Neurotoxic Effects of Linalool and β-Pinene on Tribolium castaneum Herbst
Abstract
:1. Introduction
2. Results
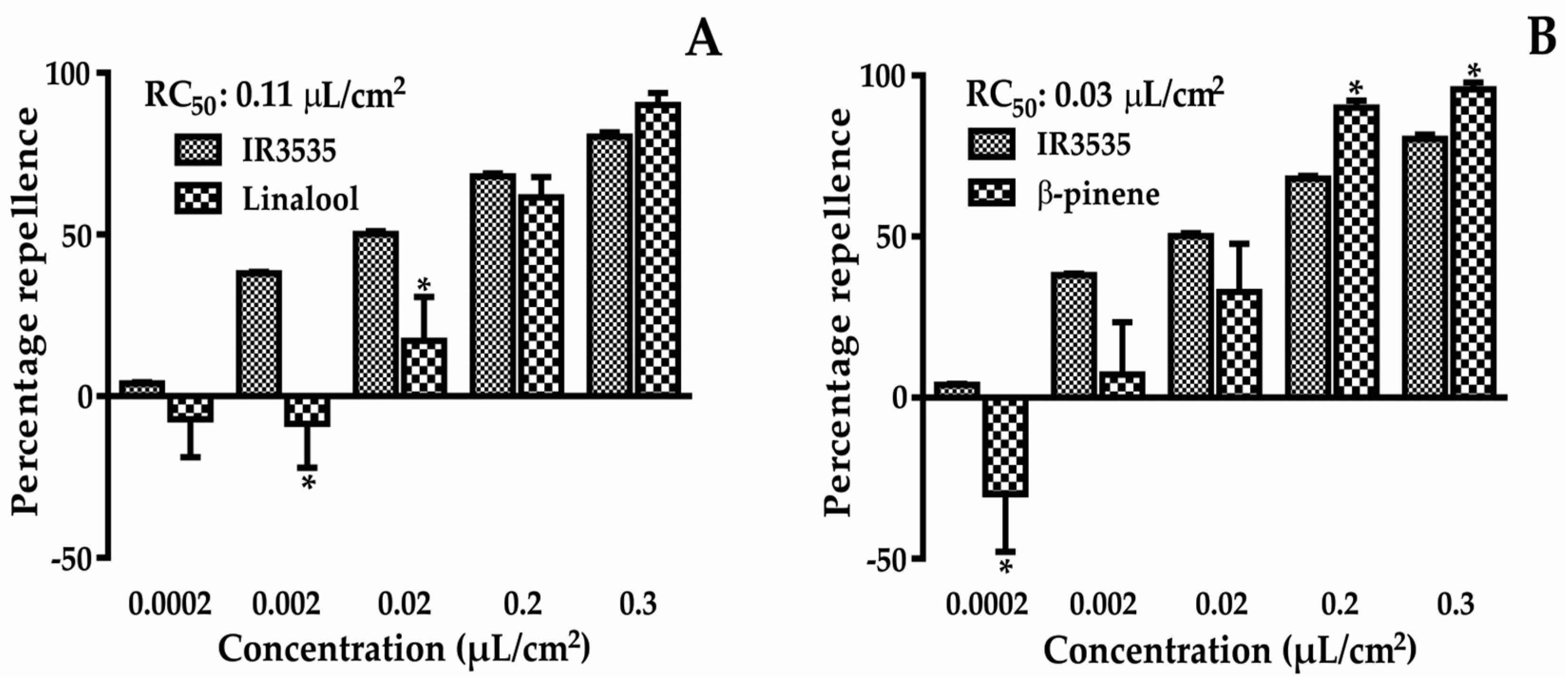
2.1. Repellent Activity
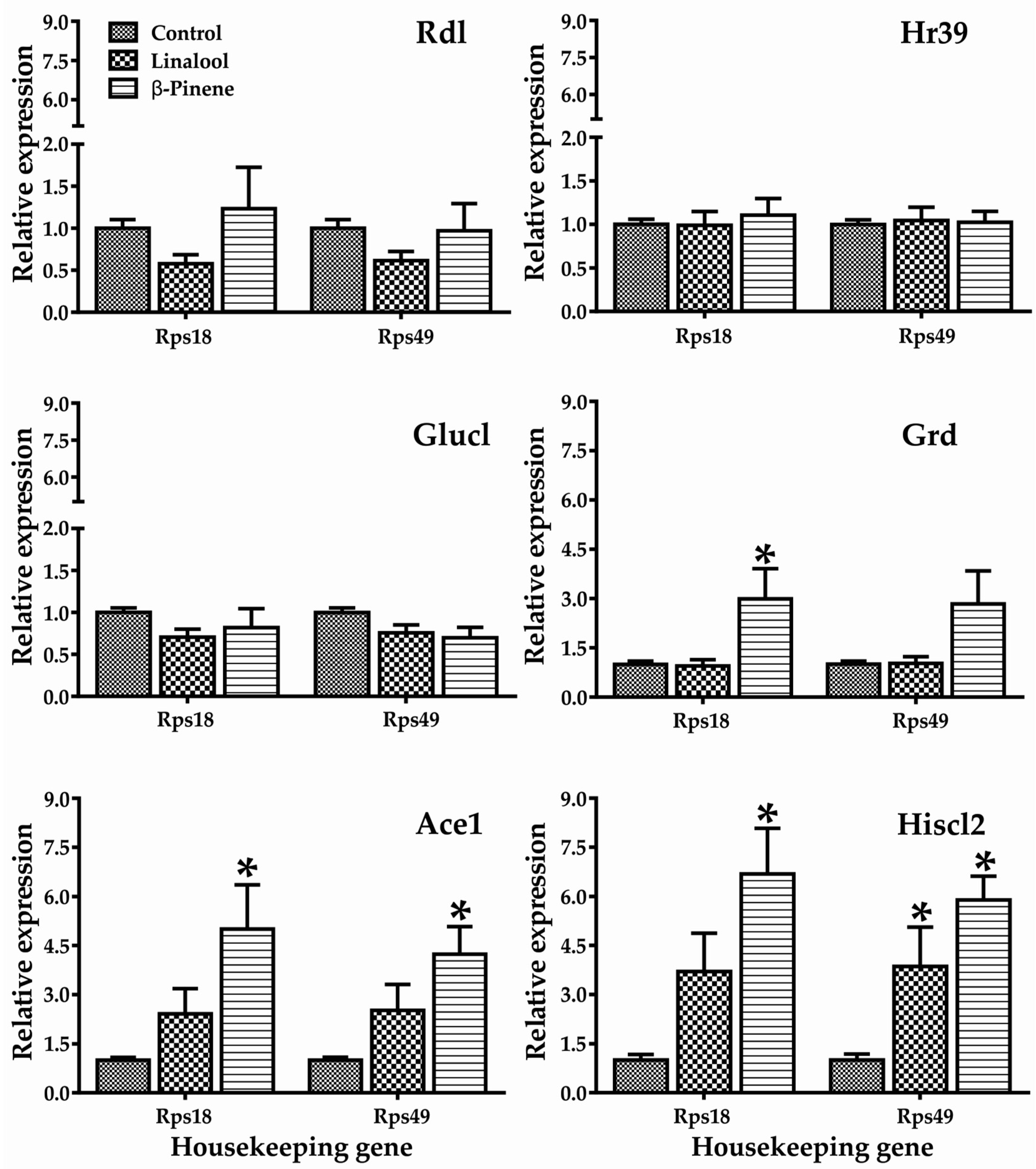
2.2. Gene Expression
2.3. Homology Modeling and Validation
2.4. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Insect Rearing
4.2. Materials
4.3. Repellent Activity
4.4. Gene Expression Assays
4.5. Homology Modeling and Validation
4.6. Molecular Docking
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rattan, R.S. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 2010, 29, 913–920. [Google Scholar] [CrossRef]
- Caballero-Gallardo, K.; Olivero-Verbel, J.; Stashenko, E.E. Repellent Activity of essential oils and some of their individual constituents against Tribolium castaneum Herbst. J. Agric. Food Chem. 2011, 59, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Blenau, W.; Rademacher, E.; Baumann, A. Plant essential oils and formamidines as insecticides/acaricides: What are the molecular targets? Apidologie 2012, 43, 334–347. [Google Scholar] [CrossRef]
- Khater, H.F. Prospects of botanical biopesticides in insect pest management. Pharmacologia 2012, 3, 641–656. [Google Scholar]
- López, M.D.; Pascual-Villalobos, M.J. Are monoterpenoids and phenylpropanoids efficient inhibitors of acetylcholinesterase from stored product insect strains? Flavour Fragr. J. 2015, 30, 108–112. [Google Scholar] [CrossRef]
- Caballero-Gallardo, K.; Olivero-Verbel, J.; Stashenko, E.E. Repellency and toxicity of essential oils from Cymbopogon martinii, Cymbopogon flexuosus and Lippia origanoides cultivated in Colombia against Tribolium castaneum. J. Stored Prod. Res. 2012, 50, 62–65. [Google Scholar] [CrossRef]
- Caballero-Gallardo, K.; Pino-Benitez, N.; Pajaro-Castro, N.; Stashenko, E.; Olivero-Verbel, J. Plants cultivated in Choco, Colombia, as source of repellents against Tribolium castaneum (Herbst). J. Asia Pac. Entomol. 2014, 17, 753–759. [Google Scholar] [CrossRef]
- Beier, R.C.; Byrd, J.A.; Kubena, L.F.; Hume, M.E.; McReynolds, J.L.; Anderson, R.C.; Nisbet, D.J. Evaluation of linalool, a natural antimicrobial and insecticidal essential oil from basil: Effects on poultry. Poult. Sci. 2014, 93, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Lynagh, T.; Lynch, J. Molecular mechanisms of Cys-loop ion channel receptor modulation by ivermectin. Front. Mol. Neurosci. 2012, 5, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elamathi, N.; Verma, V.; Sharma, V.P.; Sreehari, U.; Raghavendra, K. Neonicotinoids in vector control: In silico approach. Asian J. Biomed. Pharm. Sci. 2014, 4, 25–29. [Google Scholar]
- Kastner, K.W.; Shoue, D.A.; Estiu, G.L.; Wolford, J.; Fuerst, M.F.; Markley, L.D.; Izaguirre, J.A.; McDowell, M.A. Characterization of the Anopheles gambiae octopamine receptor and discovery of potential agonists and antagonists using a combined computational-experimental approach. Malar. J. 2014, 13, 434. [Google Scholar] [CrossRef] [PubMed]
- Kedia, A.; Prakash, B.; Mishra, P.; Singh, P.; Dubey, N. Botanicals as ECO friendly biorational alternatives of synthetic pesticides against Callosobruchus spp. (Coleoptera: Bruchidae)—A review. J. Food Sci. Technol. 2015, 52, 1239–1257. [Google Scholar] [CrossRef] [PubMed]
- Sola, P.; Mvumi, B.M.; Ogendo, J.O.; Mponda, O.; Kamanula, J.F.; Nyirenda, S.P.; Belmain, S.R.; Stevenson, P.C. Botanical pesticide production, trade and regulatory mechanisms in sub-Saharan Africa: Making a case for plant-based pesticidal products. Food Secur. 2014, 6, 369–384. [Google Scholar] [CrossRef]
- Chowhan, N.; Singh, H.; Batish, D.; Kaur, S.; Ahuja, N.; Kohli, R. β-Pinene inhibited germination and early growth involves membrane peroxidation. Protoplasma 2013, 250, 691–700. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Donadel, O.J.; Ardanaz, C.E.; Tonn, C.E.; Sosa, M.E. Toxic and repellent effects of Baccharis salicifolia essential oil on Tribolium castaneum. Pest Manag. Sci. 2005, 61, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; Upadhyay, S.; Bhuiyan, M.; Bhattacharya, P.R. A review on prospects of essential oils as biopesticide in insect-pest management. J. Pharmacogn. Phytother. 2009, 1, 52–63. [Google Scholar]
- Müller, G.C.; Junnila, A.; Butler, J.; Kravchenko, V.D.; Revay, E.E.; Weiss, R.W.; Schlein, Y. Efficacy of the botanical repellents geraniol, linalool, and citronella against mosquitoes. J. Vector Ecol. 2009, 34, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Ukeh, D.A.; Umoetok, S.B.A. Repellent effects of five monoterpenoid odours against Tribolium castaneum (Herbst) and Rhyzopertha dominica (F.) in Calabar, Nigeria. Crop Prot. 2011, 30, 1351–1355. [Google Scholar] [CrossRef]
- Re, L.; Barocci, S.; Sonnino, S.; Mencarelli, A.; Vivani, C.; Paolucci, G.; Scarpantonio, A.; Rinaldi, L.; Mosca, E. Linalool modifies the nicotinic receptor–ion channel kinetics at the mouse neuromuscular junction. Pharmacol. Res. 2000, 42, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Coats, J.R. Effects of monoterpenoid insecticides on [3H]-TBOB binding in house fly GABA receptor and 36Cl− uptake in American cockroach ventral nerve cord. Pest Biochem. Physiol. 2010, 98, 317–324. [Google Scholar] [CrossRef]
- Jones, A.; Sattelle, D. The cys-loop ligand-gated ion channel gene superfamily of the red flour beetle, Tribolium castaneum. BMC Genom. 2007, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Bloomquist, J.R. Chloride channels as tools for developing selective insecticides. Arch. Insect Biochem. Physiol. 2003, 54, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Hirschberg, B.; Yuan, J.; Wang, A.P.; Hunt, D.C.; Ludmerer, S.W.; Schmatz, D.M.; Cully, D.F. Identification of two novel Drosophila melanogaster histamine-gated chloride channel subunits expressed in the eye. J. Biol. Chem. 2002, 277, 2000–2005. [Google Scholar] [CrossRef] [PubMed]
- Wolstenholme, A.J. Glutamate-gated chloride channels. J. Biol. Chem. 2012, 287, 40232–40238. [Google Scholar] [CrossRef] [PubMed]
- Priestley, C.M.; Williamson, E.M.; Wafford, K.A.; Satelle, D.B. Thymol, a constituent of thyme essential oils, is a positive modulator of human GABA and a homo-oligosteric GABA receptor from Drosophila melanogaster. Br. J. Pharmacol. 2003, 140, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Gutiérrez, S.L.; Bonilla-Jaime, H.; Gómez-Cansino, R.; Reyes-Chilpa, R. Linalool and β-pinene exert their antidepressant-like activity through the monoaminergic pathway. Life Sci. 2015, 128, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Akiyoshi, Y.; Ju, X.-L.; Furutani, S.; Matsuda, K.; Ozoe, Y. Electrophysiological evidence for 4-isobutyl-3-isopropylbicyclophosphorothionate as a selective blocker of insect GABA-gated chloride channels. Bioorg. Med. Chem. Lett. 2013, 23, 3373–3376. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Park, Y.; Gao, X.; Zhang, X.; Yao, J.; Pang, Y.-P.; Jiang, H.; Zhu, K.Y. Cholinergic and non-cholinergic functions of two acetylcholinesterase genes revealed by gene-silencing in Tribolium castaneum. Sci. Rep. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Mamidala, P.; Wijeratne, A.J.; Wijeratne, S.; Kornacker, K.; Sudhamalla, B.; Rivera-Vega, L.J.; Hoelmer, A.; Meulia, T.; Jones, S.C.; Mittapalli, O. RNA-Seq and molecular docking reveal multi-level pesticide resistance in the bed bug. BMC Genom. 2012, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Keppanan, R.; Sivaperumal, S.; Chadra Kanta, D.; Akutse, K.S.; Wang, L. Molecular docking of protease from Metarhizium anisopliae and their toxic effect against model insect Galleria mellonella. Pestic. Biochem. Physiol. 2017, 138, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Aloui, S.; Raboudi, F.; Ghazouani, T.; Salghi, R.; Hamdaoui, M.H.; Fattouch, S. Use of molecular and in silico bioinformatic tools to investigate pesticide binding to insect (Lepidoptera) phenoloxidases (PO): Insights to toxicological aspects. J. Environ. Sci. Health Part B 2014, 49, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Wallner, B.; Elofsson, A. Can correct protein models be identified? Protein Sci. 2003, 12, 1073–1086. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Gupta, S.; Chakraborty, W.; Senapati, S.; Gachhui, R. Homology modeling, molecular docking and molecular dynamics studies of the catalytic domain of chitin deacetylase from Cryptococcus laurentii strain RY1. Int. J. Biol. Macromol. 2017, 104, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Akhter, M.; Tasleem, M.; Mumtaz Alam, M.; Ali, S. In silico approach for bioremediation of arsenic by structure prediction and docking studies of arsenite oxidase from Pseudomonas stutzeri TS44. Int. Biodeterior. Biodegrad. 2017, 122, 82–91. [Google Scholar] [CrossRef]
- Aloy, P.; Pichaud, M.; Russell, R.B. Protein complexes: Structure prediction challenges for the 21st century. Curr. Opin. Struct. Biol. 2005, 15, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Chivian, D.; Kim, D.E.; Malmstrom, L.; Schonbrun, J.; Rohl, C.A.; Baker, D. Prediction of CASP6 structures using automated Robetta protocols. Proteins 2005, 61, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Alejandro, S.-P.; Maria Natalia Dias Soeiro, C.; Lisvey, G.-M.; Reider, Y.-B. Current Computational approaches towards the rational design of new insecticidal agents. Curr. Comput. Aided Drug Des. 2011, 7, 304–314. [Google Scholar]
- Cornejo, I.; Andrini, O.; Niemeyer, M.I.; Marabolí, V.; González-Nilo, F.D.; Teulon, J.; Sepúlveda, F.V.; Cid, L.P. Identification and functional expression of a glutamate- and avermectin-gated chloride channel from Caligus rogercresseyi, a Southern Hemisphere sea louse affecting farmed fish. PLoS Pathog. 2014, 10, e1004402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dönitz, J.; Grossmann, D.; Schild, I.; Schmitt-Engel, C.; Bradler, S.; Prpic, N.; Bucher, G. TrOn: An Anatomical Ontology for the Beetle Tribolium castaneum. PLoS ONE 2013, 8, e70695. [Google Scholar] [CrossRef] [PubMed]
- Lord, J.C.; Hartzer, K.; Toutges, M.; Oppert, B. Evaluation of quantitative PCR reference genes for gene expression studies in Tribolium castaneum after fungal challenge. J. Microbiol. Methods 2010, 80, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Vuerinckx, K.; Verlinden, H.; Lindemans, M.; Broeck, J.V.; Huybrechts, R. Characterization of an allatotropin-like peptide receptor in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2011, 41, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, T.J.; Neagoe, I.; Scheel, O. CLC chloride channels and transporters. Curr. Opin. Neurobiol. 2005, 15, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Orhan, G.; Fahlke, C.; Alekov, A.K. Anion- and Proton-Dependent Gating of ClC-4 Anion/Proton Transporter under Uncoupling Conditions. Biophys. J. 2011, 100, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Pang, Y.-P.; Park, Y.; Gao, X.; Yao, J.; Zhang, X.; Zhu, K.Y. Genome organization, phylogenies, expression patterns, and three-dimensional protein models of two acetylcholinesterase genes from the red flour beetle. PLoS ONE 2012, 7, e32288. [Google Scholar] [CrossRef] [PubMed]
- Arya, M.; Shergill, I.S.; Williamson, M.; Gommersall, L.; Arya, N.; Patel, H.R. Basic principles of real-time quantitative PCR. Expert Rev. Mol. Diagn. 2005, 5, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Valasek, M.A.; Repa, J.J. The power of real-time PCR. Adv. Physiol. Educ. 2005, 29, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, J.; Liu, D.; Su, Y. Normalizing genes for real-time polymerase chain reaction in epithelial and nonepithelial cells of mouse small intestine. Anal. Biochem. 2010, 399, 211–217. [Google Scholar] [CrossRef] [PubMed]
- UniProt, C. Reorganizing the protein space at the Universal Protein Resource (UniProt). Nucleic Acids Res. 2012, 40, D71–D75. [Google Scholar]
- Chen, C.-C.; Hwang, J.-K.; Yang, J.-M. (PS)2-v2: Template-based protein structure prediction server. BMC Bioinform. 2009, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Hwang, J.-K.; Yang, J.-M. (PS)2: Protein structure prediction server. Nucleic Acids Res. 2006, 34, W152–W157. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Kucukural, A.; Zhang, Y. I-TASSER: A unified platform for automated protein structure and function prediction. Nat. Protoc. 2010, 5, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Lovell, S.C.; Davis, I.W.; Arendall, W.B.; de Bakker, P.I.W.; Word, J.M.; Prisant, M.G.; Richardson, J.S.; Richardson, D.C. Structure validation by Cα geometry: ϕ, ψ, and Cβ deviation. Proteins 2003, 50, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Benkert, P.; Tosatto, S.C.E.; Schomburg, D. QMEAN: A comprehensive scoring function for model quality assessment. Proteins 2008, 71, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.Y.; He, J.E.; He, S.Q.; Shoemaker, B.A.; et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef] [PubMed]
- Pájaro-Castro, N.; Flechas, M.; Ocazionez, R.; Staschenko, E.; Olivero-Verbel, J. Potential interaction of components from essential oils with dengue virus proteins. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas 2015, 14, 141–155. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Wolber, G.; Langer, T. LigandScout: 3-D Pharmacophores derived from protein-bound ligands and their use as virtual screening filters. J. Chem. Inf. Model. 2005, 45, 160–169. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds linalool and β-pinene are available from the authors. |
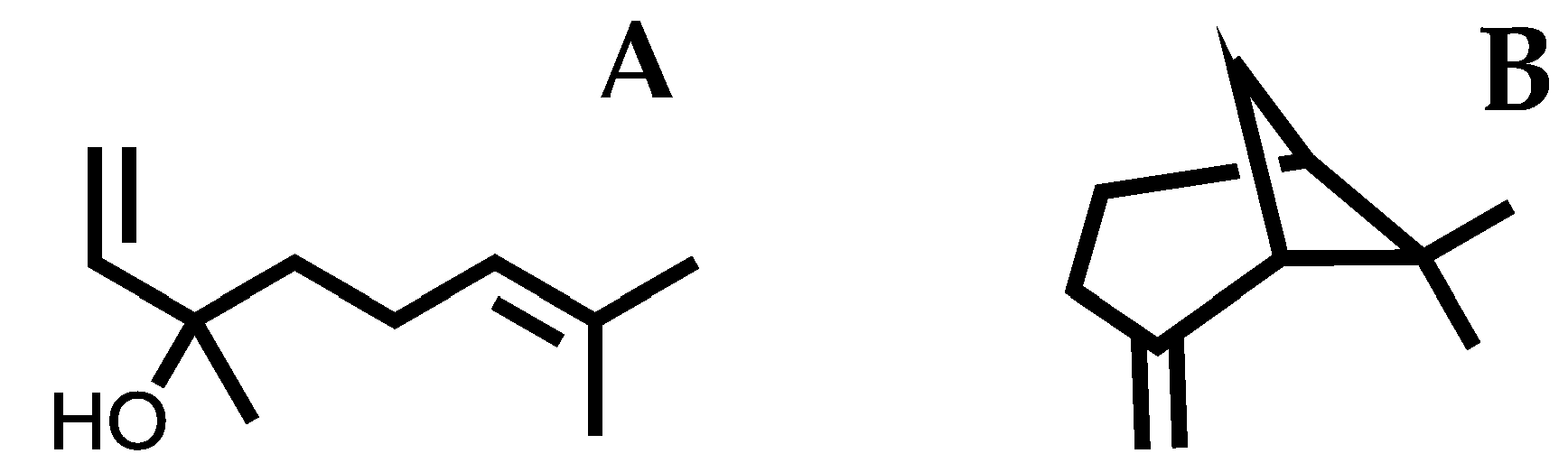
| Proteins | LGscore | MaxSub |
|---|---|---|
| Carboxylic ester hydrolase | 5.448 | 0.154 |
| Carboxylic ester hydrolase 2 (acetylcholinesterase activity) | 6.207 | 0.459 |
| Gamma-aminobutyric acid-gated anion channel splice variant 3a6a (GABA-RDL) | 1.520 | 0.087 |
| Gamma-aminobutyric acid-gated ion channel (GABA-GRD) | 2.658 | 0.120 |
| Gamma-aminobutyric acid-ligand gated chloride channel 3) (GABA-LCCH3) | 2.611 | 0.129 |
| Glutamate-gated chloride channel | 2.010 | 0.150 |
| Histamine-gated chloride channel 1 | 2.487 | 0.166 |
| Histamine-gated chloride channel 2 | 2.435 | 0.143 |
| Hormone receptor in 39-like protein | 0.947 | 0.432 |
| Nicotinic acetylcholine receptor subunit alpha1 | 1.580 | 0.090 |
| Nicotinic acetylcholine receptor subunit alpha2 | 1.707 | 0.118 |
| pH sensitive chloride channel | 1.740 | 0.139 |
| Putative octopamine/tyramine receptor | 2.150 | 0.100 |
| Proteins | Uniprot Code | Linalool | β-Pinene | Inhibitors |
|---|---|---|---|---|
| Carboxylic ester hydrolase | E7DN61 | −5.3 ± 0.1 | −5.8 ± 0.0 | |
| Carboxylic ester hydrolase 2 (acetylcholinesterase activity) | E7DN62 | −5.5 ± 0.2 | −6.1 ± 0.1 | |
| Gamma-aminobutyric acid-gated anion channel splice variant 3a6a | A8DMU1 | −4.9 ± 0.1 | −6.1 ± 0.0 | −9.8 ± 0.0 * |
| Gamma-aminobutyric acid-gated ion channel | A8DMU2 | −5.1 ± 0.1 | −6.0 ± 0.0 | −9.4 ± 0.0 * |
| Gamma-aminobutyric acid-ligand gated chloride channel 3 | A8DMU3 | −6.7 ± 0.2 | −7.2 ± 0.0 | −9.0 ± 0.0 * |
| Glutamate-gated chloride channel | A8DMU5 | −4.9 ± 0.2 | −6.6 ± 0.3 | |
| Histamine-gated chloride channel 1 | A8DMU7 | −5.3 ± 0.2 | −5.6 ± 0.0 | |
| Histamine-gated chloride channel 2 | A8DMU8 | −5.0 ± 0.2 | −5.7 ± 0.0 | |
| Hormone receptor in 39-like protein | D2A6H1 | −5.2 ± 0.2 | −6.0 ± 0.0 | |
| Nicotinic acetylcholine receptor subunit alpha1 | A8DIP3 | −7.1 ± 0.2 | −7.4 ± 0.1 | −8.8 ± 0.0 + |
| Nicotinic acetylcholine receptor subunit alpha2 | A8DIQ7 | −6.8 ± 0.3 | −7.6 ± 0.0 | −8.5 ± 0.0 + |
| pH sensitive chloride channel | A8DMU9 | −5.4 ± 0.2 | −5.0 ± 0.0 | |
| Putative octopamine/tyramine receptor | D6WB14 | −7.2 ± 0.2 | −7.7±0.0 | −8.2 ± 0.0 ‡ |
| Gene Name | Gene Symbol | Entrez Gene ID | Forward (5′-3′) | Reverse (5′-3′) | Amplicon Size |
|---|---|---|---|---|---|
| Genes Evaluated | |||||
| Acetylcholinesterase | Ace1 | HQ260968.1 | CCGTTCGTCCCAGTCATTG | AGTAGTAGCCTTCTTCTGTGTTAG | 121 |
| GABA-gated anion channel splice variant 3a6a | Rdl | NM_001114292.1 | ACTTGGGCGACGTCAACATA | ACGTGAAATCCATCTGGACC | 159 |
| GABA-gated ion channel | Grd | NM_001114300.1 | GGTCTCCTTCTGGCTGAACC | TGGACCACAGCGAACTGAAT | 198 |
| Glutamate-gated chloride channel | Glucl | NM_001114304.1 | TGAATGGCACAGATGGTCCC | CCAGACTCGACTGGCTTCAG | 194 |
| Histamine-gated chloride channel 2 | Hiscl2 | NM_001109951.1 | TGGATGTCCAGTTGTTCGGT | TGTGGCTGAATAGGCAAGTCAT | 176 |
| Hormone receptor in 39-like protein | Hr39 | XR_043083.1 | CGACCGTCGACTGTACAAAA | AGTCGACATGGAACGGAAAC | 145 |
| Housekeeping Gene | |||||
| Ribosomal protein 49 | Rps49 | XM_964471.2 | TGGCAAACTCAAACGCAACT | AGCGCCTACGAACCCTGTT | 62 |
| Ribosomal protein 18 | Rps18 | XM_968539.2 | CGAAGAGGTCGAGAAAATCG | CGTGGTCTTGGTGTGTTGAC | 235 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pajaro-Castro, N.; Caballero-Gallardo, K.; Olivero-Verbel, J. Neurotoxic Effects of Linalool and β-Pinene on Tribolium castaneum Herbst. Molecules 2017, 22, 2052. https://doi.org/10.3390/molecules22122052
Pajaro-Castro N, Caballero-Gallardo K, Olivero-Verbel J. Neurotoxic Effects of Linalool and β-Pinene on Tribolium castaneum Herbst. Molecules. 2017; 22(12):2052. https://doi.org/10.3390/molecules22122052
Chicago/Turabian StylePajaro-Castro, Nerlis, Karina Caballero-Gallardo, and Jesus Olivero-Verbel. 2017. "Neurotoxic Effects of Linalool and β-Pinene on Tribolium castaneum Herbst" Molecules 22, no. 12: 2052. https://doi.org/10.3390/molecules22122052





