Plasma and Urinary Phenolic Profiles after Acute and Repetitive Intake of Wild Blueberry
Abstract
:1. Introduction
2. Results
2.1. Characteristics Study Population
2.2. (Poly)phenol Content of Blueberry Intervention
2.3. Plasma Blueberry (Poly)phenol Metabolites after Acute and Repetitive Intake
2.4. Urinary Excretion of Blueberry (Poly)phenols after Acute and Repetitive Intake
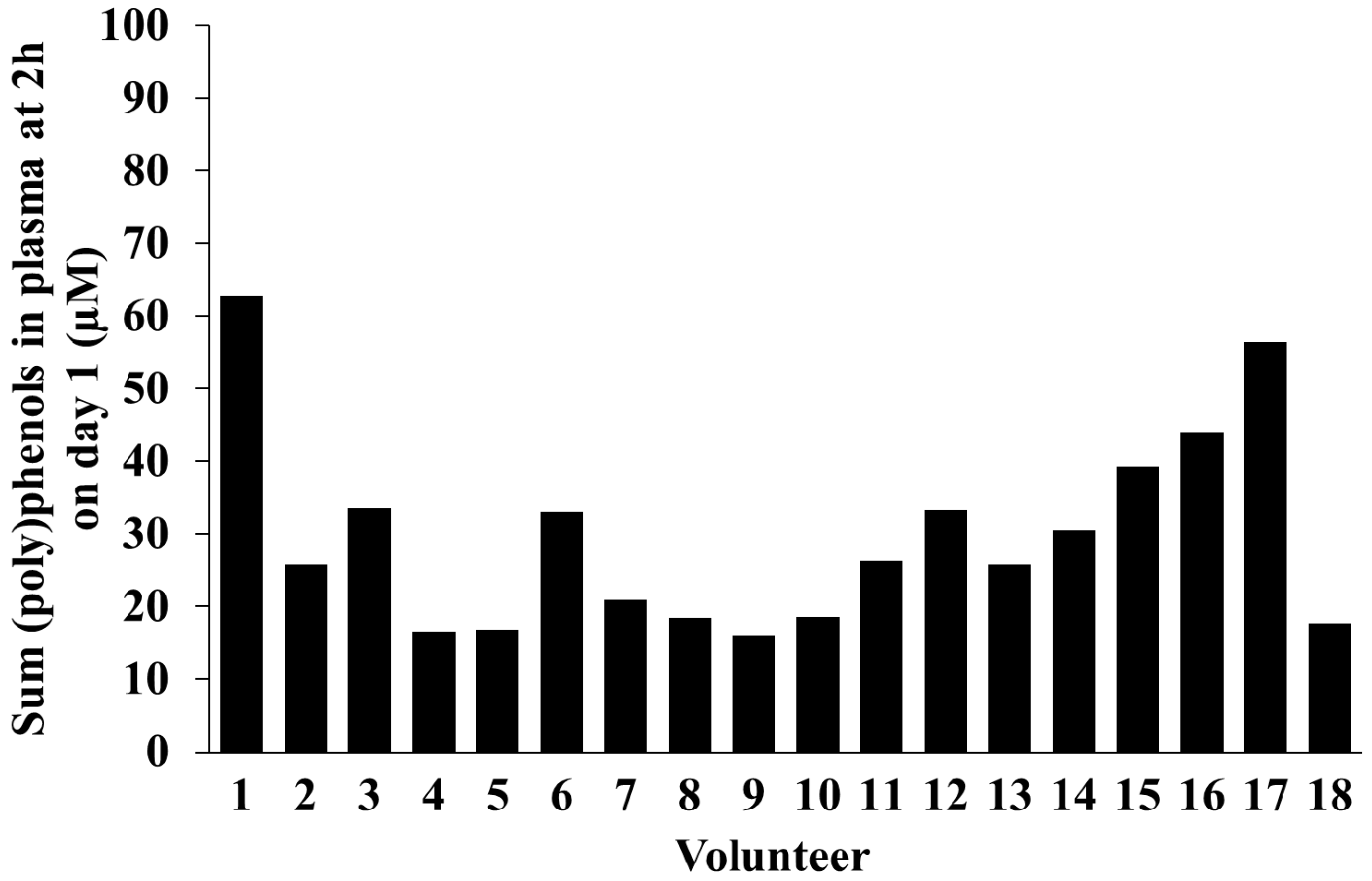
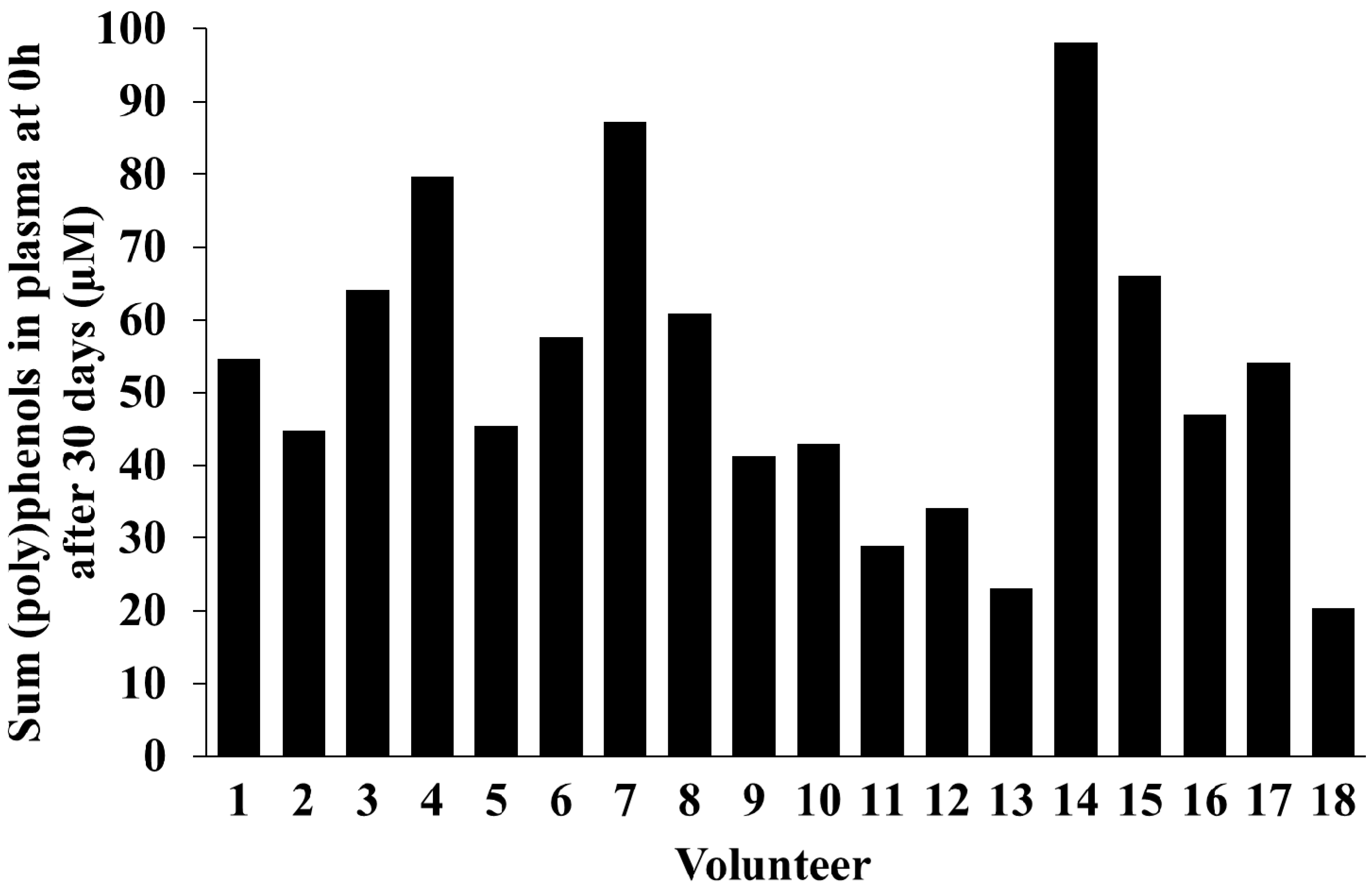
2.5. Inter-Individual Variability in Plasma and Urinary Levels of Blueberry (Poly)phenol Metabolites
3. Discussion
4. Materials and Methods
4.1. Intervention Study Subjects
4.2. Human Study Design
4.3. Blueberry Test Drinks
4.4. Plasma and Urine Collection
4.5. UPLC-Q-TOF MS Analysis of Plasma (Poly)phenols
4.6. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Figueroa, A.; Navaei, N.; Wong, A.; Kalfon, R.; Ormsbee, L.T.; Feresin, R.G.; Elam, M.L.; Hooshmand, S.; Payton, M.E.; et al. Daily Blueberry Consumption Improves Blood Pressure and Arterial Stiffness in Postmenopausal Women with Pre- and Stage 1-Hypertension: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Acad. Nutr. Diet. 2015, 115, 369–377. [Google Scholar] [CrossRef] [PubMed]
- McAnulty, L.S.; Collier, S.R.; Landram, M.J.; Whittaker, D.S.; Isaacs, S.E.; Klemka, J.M.; Cheek, S.L.; Arms, J.C.; McAnulty, S.R. Six weeks daily ingestion of whole blueberry powder increases natural killer cell counts and reduces arterial stiffness in sedentary males and females. Nutr. Res. 2014, 34, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Stull, A.J.; Cash, K.C.; Champagne, C.M.; Gupta, A.K.; Boston, R.; Beyl, R.A.; Johnson, W.D.; Cefalu, W.T. Blueberries improve endothelial function, but not blood pressure, in adults with metabolic syndrome: A randomized, double-blind, placebo-controlled clinical trial. Nutrients 2015, 7, 4107–4123. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Du, M.; Leyva, M.J.; Sanchez, K.; Betts, N.M.; Wu, M.; Aston, C.E.; Lyons, T.J. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J. Nutr. 2010, 140, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Pino-Garcia, R.D.; George, T.W.; Vidal-Diez, A.; Heiss, C.; Spencer, J.P.E. Impact of processing on the bioavailability and vascular effects of blueberry (poly)phenols. Mol. Nutr. Food Res. 2014, 58, 1952–1961. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Rendeiro, C.; Bergillos-Meca, T.; Tabatabaee, S.; George, T.W.; Heiss, C.; Spencer, J.P. Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: A randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am. J. Clin. Nutr. 2013, 98, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Whyte, A.R.; Schafer, G.; Williams, C.M. Cognitive effects following acute wild blueberry supplementation in 7- to 10-year-old children. Eur. J. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqvist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry supplementation improves memory in older adults. J. Agric. Food Chem. 2010, 58, 3996–4000. [Google Scholar] [CrossRef] [PubMed]
- De Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef] [PubMed]
- Mazza, G.; Kay, C.D.; Cottrell, T.; Holub, B.J. Absorption of anthocyanins from blueberries and serum antioxidant status in human subjects. J. Agric. Food Chem. 2002, 50, 7731–7737. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Chen, C.Y.O.; Zampariello, C.A.; Blumberg, J.B. Flavonoids and phenolic acids from cranberry juice are bioavailable and bioactive in healthy older adults. Food Chem. 2015, 168, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A (13)C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurmi, T.; Mursu, J.; Heinonen, M.; Nurmi, A.; Hiltunen, R.; Voutilainen, S. Metabolism of berry anthocyanins to phenolic acids in humans. J. Agric. Food Chem. 2009, 57, 2274–2281. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Donnarumma, G.; Napolitano, A.; Galvano, F.; Gallo, A.; Scalfi, L.; Fogliano, V. Protocatechuic Acid Is the Major Human Metabolite of Cyanidin-Glucosides. J. Nutr. 2007, 137, 2043–2048. [Google Scholar] [PubMed]
- Rodriguez-Mateos, A.; Cifuentes-Gomez, T.; Tabatabaee, S.; Lecras, C.; Spencer, J.P. Procyanidin, anthocyanin, and chlorogenic acid contents of highbush and lowbush blueberries. J. Agric. Food Chem. 2012, 60, 5772–5778. [Google Scholar] [CrossRef] [PubMed]
- Scalzo, J.; Currie, A.; Stephens, J.; Alspach, P.; McGhie, T. The anthocyanin composition of different Vaccinium, Ribes and Rubus genotypes. Biofactors 2008, 34, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, R.P.; Krueger, C.G.; Reed, J.D. Methods to determine effects of cranberry proanthocyanidins on extraintestinal infections: Relevance for urinary tract health. Mol. Nutr. Food Res. 2015, 59, 1292–1306. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Feliciano, R.P.; Cifuentes-Gomez, T.; Spencer, J.P.E. Bioavailability of wild blueberry (poly)phenols at different levels of intake. J. Berry Res. 2016, 6, 137–148. [Google Scholar] [CrossRef]
- Feliciano, R.P.; Boeres, A.; Massacessi, L.; Istas, G.; Ventura, M.R.; Nunes dos Santos, C.; Heiss, C.; Rodriguez-Mateos, A. Identification and quantification of novel cranberry-derived plasma and urinary (poly)phenols. Arch. Biochem. Biophys. 2016, 599, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Pimpao, R.C.; Ventura, M.R.; Ferreira, R.B.; Williamson, G.; Santos, C.N. Phenolic sulfates as new and highly abundant metabolites in human plasma after ingestion of a mixed berry fruit puree. Br. J. Nutr. 2015, 113, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Mosele, J.I.; Gosalbes, M.J.; Macia, A.; Rubio, L.; Vazquez-Castellanos, J.F.; Jimenez Hernandez, N.; Moya, A.; Latorre, A.; Motilva, M.J. Effect of daily intake of pomegranate juice on fecal microbiota and feces metabolites from healthy volunteers. Mol. Nutr. Food Res. 2015, 59, 1942–1953. [Google Scholar] [CrossRef] [PubMed]
- Hanhineva, K.; Lankinen, M.A.; Pedret, A.; Schwab, U.; Kolehmainen, M.; Paananen, J.; de Mello, V.; Sola, R.; Lehtonen, M.; Poutanen, K.; et al. Nontargeted metabolite profiling discriminates diet-specific biomarkers for consumption of whole grains, fatty fish, and bilberries in a randomized controlled trial. J. Nutr. 2015, 145, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Vetrani, C.; Rivellese, A.A.; Annuzzi, G.; Adiels, M.; Boren, J.; Mattila, I.; Oresic, M.; Aura, A.M. Metabolic transformations of dietary polyphenols: Comparison between in vitro colonic and hepatic models and in vivo urinary metabolites. J. Nutr. Biochem. 2016, 33, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Schrager, M.A.; Hilton, J.; Gould, R.; Kelly, V.E. Effects of blueberry supplementation on measures of functional mobility in older adults. Appl. Physiol. Nutr. Metab. 2015, 40, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Koli, R.; Erlund, I.; Jula, A.; Marniemi, J.; Mattila, P.; Alfthan, G. Bioavailability of various polyphenols from a diet containing moderate amounts of berries. J. Agric. Food Chem. 2010, 58, 3927–3932. [Google Scholar] [CrossRef] [PubMed]
- Egert, S.; Wolffram, S.; Bosy-Westphal, A.; Boesch-Saadatmandi, C.; Wagner, A.E.; Frank, J.; Rimbach, G.; Mueller, M.J. Daily quercetin supplementation dose-dependently increases plasma quercetin concentrations in healthy humans. J. Nutr. 2008, 138, 1615–1621. [Google Scholar] [PubMed]
- Ottaviani, J.I.; Balz, M.; Kimball, J.; Ensunsa, J.L.; Fong, R.; Momma, T.Y.; Kwik-Uribe, C.; Schroeter, H.; Keen, C.L. Safety and efficacy of cocoa flavanol intake in healthy adults: A randomized, controlled, double-masked trial. Am. J. Clin. Nutr. 2015, 102, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- De Ferrars, R.M.; Cassidy, A.; Curtis, P.; Kay, C.D. Phenolic metabolites of anthocyanins following a dietary intervention study in post-menopausal women. Mol. Nutr. Food Res. 2014, 58, 490–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, K.A.; Dew, T.P.; Watson, R.E.; Farrar, M.D.; Bennett, S.; Nicolaou, A.; Rhodes, L.E.; Williamson, G. High performance liquid chromatography tandem mass spectrometry dual extraction method for identification of green tea catechin metabolites excreted in human urine. J. Chromatogr. B 2014, 972, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.S.; Peng, Y.M.; McGee, D.L.; Alberts, D.S. Carotenoids, tocopherols, and retinoids in human buccal mucosal cells: Intra- and interindividual variability and storage stability. Am. J. Clin. Nutr. 1994, 59, 636–643. [Google Scholar] [PubMed]
- Borel, P.; Desmarchelier, C.; Nowicki, M.; Bott, R. A Combination of Single-Nucleotide Polymorphisms Is Associated with Interindividual Variability in Dietary beta-Carotene Bioavailability in Healthy Men. J. Nutr. 2015, 145, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Desmarchelier, C.; Nowicki, M.; Bott, R.; Morange, S.; Lesavre, N. Interindividual variability of lutein bioavailability in healthy men: Characterization, genetic variants involved, and relation with fasting plasma lutein concentration. Am. J. Clin. Nutr. 2014, 100, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032. [Google Scholar]
- Actis-Goretta, L.; Leveques, A.; Giuffrida, F.; Romanov-Michailidis, F.; Viton, F.; Barron, D.; Duenas-Paton, M.; Gonzalez-Manzano, S.; Santos-Buelga, C.; Williamson, G.; et al. Elucidation of (−)-epicatechin metabolites after ingestion of chocolate by healthy humans. Free Radic. Biol. Med. 2012, 53, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Cifuentes-Gomez, T.; Gonzalez-Salvador, I.; Ottaviani, J.I.; Schroeter, H.; Kelm, M.; Heiss, C.; Spencer, J.P. Influence of age on the absorption, metabolism, and excretion of cocoa flavanols in healthy subjects. Mol. Nutr. Food Res. 2015, 59, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.; Jacobs, D.M.; Peters, S.; Possemiers, S.; van Duynhoven, J.; Vaughan, E.E.; van de Wiele, T. In vitro bioconversion of polyphenols from black tea and red wine/grape juice by human intestinal microbiota displays strong interindividual variability. J. Agric. Food Chem. 2010, 58, 10236–10246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, I.A.; Mena, P.; Calani, L.; Borges, G.; Pereira-Caro, G.; Bresciani, L.; Del Rio, D.; Lean, M.E.; Crozier, A. New insights into the bioavailability of red raspberry anthocyanins and ellagitannins. Free Radic. Biol. Med. 2015, 89, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Barberan, F.A.; Garcia-Villalba, R.; Gonzalez-Sarrias, A.; Selma, M.V.; Espin, J.C. Ellagic acid metabolism by human gut microbiota: Consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J. Agric. Food Chem. 2014, 62, 6535–6538. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Kusuhara, H.; Kumagai, Y.; Ieiri, I.; Mori, H.; Ito, S.; Nakai, Y.; Maeda, K.; Sugiyama, Y. Association of multidrug resistance-associated protein 2 single nucleotide polymorphism rs12762549 with the basal plasma levels of phase II metabolites of isoflavonoids in healthy Japanese individuals. Pharmacogenet. Genom. 2012, 22, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Cerda, B.; Tomas-Barberan, F.A.; Espin, J.C. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: Identification of biomarkers and individual variability. J. Agric. Food Chem. 2005, 53, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Queipo-Ortuno, M.I.; Boto-Ordonez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona Diaz, F.; Andres-Lacueva, C.; Tinahones, F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Boto-Ordonez, M.; Urpi-Sarda, M.; Queipo-Ortuno, M.I.; Tulipani, S.; Tinahones, F.J.; Andres-Lacueva, C. High levels of Bifidobacteria are associated with increased levels of anthocyanin microbial metabolites: A randomized clinical trial. Food Funct. 2014, 5, 1932–1938. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Romo-Vaquero, M.; Garcia-Villalba, R.; Gonzalez-Sarrias, A.; Tomas-Barberan, F.A.; Espin, J.C. The human gut microbial ecology associated with overweight and obesity determines ellagic acid metabolism. Food Funct. 2016, 7, 1769–1774. [Google Scholar] [CrossRef] [PubMed]
- Jalanka, J.; Salonen, A.; Fuentes, S.; de Vos, W.M. Microbial signatures in post-infectious irritable bowel syndrome—Toward patient stratification for improved diagnostics and treatment. Gut Microbes 2015, 6, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.

| Mean ± SD | |
|---|---|
| Age (years) | 33 ± 18 |
| BMI (kg/m2) | 24 ± 3 |
| Systolic blood pressure (mm·Hg) | 125 ± 10 |
| Diastolic blood pressure (mm·Hg) | 73 ± 9 |
| Heart rate (bpm) | 65 ± 9 |
| Total cholesterol (mg/dL) | 175 ± 33 |
| Triglycerides (mg/dL) | 90 ± 49 |
| HDL-cholesterol (mg/dL) | 55 ± 10 |
| LDL-cholesterol (mg/dL) | 114 ± 33 |
| Fasting plasma glucose (mg/dL) | 84 ± 14 |
| Plasma Concentration (nM) | ||||
|---|---|---|---|---|
| Day 1 | Day 30 | |||
| 0 h | 2 h | 0 h | 2 h | |
| Benzoic acid Derivatives | ||||
| Benzoic acid | 794 ± 56 | 932 ± 63 | 981 ± 72 b | 926 ± 47 |
| 2-Hydroxybenzoic acid | 80 ± 22 | 131 ± 60 | 103 ± 15 | 106 ± 15 |
| 3-Hydroxybenzoic acid | 17 ± 3 | 13 ± 2 | 21 ± 5 | 17 ± 3 |
| 4-Hydroxybenzoic acid | 27 ± 8 | 23 ± 6 | 18 ± 4 | 18 ± 3 |
| 2,3-Dihydroxybenzoic acid | 8891± 1550 | 7430 ± 1323 | 8070 ± 1210 | 8658 ± 1642 |
| 2,4-Dihydroxybenzoic acid | 13 ± 5 | 11 ± 3 | 25 ± 7 | 18 ± 3 |
| 2,5-Dihydroxybenzoic acid | 71 ± 10 | 54 ± 6 | 101 ± 10 b | 94 ± 11 |
| Protocatechuic acid | 22 ± 6 | 18 ± 6 | 11 ± 4 | 8 ± 3 |
| Syringic acid | 4 ± 1 | 8 ± 2 | 9 ± 3 | 11 ± 3 |
| Vanillic acid | 290 ± 48 | 397 ± 53 | 644 ± 122 b | 730 ± 109 |
| Vanillic acid-4-O-sulfate | 30 ± 6 | 29 ± 6 | 34 ± 6 | 34 ± 6 |
| Isovanillic acid | 429 ± 51 | 447 ± 58 | 426 ± 46 | 430 ± 57 |
| 4-Methylgallic acid-3-O-sulfate | 43 ± 28 | 48 ± 11 | 28 ± 7 | 58 ± 15 c |
| Phenylacetic acid derivatives | ||||
| Homovanillic acid | 74 ± 7 | 52 ± 3 | 86 ± 14 | 71 ± 11 |
| Homovanillic acid sulfate | 4 ± 1 | 4 ± 1 | 6 ± 1 | 4 ± 1 |
| Phenylacetic acid | 2854 ± 1319 | 2599 ± 1578 | 1634 ± 603 | 1130 ± 315 |
| 3,4-Dihydroxyphenylacetic acid | 91 ± 13 | 76 ± 9 | 89 ± 14 | 87 ± 11 |
| 3-Hydroxyphenylacetic acid | 142 ± 32 | 103 ± 25 | 174 ± 29 | 152 ± 27 |
| 4-Hydroxyphenylacetic acid | 333 ± 50 | 215 ± 31 | 289 ± 61 | 230 ± 49 |
| Propionic acid derivatives | ||||
| 2-(4-hydroxyphenoxy)propionic acid | 4 ± 1 | 3 ± 1 | 2 ± 0 | 2 ± 1 |
| Benzaldehyde derivatives | ||||
| 4-Hydroxybenzaldehyde | 62 ± 17 | 58 ± 16 | 55 ± 10 | 46 ± 10 |
| 3,4-Dihydroxybenzaldehyde | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 |
| Pyrogallol derivatives | ||||
| Pyrogallol-O-1-sulfate | 22 ± 6 | 15 ± 2 | 23 ± 5 | 15 ± 2 |
| Pyrogallol-O-2-sulfate | 134 ± 60 | 99 ± 48 | 104 ± 44 | 62 ± 23 |
| 1-Methylpyrogallol-O-sulfate | 87 ± 24 | 57 ± 16 | 112 ± 36 | 90 ± 31 |
| 2-Methylpyrogallol-O-sulfate | 61 ± 18 | 58 ± 18 | 29 ± 7 | 22 ± 4 |
| Catechol derivatives | ||||
| Catechol-O-sulfate | 1228 ± 231 | 1759 ± 179 | 2726 ± 440 b | 1565 ± 195 |
| 4-Methylcatechol-O-sulfate | 693 ± 104 | 468 ± 81 | 860 ± 250 | 775 ± 234 |
| Hippuric acid derivatives | ||||
| Hippuric acid | 17,445 ± 3746 | 12,367 ± 2464 | 32545 ± 5252 b | 29,644 ± 5587 |
| 2-Hydroxyhippuric acid | 5 ± 2 | 9 ± 4 | 8 ± 2 | 8 ± 2 |
| 3-Hydroxyhippuric acid | 290 ± 72 | 320 ± 84 | 708 ± 159 b | 416 ± 87 |
| 4-Hydroxyhippuric acid | 77 ± 12 | 59 ± 8 | 76 ± 12 | 64 ± 8 |
| α-Hydroxyhippuric acid | 485 ± 66 | 385 ± 43 | 539 ± 82 | 463 ± 62 |
| Cinnamic acid derivatives | ||||
| Cinnamic acid | 22 ± 5 | 22 ± 5 | 20 ± 4 | 20 ± 6 |
| Caffeic acid | 7 ± 2 | 7 ± 2 | 6 ± 2 | 7 ± 2 |
| Caffeic acid 3-O-β-d-glucuronide | 2 ± 1 | 2 ± 1 | 4 ± 3 | 3 ± 1 |
| Caffeic acid 4-O-β-d-glucuronide | 1 ± 0 | 1 ± 0 | 2 ± 2 | 2 ± 1 |
| Dihydrocaffeic acid | 24 ± 6 | 17 ± 4 | 27 ± 6 | 23 ± 5 |
| Dihydrocaffeic acid 3-O-sulfate | 53 ± 12 | 41 ± 11 | 104 ± 28 b | 73 ± 15 |
| Dihydrocaffeic acid 3-O-β-d-glucuronide | 9 ± 1 | 8 ± 1 | 11 ± 2 | 10 ± 2 |
| Ferulic acid | 6 ± 2 | 6 ± 2 | 7 ± 2 | 6 ± 1 |
| Ferulic acid 4-O-glucuronide | 156 ± 40 | 182 ± 32 | 195 ± 76 | 223 ± 67 |
| Ferulic acid 4-O-sulfate | 90 ± 33 | 106 ± 40 | 74 ± 26 | 80 ± 17 |
| Dihydroferulic acid | 100 ± 23 | 72 ± 24 | 111 ± 22 | 74 ± 15 |
| Dihydroferulic acid 4-O-sulfate | 154 ± 26 | 96 ± 19 | 138 ± 29 | 90 ± 17 |
| Dihydroferulic acid 4-O-β-d-glucuronide | 116 ± 23 | 76 ± 16 | 124 ± 31 | 100 ± 25 |
| Isoferulic acid | 1941 ± 348 | 1633 ± 312 | 1842 ± 377 | 1686 ± 319 |
| Isoferulic acid 3-O-sulfate | 19 ± 3 | 18 ± 2 | 22 ± 5 | 20 ± 4 |
| Isoferulic acid 3-O-β-d-glucuronide | 38 ± 10 | 38 ± 8 | 74 ± 13 b | 77 ± 16 |
| Dihydro isoferulic acid 3-O-sulfate | 102 ± 64 | 73 ± 39 | 44 ± 11 | 40 ± 8 |
| Dihydroisoferulic acid 3-O-β-d-glucuronide | 8 ± 2 | 6 ± 2 | 9 ± 2 | 8 ± 2 |
| m-Coumaric acid | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 |
| o-Coumaric acid | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 |
| p-Coumaric acid | 3 ± 0 | 4 ± 1 | 4 ± 1 | 4 ± 1 |
| Sinapic acid | 11 ± 2 | 7 ± 1 | 8 ± 1 | 7 ± 1 |
| Chlorogenic acid | 22 ± 10 | 60 ± 18 a | 20 ± 6 | 63 ± 22 c |
| Flavonol derivatives | ||||
| Kaempferol | 64 ± 17 | 68 ± 17 | 71 ± 22 | 75 ± 23 |
| Kaempferol-3-O-β-d-glucuronide | 1 ± 0 | 1 ± 0 | 2 ± 2 | 2 ± 0 |
| Quercetin | 32 ± 8 | 35 ± 9 | 28 ± 7 | 28 ± 7 |
| Quercetin-3-O-β-d-glucuronide | 3 ± 1 | 5 ± 1 a | 6 ± 1 | 9 ± 2 c |
| Valerolactone derivatives | ||||
| (4R)-5-(3′-hydroxyphenyl)-γ-valerolactone-4′-O-sulfate | 62 ± 26 | 31 ± 10 | 60 ± 16 | 67 ± 25 |
| Total 24 h Urinary Excretion (µg) | Day 1 | Day 30 |
|---|---|---|
| Benzoic Acid Derivatives | ||
| Benzoic acid | 204 ± 49 | 286 ± 115 |
| 2-Hydroxybenzoic acid | 50 ± 30 | 18 ± 10 |
| 3-Hydroxybenzoic acid | 22 ± 7 | 22 ± 5 |
| 4-Hydroxybenzoic acid | 519 ± 97 | 397 ± 60 |
| 2,3-Dihydroxybenzoic acid | 6590 ± 1351 | 6079 ± 727 |
| 2,4-Dihydroxybenzoic acid | 54 ± 13 | 61 ± 15 |
| 2,5-Dihydroxybenzoic acid | 668 ± 119 | 757 ± 106 |
| Protocatechuic acid | 284 ± 52 | 217 ± 47 |
| Syringic acid | 86 ± 21 | 76 ± 20 |
| Vanillic acid | 264 ± 88 | 219 ± 36 |
| Vanillic acid-4-O-sulfate | 392 ± 103 | 407 ± 147 |
| Isovanillic acid | 68 ± 10 | 69 ± 13 |
| Gallic acid | 2 ± 1 | 1 ± 0 |
| 4-Methylgallic acid-3-O-sulfate | 194 ± 56 | 149 ± 50 |
| Phenylacetic acid derivatives | ||
| Homovanillic acid | 939 ± 175 | 941 ± 143 |
| Homovanillic acid sulfate | 37 ± 9 | 48 ± 19 |
| Phenylacetic acid | 318 ± 50 | 429 ± 129 |
| 3,4-Dihydroxyphenylacetic acid | 219 ± 39 | 168 ± 29 |
| 3-Hydroxyphenylacetic acid | 900 ± 130 | 1230 ± 188 |
| 4-Hydroxyphenylacetic acid | 1910 ± 284 | 1592 ± 199 |
| Propionic acid derivatives | ||
| 2-(4-hydroxyphenoxy)propionic acid | 38 ± 19 | 39 ± 15 |
| Benzaldehyde derivatives | ||
| 4-Hydroxybenzaldehyde | 2 ± 0 | 1 ± 0 |
| 3,4-Dihydroxybenzaldehyde | 25 ± 4 | 20 ± 2 |
| Pyrogallol derivatives | ||
| Pyrogallol-O-1-sulfate | 22 ± 6 | 19 ± 6 |
| Pyrogallol-O-2-sulfate | 154 ± 41 | 92 ± 34 |
| 1-Methylpyrogallol-O-sulfate | 71 ± 19 | 74 ± 31 |
| 2-Methylpyrogallol-O-sulfate | 48 ± 15 | 35 ± 12 |
| Catechol derivatives | ||
| Catechol-O-sulfate | 325 ± 69 | 456 ± 138 |
| 4-Methylcatechol-O-sulfate | 842 ± 127 | 874 ± 181 |
| Hippuric acid derivatives | ||
| Hippuric acid | 55,827 ± 7000 | 62,840 ± 8552 |
| 2-Hydroxyhippuric acid | 651 ± 308 | 555 ± 297 |
| 3-Hydroxyhippuric acid | 5729 ± 938 | 6242 ± 1119 |
| 4-Hydroxyhippuric acid | 480 ± 116 | 397 ± 114 |
| α-Hydroxyhippuric acid | 4887 ± 1198 | 3857 ± 1052 |
| Cinnamic acid derivatives | ||
| Cinnamic acid | 2 ± 1 | 2 ± 1 |
| Caffeic acid | 18 ± 2 | 20 ± 3 |
| Caffeic acid 3-O-β-d-glucuronide | 142 ± 25 | 148 ± 37 |
| Caffeic acid 4-O-β-d-glucuronide | 67 ± 12 | 60 ± 12 |
| Dihydrocaffeic acid | 46 ± 9 | 74 ± 14 |
| Dihydrocaffeic acid 3-O-sulfate | 824 ± 194 | 1032 ± 244 |
| Dihydrocaffeic acid 3-O-β-d-glucuronide | 105 ± 20 | 114 ± 20 |
| Ferulic acid | 10 ± 3 | 13 ± 2 |
| Ferulic acid 4-O-glucuronide | 1942 ± 266 | 1924 ± 300 |
| Ferulic acid 4-O-sulfate | 3861 ± 638 | 3828 ± 597 |
| Dihydroferulic acid | 147 ± 44 | 152 ± 36 |
| Dihydroferulic acid 4-O-sulfate | 954 ± 158 | 932 ± 136 |
| Dihydroferulic acid 4-O-β-d-glucuronide | 427 ± 130 | 404 ± 79 |
| Isoferulic acid | 832 ± 243 | 889 ± 371 |
| Isoferulic acid 3-O-sulfate | 310 ± 79 | 302 ± 74 |
| Isoferulic acid 3-O-β-d-glucuronide | 290 ± 58 | 284 ± 55 |
| Dihydro isoferulic acid 3-O-sulfate | 121 ± 27 | 184 ± 64 |
| Dihydro isoferulic acid 3-O-β-d-glucuronide | 152 ± 39 | 166 ± 47 |
| m-Coumaric acid | 9 ± 2 | 15 ± 3 |
| o-Coumaric acid | 2 ± 0 | 1 ± 0 |
| p-Coumaric acid | 3 ±1 | 2 ± 0 |
| Sinapic acid | 60 ± 16 | 46 ± 14 |
| Chlorogenic acid | 370 ± 85 | 338 ± 102 |
| Flavonol derivatives | ||
| Kaempferol | 51 ± 13 | 70 ± 18 |
| Kaempferol-3-O-β-D-glucuronide | 6 ± 2 | 4 ± 1 |
| Quercetin | 51 ± 11 | 140 ± 76 |
| Quercetin-3-O-β-D-glucuronide | 19 ± 4 | 24 ± 8 |
| Valerolactone derivatives | ||
| (4R)-5-(3′-hydroxyphenyl)-γ-valerolactone-4′-O-sulfate | 1354 ± 277 | 1246 ± 292 |
| Total amount excreted (mg) | 95 ± 11 | 101 ± 13 |
| Recovery (%) | 16 ± 2 | 17 ± 2 |
| Plasma | Urine | |||||
|---|---|---|---|---|---|---|
| Day 1 | Day 30 | Day 1 | Day 30 | |||
| 0 h | 2 h | 0 h | 2 h | 24 h | 24 h | |
| Benzoic acids | 63 | 61 | 50 | 62 | 71 | 40 |
| Phenylacetic acids | 164 | 228 | 120 | 85 | 50 | 54 |
| Propionic acids | 96 | 111 | 105 | 127 | 198 | 158 |
| Benzaldehydes | 115 | 117 | 78 | 90 | 69 | 47 |
| Pyrogallols | 87 | 133 | 141 | 125 | 77 | 134 |
| Catechols | 52 | 36 | 58 | 45 | 61 | 84 |
| Hippuric acids | 90 | 82 | 66 | 78 | 50 | 57 |
| Cinnamic acids | 65 | 63 | 79 | 74 | 57 | 65 |
| Flavonols | 81 | 83 | 91 | 88 | 79 | 151 |
| Valerolactones | 182 | 148 | 113 | 157 | 82 | 94 |
| Total (poly)phenols | 44 | 44 | 40 | 48 | 47 | 54 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feliciano, R.P.; Istas, G.; Heiss, C.; Rodriguez-Mateos, A. Plasma and Urinary Phenolic Profiles after Acute and Repetitive Intake of Wild Blueberry. Molecules 2016, 21, 1120. https://doi.org/10.3390/molecules21091120
Feliciano RP, Istas G, Heiss C, Rodriguez-Mateos A. Plasma and Urinary Phenolic Profiles after Acute and Repetitive Intake of Wild Blueberry. Molecules. 2016; 21(9):1120. https://doi.org/10.3390/molecules21091120
Chicago/Turabian StyleFeliciano, Rodrigo P., Geoffrey Istas, Christian Heiss, and Ana Rodriguez-Mateos. 2016. "Plasma and Urinary Phenolic Profiles after Acute and Repetitive Intake of Wild Blueberry" Molecules 21, no. 9: 1120. https://doi.org/10.3390/molecules21091120






