Capsaicin, Nociception and Pain
Abstract
:1. Introduction and General Concepts
1.1. Chemical Features of Capsaicin
1.2. Natural Sources of Capsaicin
1.3. Cloning, General Distribution, Functional Properties and Biological Effects of the Capsaicin Receptor in Mammals
1.3.1. Cloning and General Distribution of TRPV1
1.3.2. Functional Properties and Biological Effects of TRPV1
1.3.3. Other Molecular Targets of Capsaicin
1.4. Nociception and Pain
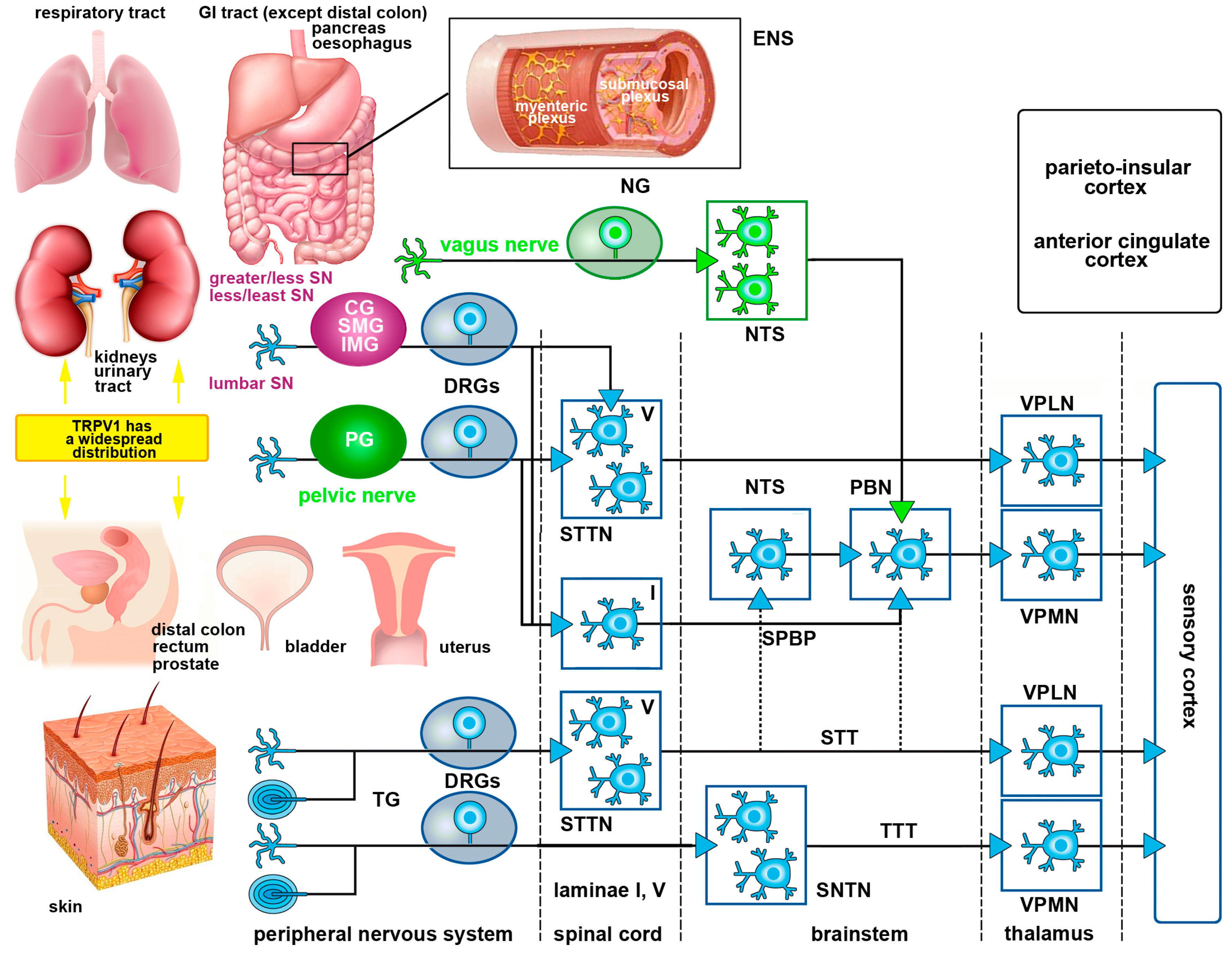
1.5. Basic Organization of Somatic and Visceral Pain Pathways
1.5.1. Somatic Pain Pathways
1.5.2. Visceral Pain Pathways
1.6. Capsaicin as an Analgesic Medication
2. The Capsaicin Receptor in Nociceptive Pathways
2.1. Structure and Physiology of the Capsaicin Receptor
2.1.1. Structure and Splice Variants of TRPV1
2.1.2. Biochemistry and Physiology of TRPV1
2.2. Localization and Activation of TRPV1 in Pain Pathways
2.2.1. PSNs and Non-Neural Cells
Activation of TRPV1 during Neurogenic Inflammation of Skin and Mucosae
Other Effects of TRPV1 Activation in PSNs
2.2.2. Somatic Pathways
First-to-Second Order Neuron Synapses and Modulation in Substantia Gelatinosa
Second-to-Third Order Neuron Synapses and Other Brain Areas Involved in Pain Control
Activation of TRPV1 during Pain Perception
2.2.3. Visceral Pathways
TRPV1 Expression and Function in the Urinary Tract—Upper Urinary Tract
TRPV1 Expression and Function in the Urinary Tract—Lower Urinary Tract
TRPV1 Expression and Function in the Digestive Apparatus
Esophagus
Stomach
Small Intestine
Large Intestine
Pancreas
TRPV1 Expression and Function in the Respiratory Tract
TRPV1 Expression and Function in the Genital Tract
3. Experimental Modeling Nociception Using Capsaicin in Vitro and ex Vivo
4. Therapeutic Use of Capsaicin
Acknowledgments
Conflicts of Interest
Abbreviations
| 12-HPETE | 12-hydroperoxyeicosatetraenoic acid |
| ATP | Adenosine triphosphate |
| CaMK II kinase | Calmodulin- dependent protein kinase II |
| CGRP | Calcitonin Gene-Related Peptide |
| CP/CPPS | Chronic Prostatitis/Chronic Pelvic Pain Syndrome |
| DNA | Deoxyribonucleic acid |
| DRG | Dorsal Root Ganglion |
| ENS | Enteric Nervous System |
| GABA | γ-Aminobutyric acid |
| GI tract | Gastrointestinal tract |
| HEK 293 | Human Embryonic Kidney 293 |
| IR | Immunoreactive |
| 15-HPETE | 15-hydroperoxyeicosatetraenoic acid |
| BDNF | Brain Derived Neurotrophic Factor |
| cDNA | complementary DNA |
| CNS | Central Nervous System |
| DkTx | Double-knot toxin |
| DOCA | Deoxycorticosterone acetate |
| DSS | Dextran sulfate sodium |
| FAF1 | Fas-Associated Factor 1 |
| GDNF | Glial cell-Derived Neurotrophic Factor |
| HCl | Acid chloride |
| ICC | Immunocytochemistry |
| KO | Knock-out |
| LB4 | Leukotriene B4 |
| LPS | Lipopolysaccharide |
| NADA | Arachidonoyl-dopamine |
| NGF | Nerve Growth Factor |
| NKA | Neurokinin A |
| NMDA receptor | N-methyl-D-aspartate receptor |
| NOS | Nitric Oxide Synthase |
| NTS | Nucleus Tractus Solitarius |
| PAG | Periaqueductal grey |
| Palvanil | N-palmitoyl-vanillamide |
| pERK | Phosphorylated Extracellular signal-Regulated Kinase |
| PI3K | Phosphatidylinositol-3-Kinase |
| PIP2 | Phosphatidylinositol 4,5-bisphosphate |
| PKB/Akt | Protein Kinase B/Akt |
| PKC | Protein Kinase C |
| PNS | Peripheral Nervous System |
| PSNs | Primary Sensory Neurons |
| RT-PCR | Reverse Transcription-Polymerase Chain Reaction |
| RTX | Resinferatoxin |
| SNTN | Spinal Nucleus of the Trigeminal Nerve |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| STT | Spinothalamic Tract |
| TG | Trigeminal Ganglion |
| TNBS | Trinitrobenzene sulphonic acid |
| TrkA | Tropomyosin kinase A receptor |
| TRP | Transient Receptor Potential |
| TRPV1 | Transient Receptor Potential cation channel Vanilloid subfamily member 1 |
| VaTx | Vanillotoxins |
| VR1 | Vanilloid Receptor subtype 1 |
| WT | Wild Type |
References
- Thresh, J.C. Isolation of capsacin. Pharm. J. Tr. 1876, 6, 941–947. [Google Scholar]
- Nelson, E.K. The constitution of capsaicin, the pungent principle of capsicum. II. J. Am. Chem. Soc. 1920, 42, 597–599. [Google Scholar]
- De, A.K. (Ed.) Capsicum: The Genus Capsicum; CRC Press: London, UK, 2003.
- Musfiroh, I.; Mutakin, M.; Angelina, T.; Muchtaridi, M. Capsaicin level of various capsicum fruits. Int. J. Pharm. Pharm. Sci. 2013, 5, 248–251. [Google Scholar]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [PubMed]
- Krause, J.E.; Chenard, B.L.; Cortright, D.N. Transient receptor potential ion channels as targets for the discovery of pain therapeutics. Curr. Opin. Investig. Drugs 2005, 6, 48–57. [Google Scholar] [PubMed]
- Hayes, P.; Meadows, H.J.; Gunthorpe, M.J.; Harries, M.H.; Duckworth, D.M.; Cairns, W.; Harrison, D.C.; Clarke, C.E.; Ellington, K.; Prinjha, R.K.; et al. Cloning and functional expression of a human orthologue of rat vanilloid receptor-1. Pain 2000, 88, 205–215. [Google Scholar] [CrossRef]
- Mezey, E.; Toth, Z.E.; Cortright, D.N.; Arzubi, M.K.; Krause, J.E.; Elde, R.; Guo, A.; Blumberg, P.M.; Szallasi, A. Distribution of mrna for vanilloid receptor subtype 1 (vr1), and vr1-like immunoreactivity, in the central nervous system of the rat and human. Proc. Natl. Acad. Sci. USA 2000, 97, 3655–3660. [Google Scholar] [CrossRef] [PubMed]
- Birder, L.A.; Kanai, A.J.; de Groat, W.C.; Kiss, S.; Nealen, M.L.; Burke, N.E.; Dineley, K.E.; Watkins, S.; Reynolds, I.J.; Caterina, M.J. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc. Natl. Acad. Sci. USA 2001, 98, 13396–13401. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, D.J.; Chesler, A.T.; Braz, J.M.; Shah, N.M.; Julius, D.; Basbaum, A.I. Restriction of transient receptor potential vanilloid-1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in nonpeptidergic neurons. J. Neurosci. 2011, 31, 10119–10127. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Hoon, M.A. Ablation of trpv1 neurons reveals their selective role in thermal pain sensation. Mol. Cell. Neurosci. 2010, 43, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Tisel, S.M.; Orestes, P.; Bhangoo, S.K.; Hoon, M.A. Trpv1-lineage neurons are required for thermal sensation. EMBO J. 2011, 30, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Cristino, L.; de Petrocellis, L.; Pryce, G.; Baker, D.; Guglielmotti, V.; di Marzo, V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience 2006, 139, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Ferrini, F.; Salio, C.; Vergnano, A.M.; Merighi, A. Vanilloid receptor-1 (trpv1)-dependent activation of inhibitory neurotransmission in spinal substantia gelatinosa neurons of mouse. Pain 2007, 129, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, D.J.; Chesler, A.T.; Jackson, A.C.; Sigal, Y.M.; Yamanaka, H.; Grant, R.; O'Donnell, D.; Nicoll, R.A.; Shah, N.M.; Julius, D.; et al. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J. Neurosci. 2011, 31, 5067–5077. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Owsianik, G. The transient receptor potential family of ion channels. Genome Biol 2011, 12, 218. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Smart, D.; Gunthorpe, M.J.; Jerman, J.C.; Nasir, S.; Gray, J.; Muir, A.I.; Chambers, J.K.; Randall, A.D.; Davis, J.B. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hvr1). Br. J. Pharmacol. 2000, 129, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.C.; Phelps, P.T.; Anthes, J.C.; Umland, S.; Greenfeder, S. Cloning and pharmacological characterization of mouse trpv1. Neurosci Lett 2004, 370, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Smart, D.; Jerman, J.C.; Gunthorpe, M.J.; Brough, S.J.; Ranson, J.; Cairns, W.; Hayes, P.D.; Randall, A.D.; Davis, J.B. Characterisation using flipr of human vanilloid vr1 receptor pharmacology. Eur. J. Pharmacol. 2001, 417, 51–58. [Google Scholar] [CrossRef]
- Takayama, Y.; Uta, D.; Furue, H.; Tominaga, M. Pain-enhancing mechanism through interaction between trpv1 and anoctamin 1 in sensory neurons. Proc. Natl. Acad. Sci. USA 2015, 112, 5213–5218. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Vij, A.S.; Sharma, M. Mechanisms and clinical uses of capsaicin. Eur. J. Pharmacol. 2013, 720, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Tympanidis, P.; Casula, M.A.; Yiangou, Y.; Terenghi, G.; Dowd, P.; Anand, P. Increased vanilloid receptor vr1 innervation in vulvodynia. Eur. J. Pain 2004, 8, 129–133. [Google Scholar] [CrossRef]
- Geppetti, P.; Trevisani, M. Activation and sensitisation of the vanilloid receptor: Role in gastrointestinal inflammation and function. Br. J. Pharmacol. 2004, 141, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Yiangou, Y.; Facer, P.; Dyer, N.H.; Chan, C.L.; Knowles, C.; Williams, N.S.; Anand, P. Vanilloid receptor 1 immunoreactivity in inflamed human bowel. Lancet 2001, 357, 1338–1339. [Google Scholar] [CrossRef]
- Bhutani, M.; Pathak, A.K.; Nair, A.S.; Kunnumakkara, A.B.; Guha, S.; Sethi, G.; Aggarwal, B.B. Capsaicin is a novel blocker of constitutive and interleukin-6-inducible stat3 activation. Clin. Cancer Res. 2007, 13, 3024–3032. [Google Scholar] [CrossRef] [PubMed]
- Donnerer, J.; Liebmann, I. The nk1 receptor antagonist sr140333 inhibits capsaicin-induced erk phosphorylation in sensory neurons. Pharmacology 2006, 77, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.Y.; Ling, Q.Z.; Chen, S.J. Identification of a potential target of capsaicin by computational target fishing. Evid. Based Complement. Alternat. Med. 2015, 2015, 983951. [Google Scholar] [CrossRef] [PubMed]
- Merighi, A.; Salio, C.; Ghirri, A.; Lossi, L.; Ferrini, F.; Betelli, C.; Bardoni, R. Bdnf as a pain modulator. Prog. Neurobiol. 2008, 85, 297–317. [Google Scholar] [CrossRef] [PubMed]
- Bonica, J.J. Evolution and current status of pain programs. J. Pain Symptom Manage. 1990, 5, 368–374. [Google Scholar] [CrossRef]
- Hunt, S.P.; Mantyh, P.W. The molecular dynamics of pain control. Nat. Rev. Neurosci. 2001, 2, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Cervero, F.; Laird, J.M. Visceral pain. Lancet 1999, 353, 2145–2148. [Google Scholar] [CrossRef]
- McMahon, S.B.; Dmitrieva, N.; Koltzenburg, M. Visceral pain. Br. J. Anaesth. 1995, 75, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, V.C.; Swett, J.E. The pattern of spinal and medullary projections from a cutaneous nerve and a muscle nerve of the forelimb of the cat: A study using the transganglionic transport of hrp. J. Comp. Neurol. 1986, 246, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.; Nadelhaft, I.; deGroat, W.C. The distribution within the spinal cord of visceral primary afferent axons carried by the lumbar colonic nerve of the cat. Brain Res. 1986, 398, 11–17. [Google Scholar] [CrossRef]
- Giamberardino, M.A. Recent and forgotten aspects of visceral pain. Eur. J. Pain 1999, 3, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Sikandar, S.; Dickenson, A.H. Visceral pain: The ins and outs, the ups and downs. Curr. Opin. Support. Palliat. Care 2012, 6, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Blackshaw, L.A. Visceral pain readouts in experimental medicine. Neurogastroenterol. Motil. 2012, 24, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, J.N. Visceral pain: The neurophysiological mechanism. Handb. Exp. Pharmacol. 2009, 31–74. [Google Scholar]
- Gebhart, G.F.J.J. Bonica lecture--2000: Physiology, pathophysiology, and pharmacology of visceral pain. Reg. Anesth. Pain Med. 2000, 25, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Gebhart, G.F. Pathobiology of visceral pain: Molecular mechanisms and therapeutic implications iv. Visceral afferent contributions to the pathobiology of visceral pain. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 278, G834–G838. [Google Scholar] [PubMed]
- Todd, A.J. Anatomy of primary afferents and projection neurones in the rat spinal dorsal horn with particular emphasis on substance p and the neurokinin 1 receptor. Exp. Physiol. 2002, 87, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Bester, H.; Menendez, L.; Besson, J.M.; Bernard, J.F. Spino (trigemino) parabrachiohypothalamic pathway: Electrophysiological evidence for an involvement in pain processes. J. Neurophysiol. 1995, 73, 568–585. [Google Scholar] [PubMed]
- Bushnell, M.C.; Duncan, G.H. Sensory and affective aspects of pain perception: Is medial thalamus restricted to emotional issues? Exp. Brain Res. 1989, 78, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Groenewegen, H.J. Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience 1988, 24, 379–431. [Google Scholar] [CrossRef]
- Mertz, H.; Morgan, V.; Tanner, G.; Pickens, D.; Price, R.; Shyr, Y.; Kessler, R. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology 2000, 118, 842–848. [Google Scholar] [CrossRef]
- Furness, J.B. Types of neurons in the enteric nervous system. J. Auton Nerv. Syst. 2000, 81, 87–96. [Google Scholar] [CrossRef]
- Furness, J.B. Novel gut afferents: Intrinsic afferent neurons and intestinofugal neurons. Auton Neurosci. 2006, 125, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Meghvansi, M.K.; Siddiqui, S.; Khan, M.H.; Gupta, V.K.; Vairale, M.G.; Gogoi, H.K.; Singh, L. Naga chilli: A potential source of capsaicinoids with broad-spectrum ethnopharmacological applications. J. Ethnopharmacol. 2010, 132, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bhagowati, R.R.; Changkija, S. Genetic variability and traditional practices in naga king chili of nagaland. Asian Agri-Hist. 2009, 13, 171–180. [Google Scholar]
- Vetter, I.; Cheng, W.; Peiris, M.; Wyse, B.D.; Roberts-Thomson, S.J.; Zheng, J.; Monteith, G.R.; Cabot, P.J. Rapid, opioid-sensitive mechanisms involved in transient receptor potential vanilloid 1 sensitization. J. Biol. Chem. 2008, 283, 19540–19550. [Google Scholar] [CrossRef] [PubMed]
- Liapi, A.; Wood, J.N. Extensive co-localization and heteromultimer formation of the vanilloid receptor-like protein trpv2 and the capsaicin receptor trpv1 in the adult rat cerebral cortex. Eur. J. Neurosci. 2005, 22, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hu, H.Z.; Colton, C.K.; Wood, J.D.; Zhu, M.X. An alternative splicing product of the murine trpv1 gene dominant negatively modulates the activity of trpv1 channels. J. Biol. Chem. 2004, 279, 37423–37430. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Zavala, K.; Fahimi, A.; Lee, J.; Xue, Q.; Eilers, H.; Schumacher, M.A. Transcription factors sp1 and sp4 regulate trpv1 gene expression in rat sensory neurons. Mol. Pain 2011, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.A.; Eilers, H. Trpv1 splice variants: Structure and function. Front. Biosci. (Landmark Ed.) 2010, 15, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Lyall, V.; Heck, G.L.; Vinnikova, A.K.; Ghosh, S.; Phan, T.H.; Alam, R.I.; Russell, O.F.; Malik, S.A.; Bigbee, J.W.; DeSimone, J.A. The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. J. Physiol. 2004, 558, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Charrua, A.; Cruz, C.D.; Narayanan, S.; Gharat, L.; Gullapalli, S.; Cruz, F.; Avelino, A. Grc-6211, a new oral specific trpv1 antagonist, decreases bladder overactivity and noxious bladder input in cystitis animal models. J. Urol. 2009, 181, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Caterina, M.J.; Malmberg, A.B.; Rosen, T.A.; Gilbert, H.; Skinner, K.; Raumann, B.E.; Basbaum, A.I.; Julius, D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 1998, 21, 531–543. [Google Scholar] [CrossRef]
- Alexander, S.P.; Catterall, W.A.; Kelly, E.; Marrion, N.; Peters, J.A.; Benson, H.E.; Faccenda, E.; Pawson, A.J.; Sharman, J.L.; Southan, C.; et al. The concise guide to pharmacology 2015/16: Voltage-gated ion channels. Br. J. Pharmacol. 2015, 172, 5904–5941. [Google Scholar] [CrossRef] [PubMed]
- Planells-Cases, R.; Garcia-Sanz, N.; Morenilla-Palao, C.; Ferrer-Montiel, A. Functional aspects and mechanisms of trpv1 involvement in neurogenic inflammation that leads to thermal hyperalgesia. Pflugers Arch. 2005, 451, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A.; Cortright, D.N.; Blum, C.A.; Eid, S.R. The vanilloid receptor trpv1: 10 years from channel cloning to antagonist proof-of-concept. Nat. Rev. Drug Discov. 2007, 6, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, D.P.; Nau, C. Regulation of ca2+-dependent desensitization in the vanilloid receptor trpv1 by calcineurin and camp-dependent protein kinase. J. Biol. Chem. 2005, 280, 13424–13432. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, D.P.; Nau, C. Desensitization of capsaicin-activated currents in the vanilloid receptor trpv1 is decreased by the cyclic amp-dependent protein kinase pathway. J. Biol. Chem. 2003, 278, 50080–50090. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, T.; Simon, S.A. Trpv1 receptors and signal transduction. In Trp ion Channel Function in Sensory Transduction and Cellular Signaling Cascades; Liedtke, W.B., Heller, S., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2007. [Google Scholar]
- Ma, W.; Quirion, R. Inflammatory mediators modulating the transient receptor potential vanilloid 1 receptor: Therapeutic targets to treat inflammatory and neuropathic pain. Expert Opin. Ther. Targets 2007, 11, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, H.M.; Zhou, X.D.; Kolosov, V.P.; Perelman, J.M. Study on trpv1-mediated mechanism for the hypersecretion of mucus in respiratory inflammation. Mol. Immunol. 2013, 53, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A.; Blumberg, P.M. Complex regulation of trpv1 by vanilloids. In Trp Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades; Liedtke, W.B., Heller, S., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2007. [Google Scholar]
- Planells-Cases, R.; Valente, P.; Ferrer-Montiel, A.; Qin, F.; Szallasi, A. Complex regulation of trpv1 and related thermo-trps: Implications for therapeutic intervention. Adv. Exp. Med. Biol. 2011, 704, 491–515. [Google Scholar] [PubMed]
- Kim, S.; Kang, C.; Shin, C.Y.; Hwang, S.W.; Yang, Y.D.; Shim, W.S.; Park, M.Y.; Kim, E.; Kim, M.; Kim, B.M.; et al. Trpv1 recapitulates native capsaicin receptor in sensory neurons in association with fas-associated factor 1. J. Neurosci. 2006, 26, 2403–2412. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.D.; Vasko, M.R. Cellular mechanisms of neurogenic inflammation. J. Pharmacol. Exp. Ther. 2002, 302, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, B.; Oortgiesen, M. The trpv1 receptor: Target of toxicants and therapeutics. Toxicol. Sci. 2006, 89, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Jordt, S.E.; Tominaga, M.; Julius, D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc. Natl. Acad. Sci. USA 2000, 97, 8134–8139. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Wada, M.; Masu, M. Potentiation of capsaicin receptor activity by metabotropic atp receptors as a possible mechanism for atp-evoked pain and hyperalgesia. Proc. Natl. Acad. Sci. USA 2001, 98, 6951–6956. [Google Scholar] [CrossRef] [PubMed]
- Ahern, G.P.; Wang, X.; Miyares, R.L. Polyamines are potent ligands for the capsaicin receptor trpv1. J. Biol. Chem. 2006, 281, 8991–8995. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.W.; Cho, H.; Kwak, J.; Lee, S.Y.; Kang, C.J.; Jung, J.; Cho, S.; Min, K.H.; Suh, Y.G.; Kim, D.; et al. Direct activation of capsaicin receptors by products of lipoxygenases: Endogenous capsaicin-like substances. Proc. Natl. Acad. Sci. USA 2000, 97, 6155–6160. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; McMaster, R.S.; Pertwee, R.G.; Roy, S.; Mahadevan, A.; Razdan, R.K.; Ross, R.A. Novel compounds that interact with both leukotriene b4 receptors and vanilloid trpv1 receptors. J. Pharmacol. Exp. Ther. 2006, 316, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Bisogno, T.; Trevisani, M.; Al-Hayani, A.; De Petrocellis, L.; Fezza, F.; Tognetto, M.; Petros, T.J.; Krey, J.F.; Chu, C.J.; et al. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid vr1 receptors. Proc. Natl. Acad. Sci. USA 2002, 99, 8400–8405. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, P.M.; Petersson, J.; Andersson, D.A.; Chuang, H.; Sorgard, M.; Di Marzo, V.; Julius, D.; Hogestatt, E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 1999, 400, 452–457. [Google Scholar] [PubMed]
- Moriyama, T.; Higashi, T.; Togashi, K.; Iida, T.; Segi, E.; Sugimoto, Y.; Tominaga, T.; Narumiya, S.; Tominaga, M. Sensitization of trpv1 by ep1 and ip reveals peripheral nociceptive mechanism of prostaglandins. Mol. Pain 2005, 1, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesare, P.; Dekker, L.V.; Sardini, A.; Parker, P.J.; McNaughton, P.A. Specific involvement of pkc-epsilon in sensitization of the neuronal response to painful heat. Neuron 1999, 23, 617–624. [Google Scholar] [CrossRef]
- Chuang, H.H.; Prescott, E.D.; Kong, H.; Shields, S.; Jordt, S.E.; Basbaum, A.I.; Chao, M.V.; Julius, D. Bradykinin and nerve growth factor release the capsaicin receptor from ptdins(4,5)p2-mediated inhibition. Nature 2001, 411, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Cho, H.; Hwang, S.W.; Jung, J.; Shin, C.Y.; Lee, S.Y.; Kim, S.H.; Lee, M.G.; Choi, Y.H.; Kim, J.; et al. Bradykinin-12-lipoxygenase-vr1 signaling pathway for inflammatory hyperalgesia. Proc. Natl. Acad. Sci. USA 2002, 99, 10150–10155. [Google Scholar] [CrossRef] [PubMed]
- Negri, L.; Lattanzi, R.; Giannini, E.; Colucci, M.; Margheriti, F.; Melchiorri, P.; Vellani, V.; Tian, H.; de Felice, M.; Porreca, F. Impaired nociception and inflammatory pain sensation in mice lacking the prokineticin receptor pkr1: Focus on interaction between pkr1 and the capsaicin receptor trpv1 in pain behavior. J. Neurosci. 2006, 26, 6716–6727. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, L.S.; Ahern, G.P. Induction of vanilloid receptor channel activity by protein kinase c. Nature 2000, 408, 985–990. [Google Scholar] [CrossRef] [PubMed]
- McNamara, F.N.; Randall, A.; Gunthorpe, M.J. Effects of piperine, the pungent component of black pepper, at the human vanilloid receptor (trpv1). Br. J. Pharmacol. 2005, 144, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Blair, N.T.; Clapham, D.E. Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J. Neurosci. 2005, 25, 8924–8937. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, E.; Yanagihara, A.; Karlsson, E.; Tytgat, J. Jellyfish and other cnidarian envenomations cause pain by affecting trpv1 channels. FEBS Lett. 2006, 580, 5728–5732. [Google Scholar] [CrossRef] [PubMed]
- Siemens, J.; Zhou, S.; Piskorowski, R.; Nikai, T.; Lumpkin, E.A.; Basbaum, A.I.; King, D.; Julius, D. Spider toxins activate the capsaicin receptor to produce inflammatory pain. Nature 2006, 444, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Bohlen, C.J.; Priel, A.; Zhou, S.; King, D.; Siemens, J.; Julius, D. A bivalent tarantula toxin activates the capsaicin receptor, trpv1, by targeting the outer pore domain. Cell 2010, 141, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Sluka, K.A.; Willis, W.D. The effects of g-protein and protein kinase inhibitors on the behavioral responses of rats to intradermal injection of capsaicin. Pain 1997, 71, 165–178. [Google Scholar] [CrossRef]
- Zou, X.; Lin, Q.; Willis, W.D. Role of protein kinase a in phosphorylation of nmda receptor 1 subunits in dorsal horn and spinothalamic tract neurons after intradermal injection of capsaicin in rats. Neuroscience 2002, 115, 775–786. [Google Scholar] [CrossRef]
- Sun, R.; Yan, J.; Willis, W.D. Activation of protein kinase b/akt in the periphery contributes to pain behavior induced by capsaicin in rats. Neuroscience 2007, 144, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Gamse, R.; Petsche, U.; Lembeck, F.; Jancso, G. Capsaicin applied to peripheral nerve inhibits axoplasmic transport of substance p and somatostatin. Brain Res. 1982, 239, 447–462. [Google Scholar] [CrossRef]
- Anand, P.; Bley, K. Topical capsaicin for pain management: Therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br. J. Anaesth. 2011, 107, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Johansen, M.E.; Reilly, C.A.; Yost, G.S. Trpv1 antagonists elevate cell surface populations of receptor protein and exacerbate trpv1-mediated toxicities in human lung epithelial cells. Toxicol. Sci. 2006, 89, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.C.; Sabnis, A.S.; Johansen, M.E.; Lanza, D.L.; Moos, P.J.; Yost, G.S.; Reilly, C.A. Transient receptor potential vanilloid 1 agonists cause endoplasmic reticulum stress and cell death in human lung cells. J. Pharmacol. Exp. Ther. 2007, 321, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.L.; Khan, G.M.; Alloway, K.D.; Chen, S.R. Resiniferatoxin induces paradoxical changes in thermal and mechanical sensitivities in rats: Mechanism of action. J. Neurosci. 2003, 23, 2911–2919. [Google Scholar] [PubMed]
- Nolano, M.; Simone, D.A.; Wendelschafer-Crabb, G.; Johnson, T.; Hazen, E.; Kennedy, W.R. Topical capsaicin in humans: Parallel loss of epidermal nerve fibers and pain sensation. Pain 1999, 81, 135–145. [Google Scholar] [CrossRef]
- Sikand, P.; Premkumar, L.S. Potentiation of glutamatergic synaptic transmission by protein kinase c-mediated sensitization of trpv1 at the first sensory synapse. J. Physiol. 2007, 581, 631–647. [Google Scholar] [CrossRef] [PubMed]
- Treede, R.D.; Meyer, R.A.; Raja, S.N.; Campbell, J.N. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog. Neurobiol. 1992, 38, 397–421. [Google Scholar] [CrossRef]
- Ringkamp, M.; Meyer, R.A. Physiology of nociceptors. Pain 2008, 97–114. [Google Scholar]
- Simone, D.A.; Ochoa, J. Early and late effects of prolonged topical capsaicin on cutaneous sensibility and neurogenic vasodilatation in humans. Pain 1991, 47, 285–294. [Google Scholar] [CrossRef]
- Willis, W.D.; Coggeshall, R.E. (Eds.) Sensory Mechanisms of the Spinal Cord. Primary Afferent Neurons and the Spinal Dorsal Horn; Plenum Press: Berlin, Germany, 2004.
- Zou, X.; Lin, Q.; Willis, W.D. Nmda or non-nmda receptor antagonists attenuate increased fos expression in spinal dorsal horn gabaergic neurons after intradermal injection of capsaicin in rats. Neuroscience 2001, 106, 171–182. [Google Scholar] [CrossRef]
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474. [Google Scholar] [CrossRef]
- Doyle, M.W.; Bailey, T.W.; Jin, Y.H.; Andresen, M.C. Vanilloid receptors presynaptically modulate cranial visceral afferent synaptic transmission in nucleus tractus solitarius. J. Neurosci. 2002, 22, 8222–8229. [Google Scholar] [PubMed]
- McGaraughty, S.; Chu, K.L.; Bitner, R.S.; Martino, B.; El Kouhen, R.; Han, P.; Nikkel, A.L.; Burgard, E.C.; Faltynek, C.R.; Jarvis, M.F. Capsaicin infused into the pag affects rat tail flick responses to noxious heat and alters neuronal firing in the rvm. J. Neurophysiol. 2003, 90, 2702–2710. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, E.; de Novellis, V.; Marabese, I.; Cuomo, D.; Rossi, F.; Berrino, L.; Rossi, F.; Maione, S. Interaction between vanilloid and glutamate receptors in the central modulation of nociception. Eur. J. Pharmacol. 2002, 439, 69–75. [Google Scholar] [CrossRef]
- Starowicz, K.; Cristino, L.; Di Marzo, V. Trpv1 receptors in the central nervous system: Potential for previously unforeseen therapeutic applications. Curr. Pharm. Des. 2008, 14, 42–54. [Google Scholar] [PubMed]
- Starowicz, K.; Maione, S.; Cristino, L.; Palazzo, E.; Marabese, I.; Rossi, F.; de Novellis, V.; Di Marzo, V. Tonic endovanilloid facilitation of glutamate release in brainstem descending antinociceptive pathways. J. Neurosci. 2007, 27, 13739–13749. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, E.; Luongo, L.; de Novellis, V.; Rossi, F.; Marabese, I.; Maione, S. Transient receptor potential vanilloid type 1 and pain development. Curr. Opin. Pharmacol. 2012, 12, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Giordano, C.; Cristino, L.; Luongo, L.; Siniscalco, D.; Petrosino, S.; Piscitelli, F.; Marabese, I.; Gatta, L.; Rossi, F.; Imperatore, R.; et al. Trpv1-dependent and -independent alterations in the limbic cortex of neuropathic mice: Impact on glial caspases and pain perception. Cereb. Cortex 2012, 22, 2495–2518. [Google Scholar] [CrossRef] [PubMed]
- de Novellis, V.; Vita, D.; Gatta, L.; Luongo, L.; Bellini, G.; De Chiaro, M.; Marabese, I.; Siniscalco, D.; Boccella, S.; Piscitelli, F.; et al. The blockade of the transient receptor potential vanilloid type 1 and fatty acid amide hydrolase decreases symptoms and central sequelae in the medial prefrontal cortex of neuropathic rats. Mol. Pain 2011, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Avelino, A.; Cruz, C.; Nagy, I.; Cruz, F. Vanilloid receptor 1 expression in the rat urinary tract. Neuroscience 2002, 109, 787–798. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.H. Protective effect of trpv1 against renal fibrosis via inhibition of tgf-beta/smad signaling in doca-salt hypertension. Mol. Med. 2011, 17, 1204–1212. [Google Scholar] [PubMed]
- Wang, Y.; Wang, D.H. Aggravated renal inflammatory responses in trpv1 gene knockout mice subjected to doca-salt hypertension. Am. J. Physiol. Ren. Physiol. 2009, 297, F1550–F1559. [Google Scholar] [CrossRef] [PubMed]
- Conte, B.; Maggi, C.A.; Giachetti, A.; Parlani, M.; Lopez, G.; Manzini, S. Intraurethral capsaicin produces reflex activation of the striated urethral sphincter in urethane-anesthetized male rats. J. Urol. 1993, 150, 1271–1277. [Google Scholar] [PubMed]
- Maggi, C.A.; Giuliani, S.; Santicioli, P.; Abelli, L.; Meli, A. Visceromotor responses to calcitonin gene-related peptide (cgrp) in the rat lower urinary tract: Evidence for a transmitter role in the capsaicin-sensitive nerves of the ureter. Eur. J. Pharmacol. 1987, 143, 73–82. [Google Scholar] [CrossRef]
- Birder, L.A. Involvement of the urinary bladder urothelium in signaling in the lower urinary tract. Proc. West. Pharmacol. Soc. 2001, 44, 85–86. [Google Scholar] [PubMed]
- Lazzeri, M.; Vannucchi, M.G.; Zardo, C.; Spinelli, M.; Beneforti, P.; Turini, D.; Faussone-Pellegrini, M.S. Immunohistochemical evidence of vanilloid receptor 1 in normal human urinary bladder. Eur. Urol. 2004, 46, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Apostolidis, A.; Brady, C.M.; Yiangou, Y.; Davis, J.; Fowler, C.J.; Anand, P. Capsaicin receptor trpv1 in urothelium of neurogenic human bladders and effect of intravesical resiniferatoxin. Urology 2005, 65, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Avelino, A.; Cruz, F. Trpv1 (vanilloid receptor) in the urinary tract: Expression, function and clinical applications. Naunyn Schmiedebergs Arch. Pharmacol. 2006, 373, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Charrua, A.; Reguenga, C.; Cordeiro, J.M.; Correiade-Sa, P.; Paule, C.; Nagy, I.; Cruz, F.; Avelino, A. Functional transient receptor potential vanilloid 1 is expressed in human urothelial cells. J. Urol. 2009, 182, 2944–2950. [Google Scholar] [CrossRef] [PubMed]
- Ost, D.; Roskams, T.; van Der Aa, F.; de Ridder, D. Topography of the vanilloid receptor in the human bladder: More than just the nerve fibers. J. Urol. 2002, 168, 293–297. [Google Scholar] [CrossRef]
- Sui, G.P.; Wu, C.; Fry, C.H. Electrical characteristics of suburothelial cells isolated from the human bladder. J. Urol. 2004, 171, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Dinis, P.; Charrua, A.; Avelino, A.; Yaqoob, M.; Bevan, S.; Nagy, I.; Cruz, F. Anandamide-evoked activation of vanilloid receptor 1 contributes to the development of bladder hyperreflexia and nociceptive transmission to spinal dorsal horn neurons in cystitis. J. Neurosci. 2004, 24, 11253–11263. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.; Guimaraes, M.; Silva, C.; Reis, M. Suppression of bladder hyperreflexia by intravesical resiniferatoxin. Lancet 1997, 350, 640–641. [Google Scholar] [CrossRef]
- Cruz, F.; Guimaraes, M.; Silva, C.; Rio, M.E.; Coimbra, A.; Reis, M. Desensitization of bladder sensory fibers by intravesical capsaicin has long lasting clinical and urodynamic effects in patients with hyperactive or hypersensitive bladder dysfunction. J. Urol. 1997, 157, 585–589. [Google Scholar] [CrossRef]
- Cruz, C.D.; Charrua, A.; Vieira, E.; Valente, J.; Avelino, A.; Cruz, F. Intrathecal delivery of resiniferatoxin (rtx) reduces detrusor overactivity and spinal expression of trpv1 in spinal cord injured animals. Exp. Neurol. 2008, 214, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Avelino, A.; Cruz, F. Peptide immunoreactivity and ultrastructure of rat urinary bladder nerve fibers after topical desensitization by capsaicin or resiniferatoxin. Auton Neurosci. 2000, 86, 37–46. [Google Scholar] [CrossRef]
- Avelino, A.; Charrua, A.; Frias, B.; Cruz, C.; Boudes, M.; de Ridder, D.; Cruz, F. Transient receptor potential channels in bladder function. Acta Physiol. (Oxf.) 2013, 207, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Charrua, A.; Cruz, C.D.; Cruz, F.; Avelino, A. Transient receptor potential vanilloid subfamily 1 is essential for the generation of noxious bladder input and bladder overactivity in cystitis. J. Urol. 2007, 177, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Mukerji, G.; Yiangou, Y.; Agarwal, S.K.; Anand, P. Transient receptor potential vanilloid receptor subtype 1 in painful bladder syndrome and its correlation with pain. J. Urol. 2006, 176, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Jaggar, S.I.; Scott, H.C.; James, I.F.; Rice, A.S. The capsaicin analogue sdz249–665 attenuates the hyper-reflexia and referred hyperalgesia associated with inflammation of the rat urinary bladder. Pain 2001, 89, 229–235. [Google Scholar] [CrossRef]
- Urban, L.; Campbell, E.A.; Panesar, M.; Patel, S.; Chaudhry, N.; Kane, S.; Buchheit, K.; Sandells, B.; James, I.F. In vivo pharmacology of sdz 249–665, a novel, non-pungent capsaicin analogue. Pain 2000, 89, 65–74. [Google Scholar] [CrossRef]
- Birder, L.A.; Nakamura, Y.; Kiss, S.; Nealen, M.L.; Barrick, S.; Kanai, A.J.; Wang, E.; Ruiz, G.; de Groat, W.C.; Apodaca, G.; et al. Altered urinary bladder function in mice lacking the vanilloid receptor trpv1. Nat. Neurosci. 2002, 5, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Avelino, A.; Cruz, F.; Coimbra, A. Intravesical resiniferatoxin desensitizes rat bladder sensory fibres without causing intense noxious excitation. A c-fos study. Eur. J. Pharmacol. 1999, 378, 17–22. [Google Scholar] [CrossRef]
- Vizzard, M.A. Alterations in spinal cord fos protein expression induced by bladder stimulation following cystitis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R1027–R1039. [Google Scholar] [PubMed]
- Brady, C.M.; Apostolidis, A.N.; Harper, M.; Yiangou, Y.; Beckett, A.; Jacques, T.S.; Freeman, A.; Scaravilli, F.; Fowler, C.J.; Anand, P. Parallel changes in bladder suburothelial vanilloid receptor trpv1 and pan-neuronal marker pgp9.5 immunoreactivity in patients with neurogenic detrusor overactivity after intravesical resiniferatoxin treatment. BJU Int. 2004, 93, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Andersson, P.O.; Malmgren, A.; Uvelius, B. Functional responses of different muscle types of the female rat urethra in vitro. Acta Physiol. Scand. 1990, 140, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Maggi, C.A.; Santicioli, P.; Abelli, L.; Parlani, M.; Capasso, M.; Conte, B.; Giuliani, S.; Meli, A. Regional differences in the effects of capsaicin and tachykinins on motor activity and vascular permeability of the rat lower urinary tract. Naunyn Schmiedebergs Arch. Pharmacol. 1987, 335, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Patterson, L.M.; Zheng, H.; Ward, S.M.; Berthoud, H.R. Vanilloid receptor (vr1) expression in vagal afferent neurons innervating the gastrointestinal tract. Cell Tissue Res. 2003, 311, 277–287. [Google Scholar] [PubMed]
- Ward, S.M.; Bayguinov, J.; Won, K.J.; Grundy, D.; Berthoud, H.R. Distribution of the vanilloid receptor (vr1) in the gastrointestinal tract. J. Comp. Neurol. 2003, 465, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P. Trpv1 and the gut: From a tasty receptor for a painful vanilloid to a key player in hyperalgesia. Eur. J. Pharmacol. 2004, 500, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Bartho, L.; Benko, R.; Patacchini, R.; Petho, G.; Holzer-Petsche, U.; Holzer, P.; Lazar, Z.; Undi, S.; Illenyi, L.; Antal, A.; et al. Effects of capsaicin on visceral smooth muscle: A valuable tool for sensory neurotransmitter identification. Eur. J. Pharmacol. 2004, 500, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.C., 3rd; Xu, L.; Gebhart, G.F. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J. Neurosci. 2005, 25, 10981–10989. [Google Scholar] [CrossRef] [PubMed]
- Rong, W.; Hillsley, K.; Davis, J.B.; Hicks, G.; Winchester, W.J.; Grundy, D. Jejunal afferent nerve sensitivity in wild-type and trpv1 knockout mice. J. Physiol. 2004, 560, 867–881. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, B.; Medda, B.K.; Lazarova, Z.; Bansal, N.; Shaker, R.; Sengupta, J.N. Effect of reflux-induced inflammation on transient receptor potential vanilloid one (trpv1) expression in primary sensory neurons innervating the oesophagus of rats. Neurogastroenterol. Motil. 2007, 19, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Hosoya, T.; Ishikawa, E.; Tashima, K.; Amagase, K.; Kato, S.; Murayama, T.; Horie, S. Distribution of transient receptor potential cation channel subfamily v member 1-expressing nerve fibers in mouse esophagus. Histochem. Cell Biol. 2014, 142, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Blackshaw, L.A.; Page, A.J.; Partosoedarso, E.R. Acute effects of capsaicin on gastrointestinal vagal afferents. Neuroscience 2000, 96, 407–416. [Google Scholar] [CrossRef]
- Schicho, R.; Florian, W.; Liebmann, I.; Holzer, P.; Lippe, I.T. Increased expression of trpv1 receptor in dorsal root ganglia by acid insult of the rat gastric mucosa. Eur. J. Neurosci. 2004, 19, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- Peles, S.; Medda, B.K.; Zhang, Z.; Banerjee, B.; Lehmann, A.; Shaker, R.; Sengupta, J.N. Differential effects of transient receptor vanilloid one (trpv1) antagonists in acid-induced excitation of esophageal vagal afferent fibers of rats. Neuroscience 2009, 161, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Blackshaw, L.A. Transient receptor potential cation channels in visceral sensory pathways. Br. J. Pharmacol. 2014, 171, 2528–2536. [Google Scholar] [CrossRef] [PubMed]
- Fujino, K.; de la Fuente, S.G.; Takami, Y.; Takahashi, T.; Mantyh, C.R. Attenuation of acid induced oesophagitis in vr-1 deficient mice. Gut 2006, 55, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; de la Monte, S.; Ma, J.; Hong, J.; Tong, M.; Cao, W.; Behar, J.; Biancani, P.; Harnett, K.M. Hcl-activated neural and epithelial vanilloid receptors (trpv1) in cat esophageal mucosa. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G135–G143. [Google Scholar] [CrossRef] [PubMed]
- Matthews, P.J.; Aziz, Q.; Facer, P.; Davis, J.B.; Thompson, D.G.; Anand, P. Increased capsaicin receptor trpv1 nerve fibres in the inflamed human oesophagus. Eur. J. Gastroenterol. Hepatol. 2004, 16, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Shieh, K.R.; Yi, C.H.; Liu, T.T.; Tseng, H.L.; Ho, H.C.; Hsieh, H.T.; Chen, C.L. Evidence for neurotrophic factors associating with trpv1 gene expression in the inflamed human esophagus. Neurogastroenterol. Motil. 2010, 22, 971–977, e252. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Y.M.; Bielefeldt, K. Capsaicin receptor (trpv1) and non-erosive reflux disease. Eur. J. Gastroenterol. Hepatol. 2006, 18, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Sharrad, D.F.; Hibberd, T.J.; Kyloh, M.A.; Brookes, S.J.; Spencer, N.J. Quantitative immunohistochemical co-localization of trpv1 and cgrp in varicose axons of the murine oesophagus, stomach and colorectum. Neurosci. Lett. 2015, 599, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Horie, S.; Michael, G.J.; Priestley, J.V. Co-localization of trpv1-expressing nerve fibers with calcitonin-gene-related peptide and substance p in fundus of rat stomach. Inflammopharmacology 2005, 13, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Faussone-Pellegrini, M.S.; Taddei, A.; Bizzoco, E.; Lazzeri, M.; Vannucchi, M.G.; Bechi, P. Distribution of the vanilloid (capsaicin) receptor type 1 in the human stomach. Histochem. Cell Biol. 2005, 124, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Aihara, E.; Nakamura, A.; Xin, H.; Matsui, H.; Kohama, K.; Takeuchi, K. Expression of vanilloid receptors in rat gastric epithelial cells: Role in cellular protection. Biochem. Pharmacol. 2003, 66, 1115–1121. [Google Scholar] [CrossRef]
- Lamb, K.; Kang, Y.M.; Gebhart, G.F.; Bielefeldt, K. Gastric inflammation triggers hypersensitivity to acid in awake rats. Gastroenterology 2003, 125, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Minowa, S.; Ishihara, S.; Tsuchiya, S.; Horie, S.; Murayama, T. Capsaicin- and anandamide-induced gastric acid secretion via vanilloid receptor type 1 (trpv1) in rat brain. Brain Res. 2005, 1039, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Minowa, S.; Tsuchiya, S.; Horie, S.; Watanabe, K.; Murayama, T. Stimulatory effect of centrally injected capsaicin, an agonist of vanilloid receptors, on gastric acid secretion in rats. Eur. J. Pharmacol. 2001, 428, 349–356. [Google Scholar] [CrossRef]
- Lee, K.J.; Vos, R.; Tack, J. Effects of capsaicin on the sensorimotor function of the proximal stomach in humans. Aliment. Pharmacol. Ther. 2004, 19, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Hammer, J.; Vogelsang, H. Characterization of sensations induced by capsaicin in the upper gastrointestinal tract. Neurogastroenterol. Motil. 2007, 19, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.L.; Bornstein, J.C.; Anderson, C.R. Distinct chemical classes of medium-sized transient receptor potential channel vanilloid 1-immunoreactive dorsal root ganglion neurons innervate the adult mouse jejunum and colon. Neuroscience 2008, 156, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, M.; Kuramoto, H.; Takaki, M. Combined determination with functional and morphological studies of origin of nerve fibers expressing transient receptor potential vanilloid 1 in the myenteric plexus of the rat jejunum. Auton Neurosci. 2004, 116, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Laird, J.M.; Martinez-Caro, L.; Garcia-Nicas, E.; Cervero, F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain 2001, 92, 335–342. [Google Scholar] [CrossRef]
- Schmidt, B.; Hammer, J.; Holzer, P.; Hammer, H.F. Chemical nociception in the jejunum induced by capsaicin. Gut 2004, 53, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Hammer, J. Effect of repeated capsaicin ingestion on intestinal chemosensation and mechanosensation. Aliment. Pharmacol. Ther. 2006, 24, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Drewes, A.M.; Schipper, K.P.; Dimcevski, G.; Petersen, P.; Gregersen, H.; Funch-Jensen, P.; Arendt-Nielsen, L. Gut pain and hyperalgesia induced by capsaicin: A human experimental model. Pain 2003, 104, 333–341. [Google Scholar] [CrossRef]
- Brierley, S.M.; Jones, R.C., 3rd; Xu, L.; Gebhart, G.F.; Blackshaw, L.A. Activation of splanchnic and pelvic colonic afferents by bradykinin in mice. Neurogastroenterol. Motil. 2005, 17, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Hosoya, T.; Tashima, K.; Namiki, T.; Murayama, T.; Horie, S. Distribution of transient receptor potential vanilloid 1 channel-expressing nerve fibers in mouse rectal and colonic enteric nervous system: Relationship to peptidergic and nitrergic neurons. Neuroscience 2011, 172, 518–534. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Kurosawa, E.; Terui, H.; Hosoya, T.; Tashima, K.; Murayama, T.; Priestley, J.V.; Horie, S. Localization of trpv1 and contractile effect of capsaicin in mouse large intestine: High abundance and sensitivity in rectum and distal colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G348–G360. [Google Scholar] [CrossRef] [PubMed]
- Christianson, J.A.; Traub, R.J.; Davis, B.M. Differences in spinal distribution and neurochemical phenotype of colonic afferents in mouse and rat. J. Comp. Neurol. 2006, 494, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Malin, S.A.; Christianson, J.A.; Bielefeldt, K.; Davis, B.M. Tprv1 expression defines functionally distinct pelvic colon afferents. J. Neurosci. 2009, 29, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Kimball, E.S.; Wallace, N.H.; Schneider, C.R.; D'Andrea, M.R.; Hornby, P.J. Vanilloid receptor 1 antagonists attenuate disease severity in dextran sulphate sodium-induced colitis in mice. Neurogastroenterol. Motil. 2004, 16, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.L.; Facer, P.; Davis, J.B.; Smith, G.D.; Egerton, J.; Bountra, C.; Williams, N.S.; Anand, P. Sensory fibres expressing capsaicin receptor trpv1 in patients with rectal hypersensitivity and faecal urgency. Lancet 2003, 361, 385–391. [Google Scholar] [CrossRef]
- Kihara, N.; de la Fuente, S.G.; Fujino, K.; Takahashi, T.; Pappas, T.N.; Mantyh, C.R. Vanilloid receptor-1 containing primary sensory neurones mediate dextran sulphate sodium induced colitis in rats. Gut 2003, 52, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.; Nordstrom, E.; Mannem, A.; Smith, C.; Banerjee, B.; Sengupta, J.N. The role of transient receptor potential vanilloid 1 in mechanical and chemical visceral hyperalgesia following experimental colitis. Neuroscience 2007, 148, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Plourde, V.; St-Pierre, S.; Quirion, R. Calcitonin gene-related peptide in viscerosensitive response to colorectal distension in rats. Am. J. Physiol. 1997, 273, G191–G196. [Google Scholar] [PubMed]
- Delafoy, L.; Raymond, F.; Doherty, A.M.; Eschalier, A.; Diop, L. Role of nerve growth factor in the trinitrobenzene sulfonic acid-induced colonic hypersensitivity. Pain 2003, 105, 489–497. [Google Scholar] [CrossRef]
- Hughes, P.A.; Brierley, S.M.; Martin, C.M.; Brookes, S.J.; Linden, D.R.; Blackshaw, L.A. Post-inflammatory colonic afferent sensitisation: Different subtypes, different pathways and different time courses. Gut 2009, 58, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Akbar, A.; Yiangou, Y.; Facer, P.; Brydon, W.G.; Walters, J.R.; Anand, P.; Ghosh, S. Expression of the trpv1 receptor differs in quiescent inflammatory bowel disease with or without abdominal pain. Gut 2010, 59, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Facer, P.; Knowles, C.H.; Tam, P.K.; Ford, A.P.; Dyer, N.; Baecker, P.A.; Anand, P. Novel capsaicin (vr1) and purinergic (p2x3) receptors in hirschsprung's intestine. J. Pediatr. Surg. 2001, 36, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Fasanella, K.E.; Christianson, J.A.; Chanthaphavong, R.S.; Davis, B.M. Distribution and neurochemical identification of pancreatic afferents in the mouse. J. Comp. Neurol. 2008, 509, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Wick, E.C.; Hoge, S.G.; Grahn, S.W.; Kim, E.; Divino, L.A.; Grady, E.F.; Bunnett, N.W.; Kirkwood, K.S. Transient receptor potential vanilloid 1, calcitonin gene-related peptide, and substance p mediate nociception in acute pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G959–G969. [Google Scholar] [CrossRef] [PubMed]
- Liddle, R.A. The role of transient receptor potential vanilloid 1 (trpv1) channels in pancreatitis. Biochim. Biophys. Acta 2007, 1772, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Wick, E.C.; Pikios, S.; Grady, E.F.; Kirkwood, K.S. Calcitonin gene-related peptide partially mediates nociception in acute experimental pancreatitis. Surgery 2006, 139, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Figini, M.; Emanueli, C.; Grady, E.F.; Kirkwood, K.; Payan, D.G.; Ansel, J.; Gerard, C.; Geppetti, P.; Bunnett, N. Substance p and bradykinin stimulate plasma extravasation in the mouse gastrointestinal tract and pancreas. Am. J. Physiol. 1997, 272, G785–G793. [Google Scholar] [PubMed]
- Bhatia, M.; Saluja, A.K.; Hofbauer, B.; Frossard, J.L.; Lee, H.S.; Castagliuolo, I.; Wang, C.C.; Gerard, N.; Pothoulakis, C.; Steer, M.L. Role of substance p and the neurokinin 1 receptor in acute pancreatitis and pancreatitis-associated lung injury. Proc. Natl. Acad. Sci. USA 1998, 95, 4760–4765. [Google Scholar] [CrossRef] [PubMed]
- Nathan, J.D.; Peng, R.Y.; Wang, Y.; McVey, D.C.; Vigna, S.R.; Liddle, R.A. Primary sensory neurons: A common final pathway for inflammation in experimental pancreatitis in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G938–G946. [Google Scholar] [CrossRef] [PubMed]
- Nathan, J.D.; Patel, A.A.; McVey, D.C.; Thomas, J.E.; Prpic, V.; Vigna, S.R.; Liddle, R.A. Capsaicin vanilloid receptor-1 mediates substance p release in experimental pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G1322–G1328. [Google Scholar] [PubMed]
- Hoogerwerf, W.A.; Zou, L.; Shenoy, M.; Sun, D.; Micci, M.A.; Lee-Hellmich, H.; Xiao, S.Y.; Winston, J.H.; Pasricha, P.J. The proteinase-activated receptor 2 is involved in nociception. J. Neurosci. 2001, 21, 9036–9042. [Google Scholar] [PubMed]
- Watanabe, N.; Horie, S.; Michael, G.J.; Spina, D.; Page, C.P.; Priestley, J.V. Immunohistochemical localization of vanilloid receptor subtype 1 (trpv1) in the guinea pig respiratory system. Pulm. Pharmacol. Ther. 2005, 18, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Horie, S.; Michael, G.J.; Keir, S.; Spina, D.; Page, C.P.; Priestley, J.V. Immunohistochemical co-localization of transient receptor potential vanilloid (trpv)1 and sensory neuropeptides in the guinea-pig respiratory system. Neuroscience 2006, 141, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Pingle, S.C.; Matta, J.A.; Ahern, G.P. Capsaicin receptor: Trpv1 a promiscuous trp channel. In Transient Receptor Potential (TRP) Channels; Springer: Berlin, Germany, 2007; pp. 155–171. [Google Scholar]
- Jia, Y.; McLeod, R.L.; Hey, J.A. Trpv1 receptor: A target for the treatment of pain, cough, airway disease and urinary incontinence. Drug News Perspect. 2005, 18, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.J.; Santos, S.; Nagatomi, J.; Hayashi, Y.; Minnery, B.S.; Xavier, M.; Patel, A.S.; Nelson, J.B.; Futrell, W.J.; Yoshimura, N.; et al. Cool (trpm8) and hot (trpv1) receptors in the bladder and male genital tract. J. Urol. 2004, 172, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Auzanneau, C.; Norez, C.; Antigny, F.; Thoreau, V.; Jougla, C.; Cantereau, A.; Becq, F.; Vandebrouck, C. Transient receptor potential vanilloid 1 (trpv1) channels in cultured rat sertoli cells regulate an acid sensing chloride channel. Biochem. Pharmacol. 2008, 75, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Mizrak, S.C.; Gadella, B.M.; Erdost, H.; Ozer, A.; van Pelt, A.M.; van Dissel-Emiliani, F.M. Spermatogonial stem cell sensitivity to capsaicin: An in vitro study. Reprod. Biol. Endocrinol. 2008, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Dinis, P.; Charrua, A.; Avelino, A.; Nagy, I.; Quintas, J.; Ribau, U.; Cruz, F. The distribution of sensory fibers immunoreactive for the trpv1 (capsaicin) receptor in the human prostate. Eur. Urol. 2005, 48, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Litwin, M.S.; McNaughton-Collins, M.; Fowler, F.J., Jr.; Nickel, J.C.; Calhoun, E.A.; Pontari, M.A.; Alexander, R.B.; Farrar, J.T.; O'Leary, M.P. The national institutes of health chronic prostatitis symptom index: Development and validation of a new outcome measure. Chronic prostatitis collaborative research network. J. Urol. 1999, 162, 369–375. [Google Scholar] [CrossRef]
- Turini, D.; Beneforti, P.; Spinelli, M.; Malagutti, S.; Lazzeri, M. Heat/burning sensation induced by topical application of capsaicin on perineal cutaneous area: New approach in diagnosis and treatment of chronic prostatitis/chronic pelvic pain syndrome? Urology 2006, 67, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Czifra, G.; Varga, A.; Nyeste, K.; Marincsak, R.; Toth, B.I.; Kovacs, I.; Kovacs, L.; Biro, T. Increased expressions of cannabinoid receptor-1 and transient receptor potential vanilloid-1 in human prostate carcinoma. J. Cancer Res. Clin. Oncol. 2009, 135, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.G.; Sanchez, A.M.; Collado, B.; Malagarie-Cazenave, S.; Olea, N.; Carmena, M.J.; Prieto, J.C.; Diaz-Laviada, I.I. Expression of the transient receptor potential vanilloid 1 (trpv1) in lncap and pc-3 prostate cancer cells and in human prostate tissue. Eur. J. Pharmacol. 2005, 515, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.P.; Pu, X.Y.; Wang, X.H. Distribution profiles of transient receptor potential melastatin-related and vanilloid-related channels in prostatic tissue in rat. Asian J. Androl. 2007, 9, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Pierce, A.N.; Zhang, Z.; Fuentes, I.M.; Wang, R.; Ryals, J.M.; Christianson, J.A. Neonatal vaginal irritation results in long-term visceral and somatic hypersensitivity and increased hypothalamic-pituitary-adrenal axis output in female mice. Pain 2015, 156, 2021–2031. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Conklin, D.; Clyne, B.B.; Stanislaus, J.D.; Eisenach, J.C. Uterine cervical afferents in thoracolumbar dorsal root ganglia express transient receptor potential vanilloid type 1 channel and calcitonin gene-related peptide, but not p2x3 receptor and somatostatin. Anesthesiology 2006, 104, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Liu, B.; Du, D.; Eisenach, J.C.; Tong, C. Estrogen amplifies pain responses to uterine cervical distension in rats by altering transient receptor potential-1 function. Anesth. Analg. 2007, 104, 1246–1250, tables of contents. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.Y.; Chang, H.M.; Lee, S.D.; Huang, P.C.; Chen, G.D.; Lai, C.H.; Lai, C.Y.; Chiu, C.H.; Tung, K.C.; Lin, T.B. Trpv1 mediates the uterine capsaicin-induced nmda nr2b-dependent cross-organ reflex sensitization in anesthetized rats. Am. J. Physiol. Renal Physiol. 2008, 295, F1324–F1335. [Google Scholar] [CrossRef] [PubMed]
- Tingaker, B.K.; Ekman-Ordeberg, G.; Facer, P.; Irestedt, L.; Anand, P. Influence of pregnancy and labor on the occurrence of nerve fibers expressing the capsaicin receptor trpv1 in human corpus and cervix uteri. Reprod Biol. Endocrinol. 2008, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.; Wolf-Johnston, A.S.; Shinde, S.; Cruz, C.D.; Cruz, F.; Avelino, A.; Birder, L.A. Urinary bladder inflammation induces changes in urothelial nerve growth factor and trpv1 channels. Br. J. Pharmacol. 2015, 172, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, B.; De Felice, M.; Stucky, C.L.; Medler, K.A.; Luo, M.C.; Gardell, L.R.; Ibrahim, M.; Malan, T.P., Jr.; Yamamura, H.I.; Ossipov, M.H.; et al. Constitutive activity at the cannabinoid cb1 receptor is required for behavioral response to noxious chemical stimulation of trpv1: Antinociceptive actions of cb1 inverse agonists. J. Neurosci. 2008, 28, 11593–11602. [Google Scholar] [CrossRef] [PubMed]
- Ferrini, F.; Salio, C.; Lossi, L.; Gambino, G.; Merighi, A. Modulation of inhibitory neurotransmission by the vanilloid receptor type 1 (trpv1) in organotypically cultured mouse substantia gelatinosa neurons. Pain 2010, 150, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Lao, L.J.; Song, B.; Marvizon, J.C. Neurokinin release produced by capsaicin acting on the central terminals and axons of primary afferents: Relationship with n-methyl-d-aspartate and gaba(b) receptors. Neuroscience 2003, 121, 667–680. [Google Scholar] [CrossRef]
- Merighi, A. Costorage and coexistence of neuropeptides in the mammalian cns. Prog. Neurobiol. 2002, 66, 161–190. [Google Scholar] [CrossRef]
- Kanai, Y.; Nakazato, E.; Fujiuchi, A.; Hara, T.; Imai, A. Involvement of an increased spinal trpv1 sensitization through its up-regulation in mechanical allodynia of cci rats. Neuropharmacology 2005, 49, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.Z.; Pan, H.L. Primary afferent stimulation differentially potentiates excitatory and inhibitory inputs to spinal lamina ii outer and inner neurons. J. Neurophysiol. 2004, 91, 2413–2421. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Gu, J.G. A p2x receptor-mediated nociceptive afferent pathway to lamina i of the spinal cord. Mol. Pain 2005, 1, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawasaki, Y.; Kohno, T.; Zhuang, Z.Y.; Brenner, G.J.; Wang, H.; Van Der Meer, C.; Befort, K.; Woolf, C.J.; Ji, R.R. Ionotropic and metabotropic receptors, protein kinase a, protein kinase c, and src contribute to c-fiber-induced erk activation and camp response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J. Neurosci. 2004, 24, 8310–8321. [Google Scholar] [CrossRef] [PubMed]
- Ferrini, F.; Russo, A.; Salio, C. Fos and perk immunoreactivity in spinal cord slices: Comparative analysis of in vitro models for testing putative antinociceptive molecules. Ann. Anat. 2014, 196, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Salio, C.; Ferrini, F.; Muthuraju, S.; Merighi, A. Presynaptic modulation of spinal nociceptive transmission by glial cell line-derived neurotrophic factor (gdnf). J. Neurosci. 2014, 34, 13819–13833. [Google Scholar] [CrossRef] [PubMed]
- Merighi, A.; Bardoni, R.; Salio, C.; Lossi, L.; Ferrini, F.; Prandini, M.; Zonta, M.; Gustincich, S.; Carmignoto, G. Presynaptic functional trkb receptors mediate the release of excitatory neurotransmitters from primary afferent terminals in lamina ii (substantia gelatinosa) of postnatal rat spinal cord. Dev. Neurobiol. 2008, 68, 457–475. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Kiritoshi, T.; Murase, K. Effect of excitatory and inhibitory agents and a glial inhibitor on optically-recorded primary-afferent excitation. Mol. Pain 2008, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Qutenza. Official website of qutenza (trademarked) patch. Avaliable online: http://www.qutenza.com/_docs/qutenza_full_PI_.pdf (accessed on 17 June 2016).
- Kulkantrakorn, K.; Lorsuwansiri, C.; Meesawatsom, P. 0.025% capsaicin gel for the treatment of painful diabetic neuropathy: A randomized, double-blind, crossover, placebo-controlled trial. Pain Pract. 2013, 13, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Derry, S.; Moore, R.A. Topical capsaicin (low concentration) for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2012, 9, CD010111. [Google Scholar] [PubMed]
- Teixeira, M.J.; Menezes, L.M.; Silva, V.; Galhardoni, R.; Sasson, J.; Okada, M.; Duarte, K.P.; Yeng, L.T.; Andrade, D.C. Liposomal topical capsaicin in post-herpetic neuralgia: A safety pilot study. Arq. Neuropsiquiatr. 2015, 73, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Casanueva, B.; Rodero, B.; Quintial, C.; Llorca, J.; Gonzalez-Gay, M.A. Short-term efficacy of topical capsaicin therapy in severely affected fibromyalgia patients. Rheumatol. Int. 2013, 33, 2665–2670. [Google Scholar] [CrossRef] [PubMed]
- Griebeler, M.L.; Morey-Vargas, O.L.; Brito, J.P.; Tsapas, A.; Wang, Z.; Carranza Leon, B.G.; Phung, O.J.; Montori, V.M.; Murad, M.H. Pharmacologic interventions for painful diabetic neuropathy: An umbrella systematic review and comparative effectiveness network meta-analysis. Ann. Intern. Med. 2014, 161, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.D.; Barthel, H.R. Topical therapies for osteoarthritis. Drugs 2011, 71, 1259–1279. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, J.M.; Ringsted, T.K.; Petersen, M.; Sommer, C.; Uceyler, N.; Werner, M.U. A capsaicin (8%) patch in the treatment of severe persistent inguinal postherniorrhaphy pain: A randomized, double-blind, placebo-controlled trial. PLoS ONE 2014, 9, e109144. [Google Scholar] [CrossRef] [PubMed]
- Hartrick, C.T.; Pestano, C.; Carlson, N.; Hartrick, S. Capsaicin instillation for postoperative pain following total knee arthroplasty: A preliminary report of a randomized, double-blind, parallel-group, placebo-controlled, multicentre trial. Clin. Drug Investig. 2011, 31, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, M.; Porta, S. Effect of red pepper on symptoms of irritable bowel syndrome: Preliminary study. Dig. Dis. Sci. 2011, 56, 3288–3295. [Google Scholar] [CrossRef] [PubMed]
- Salat, K.; Jakubowska, A.; Kulig, K. Zucapsaicin for the treatment of neuropathic pain. Expert Opin. Investig. Drugs 2014, 23, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Maihofner, C.; Heskamp, M.L. Prospective, non-interventional study on the tolerability and analgesic effectiveness over 12 weeks after a single application of capsaicin 8% cutaneous patch in 1044 patients with peripheral neuropathic pain: First results of the quepp study. Curr. Med. Res. Opin. 2013, 29, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Simpson, D.M.; Moyle, G.; Brew, B.J.; Schifitto, G.; Larbalestier, N.; Orkin, C.; Fisher, M.; Vanhove, G.F.; Tobias, J.K. Ngx-4010, a capsaicin 8% patch, for the treatment of painful hiv-associated distal sensory polyneuropathy: Integrated analysis of two phase iii, randomized, controlled trials. AIDS Res. Ther. 2013, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.M.; Brown, S.; Tobias, J.K.; Vanhove, G.F.; Group, N.-C.S. Ngx-4010, a capsaicin 8% dermal patch, for the treatment of painful hiv-associated distal sensory polyneuropathy: Results of a 52-week open-label study. Clin. J. Pain 2014, 30, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Clifford, D.B.; Simpson, D.M.; Brown, S.; Moyle, G.; Brew, B.J.; Conway, B.; Tobias, J.K.; Vanhove, G.F.; Group, N.-C.S. A randomized, double-blind, controlled study of ngx-4010, a capsaicin 8% dermal patch, for the treatment of painful hiv-associated distal sensory polyneuropathy. J. Acquir. Immune Defic. Syndr. 2012, 59, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Irving, G.; Backonja, M.; Rauck, R.; Webster, L.R.; Tobias, J.K.; Vanhove, G.F. Ngx-4010, a capsaicin 8% dermal patch, administered alone or in combination with systemic neuropathic pain medications, reduces pain in patients with postherpetic neuralgia. Clin. J. Pain 2012, 28, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Maihofner, C.G.; Heskamp, M.L. Treatment of peripheral neuropathic pain by topical capsaicin: Impact of pre-existing pain in the quepp-study. Eur. J. Pain 2014, 18, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Martini, C.H.; Yassen, A.; Krebs-Brown, A.; Passier, P.; Stoker, M.; Olofsen, E.; Dahan, A. A novel approach to identify responder subgroups and predictors of response to low- and high-dose capsaicin patches in postherpetic neuralgia. Eur. J. Pain 2013, 17, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Mou, J.; Paillard, F.; Turnbull, B.; Trudeau, J.; Stoker, M.; Katz, N.P. Qutenza (capsaicin) 8% patch onset and duration of response and effects of multiple treatments in neuropathic pain patients. Clin. J. Pain 2014, 30, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Mou, J.; Paillard, F.; Turnbull, B.; Trudeau, J.; Stoker, M.; Katz, N.P. Efficacy of qutenza(r) (capsaicin) 8% patch for neuropathic pain: A meta-analysis of the qutenza clinical trials database. Pain 2013, 154, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Hoper, J.; Helfert, S.; Heskamp, M.L.; Maihofner, C.G.; Baron, R. High concentration capsaicin for treatment of peripheral neuropathic pain: Effect on somatosensory symptoms and identification of treatment responders. Curr. Med. Res. Opin. 2014, 30, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Webster, L.R.; Peppin, J.F.; Murphy, F.T.; Lu, B.; Tobias, J.K.; Vanhove, G.F. Efficacy, safety, and tolerability of ngx-4010, capsaicin 8% patch, in an open-label study of patients with peripheral neuropathic pain. Diabetes Res. Clin. Pract. 2011, 93, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.S.; Hoye, K.; Fricova, J.; Vanelderen, P.; Ernault, E.; Siciliano, T.; Marques, S. Tolerability of the capsaicin 8% patch following pretreatment with lidocaine or tramadol in patients with peripheral neuropathic pain: A multicentre, randomized, assessor-blinded study. Eur. J. Pain 2014, 18, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Treede, R.D.; Wagner, T.; Kern, K.U.; Husstedt, I.W.; Arendt, G.; Birklein, F.; Cegla, T.; Freynhagen, R.; Gockel, H.H.; Heskamp, M.L.; et al. Mechanism- and experience-based strategies to optimize treatment response to the capsaicin 8% cutaneous patch in patients with localized neuropathic pain. Curr. Med. Res. Opin. 2013, 29, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Derry, S.; Sven-Rice, A.; Cole, P.; Tan, T.; Moore, R.A. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2013, 2, CD007393. [Google Scholar] [PubMed]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpaa, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef]
- Remadevi, R.; Szallisi, A. Adlea (algrx-4975), an injectable capsaicin (trpv1 receptor agonist) formulation for longlasting pain relief. IDrugs 2008, 11, 120–132. [Google Scholar] [PubMed]
- Diamond, E.; Richards, P.; Miller, T. Algrx 4975 reduces pain of intermetatarsal neuroma: Preliminary results from a randomized, double-blind, placebo-controlled, phase ii multicenter clinical trial. J. Pain 2006, 7, S41. [Google Scholar] [CrossRef]
- Richards, P.; Vasko, G.; Stasko, I.; Lacko, M.; Hewson, G. Algrx 4975 reduces pain of acute lateral epicondylitis: Preliminary results from a randomized, double-blind, placebo-controlled, phase ii multicenter clinical trial. J. Pain 2006, 7, S3. [Google Scholar] [CrossRef]
- Cantillon, M.; Vause, E.; Sykes, D.; Russel, R.; Moon, A.; Hughes, S. Preliminary safety, tolerability and efficacy of algrx 4975 in osteoarthristis (oa) of the knee. J. Pain 2005, 6, S39. [Google Scholar]
- Silberberg, A.; Moeller-Bertram, T.; Wallace, M.S. A randomized, double-blind, crossover study to evaluate the depth response relationship of intradermal capsaicin-induced pain and hyperalgesia in healthy adult volunteers. Pain Med. 2015, 16, 745–752. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Guida, F.; Moriello, A.S.; De Chiaro, M.; Piscitelli, F.; de Novellis, V.; Maione, S.; Di Marzo, V. N-palmitoyl-vanillamide (palvanil) is a non-pungent analogue of capsaicin with stronger desensitizing capability against the trpv1 receptor and anti-hyperalgesic activity. Pharmacol. Res. 2011, 63, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Luongo, L.; Costa, B.; D'Agostino, B.; Guida, F.; Comelli, F.; Gatta, L.; Matteis, M.; Sullo, N.; De Petrocellis, L.; de Novellis, V.; et al. Palvanil, a non-pungent capsaicin analogue, inhibits inflammatory and neuropathic pain with little effects on bronchopulmonary function and body temperature. Pharmacol. Res. 2012, 66, 243–250. [Google Scholar] [CrossRef] [PubMed]


| Activators of TRPV1 Receptor | Action | Refs | ||
|---|---|---|---|---|
| Physical activators | Depolarization (V½ ~ 0 mV at 35 °C) | activator | [59] | |
| Noxious heat (> 43 °C at pH 7.4) | activator | [59] | ||
| Endogenous activators | Protons | Mild acidification (extracellular H+ pEC50 5.4 at 37 °C) | activator | [58,59,72] |
| Small molecules | Adenosine and ATP | activator | [73] | |
| Polyamines | activator | [74] | ||
| Lipids, lipid metabolites or derivatives | lipoxygenase products (12-HPETE, 15-HPETE) | agonist | [75] | |
| leukotriene B4 | [76] | |||
| 5-(S)-hydroxyeicosatetraenoic acid | [59] | |||
| NADA N-oleoyldopamine | channel blocker | [77] | ||
| anandamide (arachidonoylethanolamide) | channel blocker | [78] | ||
| prostaglandins | activator | [79] | ||
| Peptides, proteins and growth factors | bradykinin | activator | [80,81,82] | |
| prokineticin | activator | [83] | ||
| protein kinase C | activator | [84] | ||
| NGF | [81] | |||
| Exogenous activators | Plant products or derivatives | RTX (active compound from the cactus Euphorbia resinifera) | agonist | [20] |
| piperine (pungent component in black pepper) | agonist | [85] | ||
| camphor (terpenoid extracted from Cinnamomum camphora) | agonist | [86] | ||
| Venoms | from jellyfish (crude extracts from Aiptasia pulchella, Cyanea capillata, Physalia physalis and Chironex fleckeri) | agonist | [87] | |
| VaTx1-3 (Tarantulas’ toxins from Psalmopoeus cambridgei) | agonist | [88] | ||
| DkTx (from the Chinese earth tiger tarantula Chilobrachys guangxiensis) | activator | [89] | ||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frias, B.; Merighi, A. Capsaicin, Nociception and Pain. Molecules 2016, 21, 797. https://doi.org/10.3390/molecules21060797
Frias B, Merighi A. Capsaicin, Nociception and Pain. Molecules. 2016; 21(6):797. https://doi.org/10.3390/molecules21060797
Chicago/Turabian StyleFrias, Bárbara, and Adalberto Merighi. 2016. "Capsaicin, Nociception and Pain" Molecules 21, no. 6: 797. https://doi.org/10.3390/molecules21060797





