Coffee Consumption and Oxidative Stress: A Review of Human Intervention Studies
Abstract
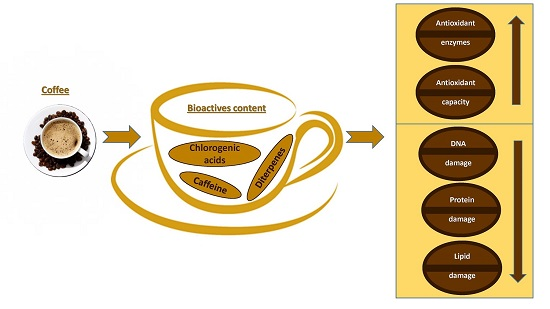
:1. Introduction
2. Objective and Literature Search Strategy
3. Results
3.1. Total Plasma Antioxidant Capacity and Antioxidant Enzymes
3.2. Protein Damage
3.3. Lipid Damage
3.4. DNA Damage
4. Conclusions
Author Contributions
Conflicts of Interest
References
- International Coffee Organization. Infographics on the Global Coffee Trade. Available online: http://www.ico.org/coffee-trade-statistics-infographics.asp?section=Statistics (accessed on 1 March 2016).
- International Coffee Council. Trends in Coffee Consumption in Selected Importing Countries. Available online: http://www.ico.org/documents/icc-109-8e-trends-consumption.pdf (accessed on 4 June 2016).
- WHO. Coffee, Tea, Mate, Methylxanthines and Methylglyoxal; International Agency for Research on Cancer: Lyon, France, 1991. [Google Scholar]
- Loomis, D.; Guyton, K.Z.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaaa, L.; Guhaa, N.; Mattocka, H.; Straifa, K. Carcinogenicity of drinking coffee, mate, and very hot beverages. Lancet Oncol. 2016, 17, 877–878. [Google Scholar] [CrossRef]
- Vinson, J.A. Polyphenols: Total amounts in foods and beverages and U.S. per capital consumption. Abstract number AGFD 10. In Proceedings of the American Chemical Society 230th National Meeting, Washington, DC, USA, 28 August 2005.
- Farah, A. Coffee Constituents. In Coffee: Emerging Health Effects and Disease Prevention; Chu, Y.F., Ed.; Wiley-Blackwell: Oxford, UK, 2012; pp. 21–58. [Google Scholar]
- Ludwig, I.A.; Mena, P.; Calani, L.; Cid, C.; Del Rio, D.; Lean, M.E.; Crozier, A. Variations in caffeine and chlorogenic acid contents of coffees: What are we drinking? Food Funct. 2014, 5, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Dieminger, N.; Beusch, A.; Lee, Y.M.; Dunkel, A.; Suess, B.; Skurk, T.; Wahl, A.; Hauner, H.; Hofmann, T. Bioappearance and pharmacokinetics of bioactives upon coffee consumption. Anal. Bioanal. Chem. 2013, 405, 8487–8503. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E.J.; Ashihara, H.; Clifford, M.N.; Crozier, A. Purine Alkaloids: A Focus on Caffeine and Related Compounds in Beverages. In Teas, Cocoa and Coffee: Plant Secondary Metabolites and Health; Crozier, A., Ashihara, H., Tomás-Barbéran, F., Eds.; Wiley-Blackwell: Oxford, UK, 2011; pp. 25–44. [Google Scholar]
- Godos, J.; Pluchinotta, F.R.; Marventano, S.; Buscemi, S.; Li Volti, G.; Galvano, F.; Grosso, G. Coffee components and cardiovascular risk: Beneficial and detrimental effects. Int. J. Food Sci. Nutr. 2014, 65, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Cappelletti, S.; Piacentino, D.; Sani, G.; Aromatario, M. Caffeine: Cognitive and physical performance enhancer or psychoactive drug? Curr. Neuropharmacol. 2015, 13, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Owens, J.A.; Mindell, J.; Baylor, A. Effect of energy drink and caffeinated beverage consumption on sleep, mood, and performance in children and adolescents. Nutr. Rev. 2014, 72 (Suppl. S1), 65–71. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products Nutrition and Allergies (EFSA NDA Panel). Scientific Opinion on the substantiation of health claims related to caffeine and increase in physical performance during short-term high-intensity exercise (ID 737, 1486, 1489), increase in endurance performance (ID 737, 1486), increase in endurance capacity (ID 1488) and reduction in the rated perceived exertion/effort during exercise (ID 1488, 1490) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2053. [Google Scholar]
- Urgert, R.; Katan, M.B. The cholesterol-raising factor from coffee beans. Ann. Rev. Nutr. 1997, 17, 305–324. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Medina-Remón, A.; Estruch, R.; Lamuela-Raventós, R.M. Coffee Polyphenols and High Cardiovascular Risk Parameters. In Coffee and Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 387–394. [Google Scholar]
- Arai, K.; Terashima, H.; Aizawa, S.I.; Taga, A.; Yamamoto, A.; Tsutsumiuchi, K.; Kodama, S. Simultaneous determination of trigonelline, caffeine, chlorogenic acid and their related compounds in instant coffee samples by HPLC using an acidic mobile phase containing octanesulfonate. Anal. Sci. 2015, 31, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Mullen, W.; Barron, D.; Uchida, K.; Yokota, T.; Cavin, C.; Steiling, H.; Williamson, G.; Crozier, A. Metabolite profiling of hydroxycinnamate derivatives in plasma and urine after the ingestion of coffee by humans: Identification of biomarkers of coffee consumption. Drug Metab. Dispos. 2009, 37, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Farah, A.; Donangelo, C.M. Phenolic compounds in coffee. Braz. J. Plant Physiol. 2006, 18, 23–36. [Google Scholar] [CrossRef]
- Ky, C.L.; Louarn, J.; Dussert, S.; Guyot, B.; Hamon, S.; Noirot, M. Caffeine, trigonelline, chlorogenic acids and sucrose diversity in wild Coffea arabica L. and C. canephora P. accessions. Food Chem. 2001, 75, 223–230. [Google Scholar] [CrossRef]
- Komes, D.; Aleksandra, V. Effects of varieties and growing conditions on antioxidant capacity of coffee. In Processing and Impact on Antioxidants in Beverages; Preedy, V., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 77–85. [Google Scholar]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef] [PubMed]
- Fumeaux, R.; Menozzi-Smarrito, C.; Stalmach, A.; Munari, C.; Kraehenbuehl, K.; Steiling, H.; Crozier, A.; Williamson, G.; Barron, D. First synthesis, characterization, and evidence for the presence of hydroxycinnamic acid sulfate and glucuronide conjugates in human biological fluids as a result of coffee consumption. Org. Biomol. Chem. 2010, 8, 5199–5211. [Google Scholar] [CrossRef] [PubMed]
- Agudelo-Ochoa, G.M.; Pulgarín-Zapata, I.C.; Velásquez-Rodriguez, C.M.; Duque-Ramírez, M.; Naranjo-Cano, M.; Quintero-Ortiz, M.M.; Lara-Guzmán, O.J.; Muñoz-Durango, K. Coffee consumption increases the antioxidant capacity of plasma and has no effect on the lipid profile or vascular function in healthy adults in a randomized controlled trial. J. Nutr. 2016, 146, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Teekachunhatean, S.; Tosri, N.; Sangdee, C.; Wongpoomchai, R.; Ruangyuttikarn, W.; Puaninta, C.; Srichairatanakool, S. Antioxidant effects after coffee enema or oral coffee consumption in healthy Thai male volunteers. Hum. Exp. Toxicol. 2012, 31, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J.; Trepanowski, J.F.; Farney, T.M. Influence of acute coffee consumption on postprandial oxidative stress. Nutr. Metab. Insights 2013, 6, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Leelarungrayub, D.; Sallepan, M.; Charoenwattana, S. Effects of acute caffeinated coffee consumption on energy utilization related to glucose and lipid oxidation from short submaximal Treadmill exercise in sedentary Men. Nutr. Metab. Insights 2011, 4, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Moura-Nunes, N.; Perrone, D.; Farah, A.; Donangelo, C.M. The increase in human plasma antioxidant capacity after acute coffee intake is not associated with endogenous non-enzymatic antioxidant components. Int. J. Food Sci. Nutr. 2009, 60 (Suppl. S6), 173–181. [Google Scholar] [CrossRef] [PubMed]
- Natella, F.; Nardini, M.; Giannetti, I.; Dattilo, C.; Scaccini, C. Coffee drinking influences plasma antioxidant capacity in humans. J. Agric. Food Chem. 2002, 50, 6211–6216. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, R.; Sugiura, Y.; Shioya, Y.; Otsuka, K.; Katsuragi, Y.; Hashiguchi, T. Coffee polyphenols improve peripheral endothelial function after glucose loading in healthy male adults. Nutr. Res. 2014, 34, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, R.; Sugiura, Y.; Otsuka, K.; Katsuragi, Y.; Hashiguchi, T. Coffee bean polyphenols ameliorate postprandial endothelial dysfunction in healthy male adults. Int. J. Food Sci. Nutr. 2015, 66, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Sirota, R.; Gorelik, S.; Harris, R.; Kohen, R.; Kanner, J. Coffee polyphenols protect human plasma from postprandial carbonyl modifications. Mol. Nutr. Food Res. 2013, 57, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Mursu, J.; Voutilainen, S.; Nurmi, T.; Alfthan, G.; Virtanen, J.K.; Rissanen, T.H.; Happonen, P.; Nyyssönen, K.; Kaikkonen, J.; Salonen, R.; et al. The effects of coffee consumption on lipid peroxidation and plasma total homocysteine concentrations: A clinical trial. Free Radic. Biol. Med. 2005, 38, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Bakuradze, T.; Lang, R.; Hofmann, T.; Schipp, D.; Galan, J.; Eisenbrand, G.; Richling, E. Coffee consumption rapidly reduces background DNA strand breaks in healthy humans: Results of a short-term repeated uptake intervention study. Mol. Nutr. Food Res. 2016, 60, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, T.A.F.; Monteiro, M.P.; Mendes, T.M.N.; de Oliveira, D.M.; Rogero, M.M.; Benites, C.I.; Vinagre, C.G.; Mioto, B.M.; Tarasoutchi, D.; Tuda, V.L.; et al. Medium light and medium roast paper-filtered coffee increased antioxidant capacity in healthy volunteers: Results of a randomized trial. Plant Foods Hum. Nutr. 2012, 67, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Hoelzl, C.; Knasmüller, S.; Wagner, K.H.; Elbling, L.; Huber, W.; Kager, N.; Ferk, F.; Ehrlich, V.; Nersesyan, A.; Neubauer, O.; et al. Instant coffee with high chlorogenic acid levels protects humans against oxidative damage of macromolecules. Mol. Nutr. Food Res. 2010, 54, 1722–1733. [Google Scholar] [CrossRef] [PubMed]
- Mišík, M.; Hoelzl, C.; Wagner, K.H.; Cavin, C.; Moser, B.; Kundi, M.; Simic, T.; Elbling, L.; Kager, N.; Ferk, F.; et al. Impact of paper filtered coffee on oxidative DNA-damage: Results of a clinical trial. Mutat. Res. 2010, 692, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Revuelta-Iniesta, R.; Al-Dujaili, E.A. Consumption of green coffee reduces blood pressure and body composition by influencing 11β-HSD1 enzyme activity in healthy individuals: A pilot crossover study using green and black coffee. BioMed Res. Int. 2014, 2014, 482704. [Google Scholar] [CrossRef] [PubMed]
- Kotyczka, C.; Boettler, U.; Lang, R.; Stiebitz, H.; Bytof, G.; Lantz, I.; Hofmann, T.; Marko, D.; Somoza, V. Dark roast coffee is more effective than light roast coffee in reducing body weight, and in restoring red blood cell vitamin E and glutathione concentrations in healthy volunteers. Mol. Nutr. Food Res. 2011, 55, 1582–1586. [Google Scholar] [CrossRef] [PubMed]
- Steinkellner, H.; Hoelzl, C.; Uhl, M.; Cavin, C.; Haidinger, G.; Gsur, A.; Schmid, R.; Kundi, M.; Bichler, J.; Knasmüller, S. Coffee consumption induces GSTP in plasma and protects lymphocytes against (±)-anti-benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide induced DNA-damage: Results of controlled human intervention trials. Mutat. Res. 2005, 591, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Bakuradze, T.; Boehm, N.; Janzowski, C.; Lang, R.; Hofmann, T.; Stockis, J.P.; Albert, F.W.; Stiebitz, H.; Bytof, G.; Lantz, I.; et al. Antioxidant-rich coffee reduces DNA damage, elevates glutathione status and contributes to weight control: Results from an intervention study. Mol. Nutr. Food Res. 2011, 55, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Esposito, F.; Morisco, F.; Verde, V.; Ritieni, A.; Alezio, A.; Caporaso, N.; Fogliano, V. Moderate coffee consumption increases plasma glutathione but not homocysteine in healthy subjects. Aliment. Pharmacol. Ther. 2003, 17, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Grubben, M.J.A.L.; Den Braak, V.; Jong, D.; Rijt, V.; Ruijter, D. The effect of unfiltered coffee on potential biomarkers for colonic cancer risk in healthy volunteers: A randomized trial. Aliment. Pharmacol. Ther. 2000, 14, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Kempf, K.; Herder, C.; Erlund, I.; Kolb, H.; Martin, S.; Carstensen, M.; Koenig, W.; Sundvall, J.; Bidel, S.; Kuha, S.; et al. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: A clinical trial. Am. J. Clin. Nutr. 2010, 91, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Yukawa, G.S.; Mune, M.; Otani, H.; Tone, Y.; Liang, X.M.; Iwahashi, H.; Sakamoto, W. Effects of coffee consumption on oxidative susceptibility of low-density lipoproteins and serum lipid levels in humans. Biochemistry 2004, 69, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Bakuradze, T.; Parra, G.A.M.; Riedel, A.; Somoza, V.; Lang, R.; Dieminger, N.; Hofmann, T.; Winkler, S.; Hassmannd, U.; Marko, D.; et al. Four-week coffee consumption affects energy intake, satiety regulation, body fat, and protects DNA integrity. Food Res. Int. 2014, 63, 420–427. [Google Scholar] [CrossRef]
- Bakuradze, T.; Lang, R.; Hofmann, T.; Eisenbrand, G.; Schipp, D.; Galan, J.; Richling, E. Consumption of a dark roast coffee decreases the level of spontaneous DNA strand breaks: A randomized controlled trial. Eur. J. Nutr. 2015, 54, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Bichler, J.; Cavin, C.; Simic, T.; Chakraborty, A.; Ferk, F.; Hoelzl, C.; Schulte-Hermann, R.; Kundi, M.; Haidinger, G.; Angelis, K.; et al. Coffee consumption protects human lymphocytes against oxidative and 3-amino-1-methyl-5H-pyrido [4,3-b] indole acetate (Trp-P-2) induced DNA-damage: Results of an experimental study with human volunteers. Food Chem. Toxicol. 2007, 45, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Cardin, R.; Piciocchi, M.; Martines, D.; Scribano, L.; Petracco, M.; Farinati, F. Effects of coffee consumption in chronic hepatitis C: A randomized controlled trial. Dig. Liver Dis. 2013, 45, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H. 3-Nitrotyrosine: A biomarker of nitrogen free radical species modified proteins in systemic autoimmunogenic conditions. Hum. Immunol. 2013, 74, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.; Koppen, G.; Valdiglesias, V.; Dusinská, M.; Kruszewski, M.; Møller, P.; Rojas, E.; Dhawan, A.; Benzie, I.; Coskun, E.; et al. The comet assay as a tool for human biomonitoring studies: The ComNet project. Mutat. Res. Rev. Mutat. Res. 2014, 759, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Hoelzl, C.; Knasmüller, S.; Misík, M.; Collins, A.; Dusinská, M.; Nersesyan, A. Use of single cell gel electrophoresis assays for the detection of DNA-protective effects of dietary factors in humans: Recent results and trends. Mutat. Res. 2009, 681, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Dušinská, M.; Collins, A. Detection of oxidized purines and UV-induced photoproducts in DNA of single cells, by inclusion of lesion-specific enzymes in the comet assay. Altern. Lab. Anim. 1996, 24, 405–411. [Google Scholar]
- Collins, A.R.; Duthie, S.J.; Dobson, V.L. Direct enzymic detection of endogenous oxidative base damage in human lymphocyte DNA. Carcinogenesis 1993, 14, 1733–1735. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.S.; Evans, M.D.; Burd, R.M.; Patel, K.; Barnard, A.; Lunec, J.; Hutchinson, P.E. Induction and excretion of ultraviolet-induced 8-oxo-2′-deoxyguanosine and thymine dimers in vivo: Implications for PUVA. J. Investig. Dermatol. 2001, 116, 281–285. [Google Scholar] [CrossRef] [PubMed]


| Reference | Subjects | Type of Coffee and Composition | Doses | Study Design | Markers | |||
|---|---|---|---|---|---|---|---|---|
| Antioxidant Capacity/Enzymes | Lipid Damage | DNA Damage | Protein Damage | |||||
| Agudelo-Ochoa et al. [23] | 74 healthy subjects (38 males, 37 females), mean age of 38.5 ± 6.9 years, mean BMI 24.1 ± 2.6 kg/m2 Control group: 13 males, 12 females; 14 subjects aged 20–40 years, 11 aged 41–60 years Group MCCGA: 12 males, 13 females; 14 subjects aged 20–40 years, 11 aged 41–60 years Group HCCGA: 12 males, 12 females; 10 subjects aged 20–40 years, 14 aged 41–60 years | Coffee 1 (MCCGA): Colombian Arabica coffee Composition: total CGAs 105 ± 4.1 mg/100 mL, cafestol 0.19 ± 0.03 mg/100 mL, kahweol 0.22 ± 0.03 mg/100 mL, caffeine 47 ± 1.4 mg/100 mL Coffee 2 (HCCGA): Colombian Arabica coffee Composition: total CGAs 195 ± 6.9 mg/100 mL, cafestol 0.19 ± 0.01 mg/100 mL, kahweol 0.23 ± 0.02 mg/100 mL, caffeine 49 ± 1 mg/100 mL | Coffee group 1: 400 mL of MCCGA coffee Coffee group 2: 400 mL of HCCGA coffee Control group: no coffee | Parallel intervention | ↑AC (FRAP) | |||
| Teekachunhatean et al. [24] | 11 healthy men, (mean age 21.09 ± 7.97 years, mean BMI 20.80 ± 2.27 kg/m2) | Coffee 1: Coffee enema, prepared mixing 4 g of ground coffee beans with 100 mL of purified water. Composition: n.d Coffee 2: Coffee for oral procedure: ready-to-drink coffee beverage Composition: n.d | Coffee group 1: Coffee enema (500 mL) Coffee group 2: 180 mL ready-to-drink coffee Control group: n.d | Randomized, two-phase, crossover intervention | =GSH ↓TAC | =MDA | ||
| Bloomer et al. [25] | 16 healthy subjects (8 males, 8 females; mean age 29.2 ± 14.4 years, mean BMI 23.3 ± 2.2 kg/m2) | Coffee: caffeinated and decaffeinate Composition: 175 mg caffeine (caffeinated), 15 mg caffeine (decaffeinated) per 16 ounces | Coffee group 1: 16 ounces of freshly brewed caffeinated coffee following milk shake consumption Coffee group 2: 16 ounces of freshly brewed decaffeinated coffee following milk shake consumption Control group: 16 ounces of bottled water, following milk shake consumption | Parallel intervention | =TAC | =MDA | ||
| Leelarungrayub et al. [26] | 26 sedentary men
Group 1 (Caffeine): 10 males, mean age 20.5 ± 0.53 years, mean BMI 22.84 ± 2.65 kg/m2 Group 2 (Decaffeinated): 10 males, mean age 20.3 ± 0.48 years, mean BMI 22.27 ± 3.56 kg/m2 Group 3 (Control): 6 males, mean age 20.17 ± 0.98 years, mean BMI 23.06 ± 3.60 kg/m2 | Coffee 1: Caffeinated coffee Composition: n.d. Coffee 2: Decaffeinated coffee (Instant freeze dried) Composition: n.d. | Coffee group 1: Caffeinated (5 mg caffeine/kg bw) coffee followed by a submaximal exercise test Coffee group 2: decaffeinated coffee followed by a submaximal exercise test Control group: No coffee consumption followed by a submaximal exercise test | Parallel intervention | =TAC | ↑MDA | ||
| Moura-Nunes et al. [27] | 10 subject (3 males and 7 females), range age 22–57 years, BMI n.d. | Coffee: Instant coffee 100% Arabica prepared dissolving 8 g in 200 mL boiling water. Composition: n.d. | Coffee group: 200 mL instant coffee beverage Control group: 200 mL water | Randomized, controlled, crossover intervention | ↑AC (TRAP and FRAP) | |||
| Natella et al. [28] | 10 healthy nonsmoker subjects (5 males, 5 females), age and BMI n.d. | Coffee: Coffee Lavazza Qualità Rossa Composition: caffeine 181 ± 10 mg/cup, theobromine 28.9 ± 1.1 mg/cup, total phenols 161 ± 9 mg of GAE/cup, TRAP 10.1 ± 0.6 mM ROO·eq./cup Tea: Twining Earl Gray Composition: caffeine 130 ± 7 mg/cup, theobromine 5.9 ± 0.4 mg/cup, total phenols 87 ± 9 mg of GAE/cup, TRAP 1.3 ± 0.1 mM ROO·equiv/cup | Coffee group: 200 mL of brewed coffee Control group: 200 mL of Twining Earl Gray tea | Baseline and post-intervention | ↑AC (TRAP) ↑SH groups (ns) | |||
| Ochiai et al. [29] | 14 healthy men, (mean age 36.2 ± 7.8 years, mean BMI 22.7 ± 1.8 kg/m2) | Coffee: Coffee polyphenol (CPP) prepared from green coffee beans by hot water extraction Composition: Total CQA content 80.7% | Coffee group: CPP (600 mg CGAs, co-administered with the glucose solution) Control group: 225 mL of a 75-g Glu-equivalent test solution | Single-blind, randomized, controlled, crossover intervention | ↑MDA (no differences among treatment) ↑IsoPs (no differences among treatment) | |||
| Ochiai et al. [30] | 13 healthy men, (mean age 44.9 ± 1.4 years, mean BMI 21.9 ± 0.6 kg/m2) | Coffee: Coffee bean polyphenol (CBP) beverage Composition: 600 mg CGA/100 mL water | Coffee group: 600 mg CGAs (equivalent to two cups of coffee) in 100 mL of water after a test meal Control group: 100 mL of water after a test meal | Double-blind, randomized, crossover intervention | ↑MDA (no differences among treatment) ↓IsoPs | |||
| Sirota et al. [31] | 10 healthy subjects Characteristics of the subjects: n.d. | Coffee 1: Turkish roasted ground coffee (A) Composition: 110 mg polyphenols/g∙dm Coffee 2: Turkish roasted ground coffee (AG) enriched by 2% freeze-dried powder of green beans Composition: 123 mg polyphenols/g∙dm | 200 mL coffee A, AG or water together with 250 g red-meat cutlets | Crossover intervention | ↓MDA concentration ↓MDA absorption after coffee A, less after coffee AG | |||
| Mursu et al. [32] | 45 nonsmoking volunteer men (mean age, 26 ± 6 years and BMI < 32 kg/m2). Only 35 subjects completed the trial | Coffee: Finely ground coffee, repared by filtering through paper (7–8 g of grounds per one 150-mL cup) Composition: 80.9 ± 3.3 mg/100 mL of phenolic acids, with CGA as major compound (~90%) | Coffee group: 1–2 cups (150–300 mL, respectively) Control group: No coffee | Parallel intervention | =LDL-conjugated dienes =Plasma hydroxy fatty acids =F2-IsoPs | |||
| Bakuradze et al. [33] | 13 healthy men subjects (mean age 23 ± 2.4 years, mean BMI 23.8 ± 1.6 kg/m2) | Arabica coffee, freshly prepared in a pad machine Composition: 16.7 ± 0.7 mg/g caffeine, 10.4 ± 0.9 mg/g CGA, 1.1 ± 0.2 NMP and 3.9 ± 0.3 mg/g trigonelline | Coffee group: 200 mL Control group: n.d | Baseline and post intervention | ↓SBs | |||
| Reference | Subjects | Type of Coffee and Composition | Doses | Study Design | Markers | |||
|---|---|---|---|---|---|---|---|---|
| Antioxidant Capacity/Enzymes | Lipid Damage | DNA Damage | Protein Damage | |||||
| Agudelo-Ochoa et al. [23] | 74 healthy subjects (38 males, 37 females), mean age of 38.5 ± 6 9 years, mean BMI 24.1 ± 2.6 kg/m2 Control group: 13 males, 12 females; 14 subjects aged 20–40 years, 11 aged 41–60 years Group MCCGA: 12 males, 13 females; 14 subjects aged 20–40 years, 11 aged 41–60 years Group HCCGA: 12 males, 12 females; 10 subjects aged 20–40 years, 14 aged 41–60 years | Coffee 1 (MCCGA): Colombian Arabica coffee Composition: total CGAs 105 ± 4.1 mg/100 mL, cafestol 0.19 ± 0.03 mg/100 mL, kahweol 0.22 ± 0.03 mg/100 mL, caffeine 47 ± 1.4 mg/100 mL Coffee 2 (HCCGA): Colombian Arabica coffee Composition: total CGAs 195 ± 6.9 mg/100 mL, cafestol 0.19 ± 0.01 mg/100 mL, kahweol 0.23 ± 0.02 mg/100 mL, caffeine 49 ± 1 mg/100 mL | Coffee group 1: 400 mL/day of MCCGA for 8 weeks Coffee group 2: 400 mL/day of HCCGA for 8 weeks Control group: no coffee for 8 weeks | Parallel intervention | ↓AC (FRAP) | |||
| Teekachunhatean et al. [24] | 11 healthy men, mean age 21.09 ± 7.97 years, mean BMI 20.80 ± 2.27 kg/m2 | Coffee 1: (Enema, coffee prepared mixing 4 g of ground coffee beans with 100 mL of purified water. Composition: n.d. Coffee 2: coffee for oral procedure: ready-to-drink coffee beverage Composition: n.d. | Coffee group 1: Coffee enema (500 mL, 3 times/week for 6 visits) Coffee group 2: 180 mL ready-to-drink coffee (2/day for 11 days) Control group: n.d. | Randomized, crossover intervention | =GSH ↓TEAC | =MDA | ||
| Mursu et al. [32] | 45 nonsmoking men (mean age, 26 ± 6 years and BMI < 32 kg/m2). 43 subjects completed the trial | Coffee: finely ground coffee, repared by filtering through paper (7–8 g of grounds per one 150-mL cup) Composition: 80.9 ± 3.3 mg/100 mL of phenolic acids, with CGA as major compound (~90%) | Coffee group 1: 3 cups (450 mL/day) of coffee for 3 weeks Coffee group 2: 6 cups (900 mL/day) of coffee for 3 weeks Control group: No coffee consumption for 3 weeks | Parallel intervention | =GPx | =Serum LDL-conjugated dienes =Plasma hydroxy fatty acids =F2-IsoPs | ||
| Corrêa et al. [34] | Twenty healthy subjects (6 males, 14 females), mean age 49 ± 9 years, BMI n.d. | Coffee 1: MLR-Medium Light Roast paper-filtered coffee. 15 g per one 150-mL cup Composition: total phenolic content 11.09 ± 0.29 mg 5-CQAE/mL, total CGAs 1.98 ± 0.02 mg 5-CQAE/mL, caffeine 1.54 ± 0.01 mg/mL Coffee 2: Medium Roast (MR) paper-filtered coffee. 15 g per one 150-mL cup. Composition: total phenolic content 10.53 ± 0.56 mg 5-CQAE/mL, total CGAs 1.24 ± 0.01 mg 5-CQAE/mL, caffeine 1.63 ± 0.02 mg/mL | Coffee group 1: 150 mL MLR for 4 weeks Coffee group 2: 150 mL MR for 4 weeks Control group: n.d. | Randomized, cross-over intervention | ↑AC (TAS and ORAC) ↑GPx ↑CAT ↑SOD | =OxLDL =IsoPs | ||
| Hoelzl et al. [35] | 29 subjects (13 males: mean age 25.2 ± 5.6 years, mean BMI 23.0 ± 1.7 kg/m2; 16 females: mean age 29.3 ± 10.9 years, mean BMI 21.8 ± 2.4 kg/m2) | Coffee: mix of 35% green and 65% roasted coffee water extracts Composition: total CGA 8.91% dm | Coffee group: 800 mL coffee/day over 5 days Control group: 800 mL water/day over 5 days | Randomized, controlled, crossover intervention | =GSH =TAC | ↓8-IsoPs =OxLDL =MDA | =EndoIII and FPG sensitive sites =H2O2-induced DNA damage | ↓3NT |
| Misik et al. [36] | 38 healthy nonsmokers subjects (14 males, 24 females), mean age 27.6 ± 8.0 years, mean BMI 22.3 ± 2.8 kg/m2 | Coffee: coffee brand “Tchibo Beste Bohne” (100% Arabica) prepared by paper filtration. Composition: total CGA 125 mg/100 mL, caffeine 65 mg/100 mL and NMP 3.1 mg/100 mL | Coffee group: 800 mL coffee/day over 5 days Control group: 800 mL water/day over 5 days | Randomized, controlled, crossover intervention | =SOD =GPx =GSH =TAC | =IsoPs =OxLDL =MDA | ↓FPG-sensitive sites ↓EndoIII sensitive sites (ns) =H2O2-induced DNA damage | =3NT |
| Revuelta-Iniesta & Al-Dujaili [37] | 20 subjects (7 males, 13 females), mean BMI 24.23 ± 4.6 kg/m2, age n.d. | Coffee 1: BC (black coffee): Sainsbury’s Original Blend Cafetière Coffee Composition: polyphenols ranging from 1451 mg GAE/100 mL (Filter method) to 2475 mg GAE/100 mL (French Cafetiere) Coffee 2: GC (green coffee): Ethiopian Harrar 4 (100% Arabica) Composition: polyphenols ranging from 972 mg GAE/100 mL (French Cafetiere) to 2052 mg GAE/100 mL (Italian Cafetiere) | Coffee group 1: 40 g/day of GC for 2 weeks Coffee group 2: 40 g/day of BC for 2 weeks Control group: n.d. | Randomized, cross-over intervention | =AC (FRAP) | |||
| Kotyczka et al. [38] | 30 healthy subjects, mean age 26 ± 1 years, mean BMI 23.2 ± 0.5 kg/m2 | Coffee 1: CBs 30 g of roast powder. Dark roast coffee beverage (NMP-CB, 260 °C, 5 min) Composition: rich in NMP (785 μmol/L) and low in CGA (523 μmol/L). Coffee 2: Light roast coffee beverage (CGA-CB, 260 °C, 2 min) Composition: rich in CGA (4538 μmol/L) and low in NMP (56 μmol/L) | Coffee group 1: 500 mL/day of light roast coffee for 4 weeks Coffee group 2: 500 mL/day of dark roast coffee for 4 weeks Control group: n.d. | Randomized, longitudinally, intervention | ↑SOD (CGA-CB)
↓SOD (NMP-CB) ↑CAT ↑GPx (CGA-CB) ↓GPx (NMP-CB) ↑tGSH (CGA-CB) ↑tGSH (NMP-CB) | |||
| Steinkellner et al. [39] | First trial: 10 healthy nonsmokers subjects (3 males, 7 females), mean age 26 ± 4 years, mean bw 75 ± 9 kg Second trial: 14 subjects, mean age 25 ± 6 years, mean bw 74 ± 10 kg Third trial: subjects (number n.d.), mean age 26 ± 6 years, mean bw 72 ± 8 kg | Coffee 1: unfiltered coffee: Ground coffee (“Brasil sanft”) boiled in 10.0 L tap water for 5 min and pressed through a metal mesh Composition: n.d. Coffee 2: filtered coffee Composition: n.d. | First trial: Coffee group: 7 cups/day (in total 1 L) of unfiltered coffee over 5 days Control group: n.d. Second trial: Coffee group 1: 7 cups/day (in total 1 L) of unfiltered coffee for 3 days Coffee group 2: 7 cups/day (in total 1 L) of filtered coffee for 3 days Control group: n.d. Third trial: Coffee group: 7 cups/day (in total 1 L) of unfiltered coffee for 5 days Control group: n.d. | First trial: Baseline and post-intervention Second trial: Parallel intervention Third trial: Baseline and post-intervention | =GST in saliva ↑GST in plasma | ↓BPDE-induced DNA damage | ||
| Bakuradze et al. [40] | 33 healthy males (range age 20–44 years; mean BMI 25.6 ± 3.7 kg/m2) | Coffee: special roasted and blended Arabica coffee rich in both green and roast bean constituents, especially in CGA and NMP Composition: 72 mg/L NMP, 263.6 mg/L trigonelline, 720 mg/L caffeine | Coffee group: 750 mL/day (in three equal portions) for 4 weeks Control group: 750 mL/day water for 4 weeks | Randomized, controlled, cross-over intervention | ↑GSH =GSSG ↑GSR activity | ↓SBs ↓ FPG-sensitive sites | ||
| Esposito et al. [41] | 23 healthy subjects (18 treated and 5 controls), smokers and non smokers Coffee group: 7 males, 11 females; age range 19–25 years, mean BMI M 24.7 ± 2.9 kg/m2, F 22.8 ± 5.4 kg/m2 Control group: 2 males, 3 females; age range 20–27 years, mean BMI males 23.0 ± 1.9 kg/m2, females 22 ± 2.4 kg/m2; | Coffee: 5.1 ± 0.4 cups/day. 3.6 cups/day moka, 1.5 cups/day espresso; moka 40–50 mL/cup, espresso 25–35 mL/cup. Decaffeinated coffee intake was 24% of the total. Composition: n.d. | Coffee group: 5 cups coffee/day for 1 week Control group: No coffee consumption for 1 week | Parallel intervention | ↑GSH | |||
| Grubben et al. [42] | 64 subjects (31 males and 33 females; mean age 43 ± 11 years, mean BMI 24.5 ± 0.5 kg/m2) | Coffee: blend of arabica and robusta beans. 39 g of ground coffee into a 1 L cafetière coffee-pot (1 L equals six cups) Composition: cafestol mean 34 ± 3 mg/L, kahweol mean 26 ± 1 mg/L | Coffee group: 1 L/day of unfiltered cafetière coffee for 2 weeks Control group: water, milk, tea chocolate drink or broth for 2 weeks | Randomized, controlled, crossover intervention | ↑GSH (Colorectal mucosa and plasma) | |||
| Kempf et al. [43] | 47 subjects (11 males, 36 females), mean age 54.0 ± 9.0 years, mean BMI 29.2 ± 4.6 kg/m2 | Coffee: Juhla Mokka branded coffee, made with participants’ coffee machines at home Composition: n.d. | Coffee group 1: 4 cups (150 mL per cup) of coffee for 4 weeks Coffee group 2: 8 cups (150 mL per cup) of coffee for 4 weeks Control group: n.d. | Single blind, three stages intervention | ↓IsoPs | =3NT | ||
| Yukawa et al. [44] | 11 healthy men, range age 21–31 years | Coffee: coffee freshly prepared by mixing 8 g of Arabica coffee with 150 mL water Composition: n.d. | Coffee group: 150 mL coffee (3 times per day for 1 week) Control group: mineral water for 1 week (amount not reported) | Baseline and post intervention | ↓Susceptibility of LDL to oxidation ↓MDA | |||
| Bakuradze et al. [45] | 84 healthy subjects, mean age 25.6 ± 5.8 years, mean BMI 22.9 ± 1.9 kg/m2 | Coffee 1: blend (SB) coffee: 100% Arabica Composition: 12.39 ± 0.1 mg/g caffeine, 19.31 ± 0.3 mg/g CGA, 0.39 ± 0.0 mg/g NMP and 6.27 ± 0.1 mg/g trigonelline Coffee 2: market blend (MB) coffee, obtained from equal portions of 4 Arabica and 1 Robusta commercially available regular coffee brands Composition: 12.8 ± 0.2 mg/g caffeine, 10.01 ± 0.3 mg/g CGA, 1.20 ± 0.0 NMP and 3.42 ± 0.2 mg/g trigonelline | Coffee group: 750 mL/day of MB or SB for 4 weeks Control group: n.d. | Randomized, non-controlled, cross-over intervention | ↓SBs ↓FPG-sensitive sites | |||
| Bakuradze et al. [46] | 84 healthy men, range age 19–50 years, mean bw 80.9 ± 12.4 kg Coffee group: 42 men, mean BMI 24.9 ± 3.0 kg/m2 Control group: 42 men, mean BMI 24.4 ± 3.5 kg/m2 | Coffee: Arabica coffee, freshly prepared in a pad machine Composition: 11.78 ± 0.42 mg/g caffeine, 10.18 ± 0.33 mg/g CGA, 1.10 ± 0.05 NMP and 3.82 ± 0.09 mg/g trigonelline | Coffee group: 750 mL/day of coffee for 4 weeks Control group: 750 mL/day of water for 4 weeks | Randomized, controlled, cross-over intervention | ↓SBs | |||
| Bichler et al. [47] | 8 healthy non-smokers volunteers (age range 20–50 years, BMI n.d.) | Coffee: metal filtered coffee andpaper filtered coffee, both prepared with 50 g of ground coffee per liter Composition: n.d. | Coffee group: 600 mL coffee/day (200 mL metal filtered coffee and 400 mL paper filtered coffee) for 5 days Control group: No coffee consumption | =GPx | ↓Endo and FPG-sensitive sites ↓H2O2- and Trp-P-2-induced DNA damage | |||
| Cardin et al. [48] | 37 patients with chronic hepatitis C (29 males, 8 females), mean age 58 ± 11 years, mean BMI 26 ± 5 kg/m2 | Coffee: 100% Coffee Arabica prepared with an Italian-style coffee machine Composition: n.d. | Coffee group: 4 cups of coffee/day for 30 days Control group: no coffee consumption for 4 weeks | Randomized, controlled, cross-over intervention | ↓8-OHdG | |||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martini, D.; Del Bo’, C.; Tassotti, M.; Riso, P.; Del Rio, D.; Brighenti, F.; Porrini, M. Coffee Consumption and Oxidative Stress: A Review of Human Intervention Studies. Molecules 2016, 21, 979. https://doi.org/10.3390/molecules21080979
Martini D, Del Bo’ C, Tassotti M, Riso P, Del Rio D, Brighenti F, Porrini M. Coffee Consumption and Oxidative Stress: A Review of Human Intervention Studies. Molecules. 2016; 21(8):979. https://doi.org/10.3390/molecules21080979
Chicago/Turabian StyleMartini, Daniela, Cristian Del Bo’, Michele Tassotti, Patrizia Riso, Daniele Del Rio, Furio Brighenti, and Marisa Porrini. 2016. "Coffee Consumption and Oxidative Stress: A Review of Human Intervention Studies" Molecules 21, no. 8: 979. https://doi.org/10.3390/molecules21080979