terpinen-4-ol (21.8–29.96%), (E)-1-(3-4-dimethoxyphenyl) butadiene (0.95–16.16%) | NR | [49] |
| Z. cassumunar | Steam distillation | 6,9,9-tetramethyl-2,6,10-cycloundecatrien-1-one (60.77%), α-caryophyllene (23.92%) | Slight antimicrobial | [57] |
| Z. officinale | Steam distillation | ar-curcumene (59%), b-myrcene (14%), 1,8-cineol (8%), citral (7.5%), and α-zingiberene (7.5%) | anti-inflammatory | [39] |
| Z. zerumbet var. Darcyi | Hydrodistillation | zerumbone (69.9%), α-humulene (12.9%), humulene epoxide II (2.5%), caryophyllene oxide (1.1%), camphene (1.9%) | NR | [60] |
| Z. corallinum | Steam distillation | Sabinene (53.38%), ɑ-terpinene (3.23%), γ-terpinene (2.16%), terpinen-4-ol (22.66%), β-sesquiphellandrene (1.41%), 1,4-bis(methoxy) triquinacene (9.64%) | NR | [59] |
| Z. nimmonii | Hydrodistillation | Myrcene (5.1%), β-caryophyllene (26.9%), α-humulene (19.6%), α-cadinol (5.2%) | Larvicidal and repellent | [31] |
| Z. nimmonii | Hydrodistillation | β-caryophyllene (42.2%), α-humulene, α-caryophyllene (27.7%) | Antimicrobial | [19] |
| Z. moran | Hydrodistillation | Camphene, citral, linalool | Cytotoxic | [54] |
| Z. wrayi var. Halabala | Steam distillation | trans-anethole (96.5%) | Antibacterial | [62] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharifi-Rad, M.; Varoni, E.M.; Salehi, B.; Sharifi-Rad, J.; Matthews, K.R.; Ayatollahi, S.A.; Kobarfard, F.; Ibrahim, S.A.; Mnayer, D.; Zakaria, Z.A.; et al. Plants of the Genus Zingiber as a Source of Bioactive Phytochemicals: From Tradition to Pharmacy. Molecules 2017, 22, 2145. https://doi.org/10.3390/molecules22122145
Sharifi-Rad M, Varoni EM, Salehi B, Sharifi-Rad J, Matthews KR, Ayatollahi SA, Kobarfard F, Ibrahim SA, Mnayer D, Zakaria ZA, et al. Plants of the Genus Zingiber as a Source of Bioactive Phytochemicals: From Tradition to Pharmacy. Molecules. 2017; 22(12):2145. https://doi.org/10.3390/molecules22122145
Chicago/Turabian StyleSharifi-Rad, Mehdi, Elena Maria Varoni, Bahare Salehi, Javad Sharifi-Rad, Karl R. Matthews, Seyed Abdulmajid Ayatollahi, Farzad Kobarfard, Salam A. Ibrahim, Dima Mnayer, Zainul Amiruddin Zakaria, and et al. 2017. "Plants of the Genus Zingiber as a Source of Bioactive Phytochemicals: From Tradition to Pharmacy" Molecules 22, no. 12: 2145. https://doi.org/10.3390/molecules22122145









