1. Introduction
Rubia cordifolia L., belonging to the genus Rubia (Rubiaceae), is a type of perennial herb, distributed widely around the world, including the western and northern parts of Europe, the Mediterranean coast, temperate regions of Asia and Africa, as well as the regions from Mexico to Tropical America [
1]. The genus Rubia comprises about 70 species, among which a total of 36 species and two varieties have been reported in China. The dried roots and rhizomes are referred to in Traditional Chinese Medicine as
Rubiae Radix et Rhizoma, and is widely used for the treatment of menoxenia, rheumatism, contusions, hemorrhages, chronic bronchitis and trauma, and is officially listed in the 2015 edition of the Chinese Pharmacopoeia [
2]. Previous phytochemical investigations have shown that this plant is a source of cyclic hexapeptides, anthraquinones, and arborinane-type triterpenoids [
3].
R. cordifolia exhibited cytotoxicity in various cancer models, including leukemia, melanoma, colon cancer, laryngeal squamous cell carcinoma, as well as lung cancer [
4,
5,
6,
7,
8]. In addition,
R. cordifolia has a long history in skin care and treatment, and has been used in disorders of the urinary tract [
9].
In our previous investigations, three compounds: purpurin, munjistin, and mollugin (
Figure 1) were isolated and identified from the 70% ethanol extract of
R. cordifolia. Purpurin and mollugin are the main indicator compounds for this composition in the Chinese Pharmacopoeia [
2]. Purpurin and mollugin have been reported to possess antioxidant and enzyme inhibition activities. In particular, purpurin showed stronger antioxidant and better enzyme inhibitory effects than mollugin [
10]. Mollugin is a naturally benzochromene reputed for its anti-carcinogenic and anti-viral activities [
11]. In addition, mollugin might be a broad action inhibitor, inhibiting LPS-induced inflammatory responses and suppressing the inflammatory responses in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages [
12]. Hydroxyanthraquinones have been reported to be the predominant antioxidant phenolic constituents in the roots of
R. cordifolia [
13]. Purpurin is a novel specific inhibitor of adipocyte-derived leucine aminopeptidase (A-LAP) and could be developed as a new anti-angiogenic agent [
14]. Studies have demonstrated the potent
in vitro anti-fungal activities of purpurin in terms of inhibiting six
Candida species [
15].
Various analytical methods, including ultra-fast liquid chromatography [
16], high-performance liquid chromatography [
17,
18,
19], and ultrasound-assisted ionic liquid-reversed phase liquid chromatography [
20] have been used in the
in vitro qualitative or quantitative analysis of munjistin, purpurin, and mollugin. However, there is no report on the simultaneous determination of the three compounds using a single method. LC coupled with triple-quadrupole MS method has been widely used in pharmacokinetic studies because its multiple reaction monitoring (MRM) technique provides high sensitivity for quantification [
21,
22,
23,
24]. In this paper, we have developed for the first time a tested sensitive and selective UHPLC-MS/MS method to simultaneously determine munjistin, purpurin, and mollugin in rat plasma. The validated method has then been successfully applied to the pharmacokinetic study of the three components for the first time. The results of this pharmacokinetic study could provide some useful references for the further pharmacological study of
R. cordifolia.
3. Materials and Methods
3.1. Materials and Reagents
Reference standards of purpurin, munjistin, and mollugin were isolated from R. cordifolia and identified by our laboratory. On the basis of UV, MS, NMR and HPLC analysis, the chemical structures and the purities (purity ≥ 98%) of isolated reference standards were confirmed. Bifendate (Lots: 100192-200503) was purchased from Chengdu Must Bio-Technology Co., Ltd. (Chengdu, Sichuan, China) and used as an internal standard (I.S.).
The following solvents and chemicals were used in our experiments: J & K Medical (Beijing, China) provided the HPLC-grade methanol (MeOH) and acetonitrile (CH3CN). HPLC-grade ammonium acetate was purchased from DIKMA Technologies (Beijing, China). All other reagents were of analytical-grade. Ultrapure water was prepared using a Milli-Q water purification system (Millipore, Molsheim, France).
The dried roots and rhizomes of R. cordifolia were obtained from Bozhou (Anhui, China), the largest Chinese herbal medicine distribution center in the world, and were identified by Professor Zhenyue Wang in Heilongjiang University of Chinese Medicine. A voucher specimen (Art. No. 20160001) was deposited in the College of Pharmacy, Harbin Medical University, China.
3.2. Instruments and Analytical Conditions
The UHPLC-MS system (Agilent Technologies 1290 series) consisting of a quaternary pump, an automatic degasser, and an auto-sampler, coupled to a 6430 QQQ-MS instrument (Agilent, Santa Clara, CA, USA) with an ESI interface. Chromatographic separation was carried out using a C18 column (ACQUITY UPLC® HSS T3, 1.8 μm, 2.1 × 100 mm). The column temperature was set at 40 °C and a 5 μL aliquot of the sample solution was injected for each injection. Chromatography was performed using a gradient system consisting of mobile phase solution A (5 mM ammomium acetate in water) and solution B (methanol) at a flow rate of 0.4 mL/min. The gradient elution method was as follows: 0–6.5 min 90% B; 6.5–8 min linear increase to 98% B; 8.0–8.5 min linear decrease to 90% B. The analysis time was 8.5 min for each injection.
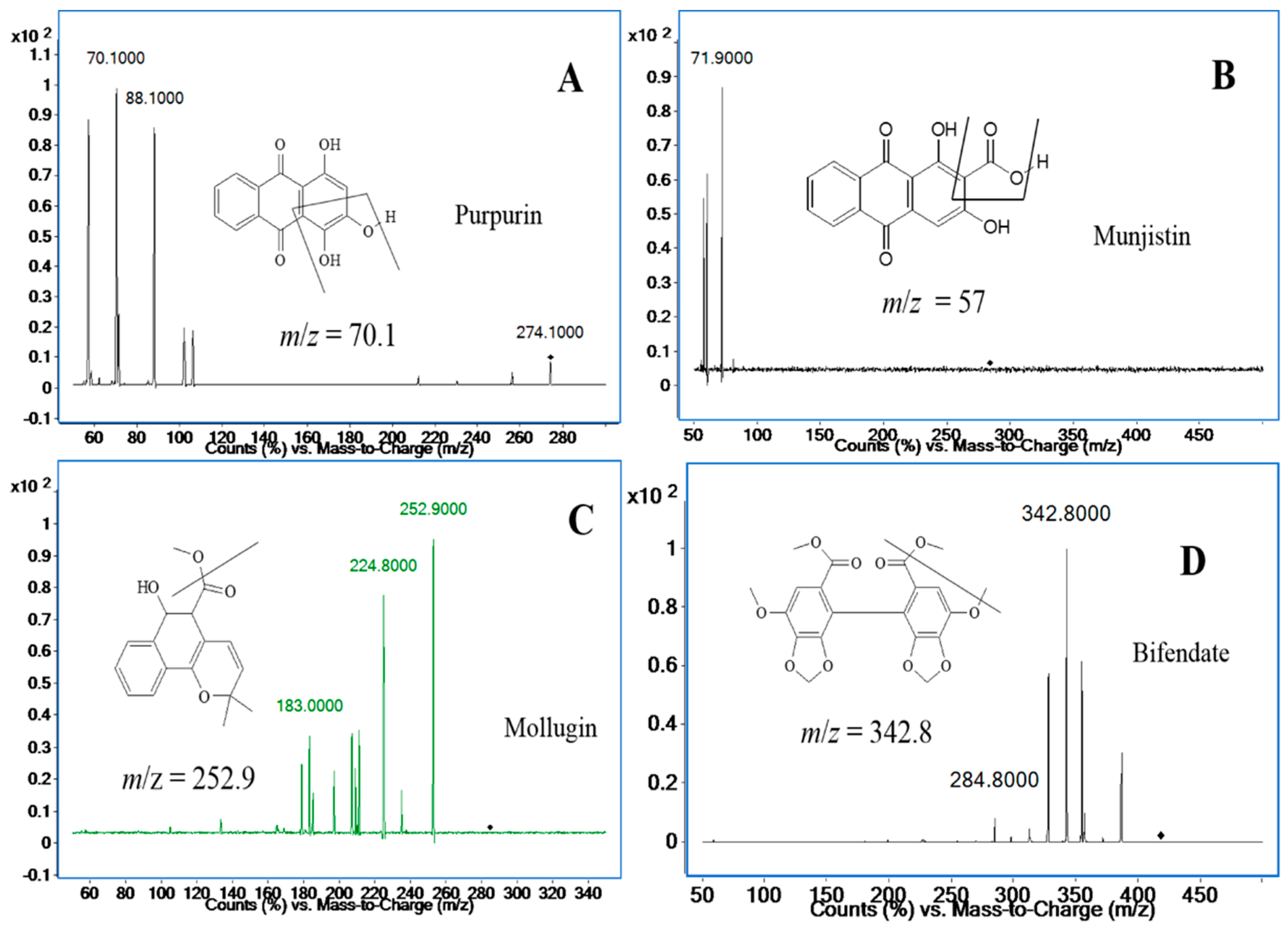
The positive ionization mode was used for compound ionization. An Agilent Mass Hunter workstation was used to control the equipment and for data acquisition and analysis. The quantification was obtained in multiple reaction monitoring (MRM) mode with the precursor-product ion transition at
m/
z 274.2→70.1 (88.1) for purpurin,
m/
z 284.3→57.0 (71.9) for munjistin,
m/
z 285.0→252.9 (183.0) for mollugin, and
m/
z 418.9→342.9 (284.8) for bifendate (I.S.), respectively. The fragment and collision energy are shown in
Table 1. The product ion scan spectra of the analytes and the I.S. are shown in
Figure 2. High-purity nitrogen (N
2) was used as the nebulizing gas, and nitrogen (N
2) was used as the drying gas at a flow rate of 12 L/min. The mass spectrometer was operated at a capillary voltage of 4000 V, source temperature of 100 °C and desolvation temperature of 350 °C.
3.3. Preparation of R. Cordifolia Extract
An ultrasonic extraction method was used for the extract preparation. Dried powder (200 g) of R. cordifolia was ultrasonically extracted twice (1 h each time) with methanol at a material to liquid ratio of 1:6 and then filtered. The combined filtrate was evaporated to dryness, and the residue was reconstituted in water to a concentration equivalent to 0.1 g/mL of the R. cordifolia extract.
3.4. Preparation of Calibration Standards and Quality Control (QC) Samples
Standard mixed stock solution of purpurin, munjistin, and mollugin were prepared in methanol at concentrations of 0.264 mg/mL, 0.515 mg/mL and 2.45 mg/mL, and then further diluted in methanol to produce the working solutions with a series of concentrations. The I.S. solution (2000 ng/mL) was obtained by diluting the stock solution in methanol for routine use. The samples for the standard calibration curves were prepared by spiking appropriate amounts of the standard solutions into 100 μL of blank plasma to yield the final calibration concentrations of 0.264, 0.528, 2.11, 5.28, 26.4, 52.8, 264 ng/mL for purpurin, 0.515, 1.03, 4.12, 10.3, 51.5, 103, 515 ng/mL for munjistin, and 2.45, 4.90, 19.6, 49.0, 245, 490, 2450 ng/mL for mollugin, respectively. The QC samples were prepared in drug-free plasma at four different concentration levels, high QC (211/412/1960 ng/mL), medium QC (26.4/51.5/245ng/mL), low QC (0.528/1.03/4.90 ng/mL), and LLOQ (0.264/0.515/2.45 ng/mL), for purpurin, munjistin, and mollugin. All the stock and preparing solutions were maintained in the freezer at 4 °C and brought to room temperature before use.
3.5. Animal Experiments
Twelve male Sprague-Dawley (SD) rats (body weight 220 ± 20 g) were obtained from the experimental animal center of Harbin Medical University and adapted to a constant-temperature (25 °C) room. The animal handling procedures were approved by the institutional ethics committee and conformed to the principles of the International Guide for the Care and Use of Laboratory Animals. Before the experiment, the rats were fasted for 12 h and had access to water during the research period. Blood (0.4 mL) was collected from the orbital venous plexus at several specific time points (0, 0.33, 0.67, 1, 1.3, 1.67, 2, 2.5, 3, 4, 6, 8, 12 and 24 h) after the oral administration of R. cordifolia (0.82 g/kg). After centrifugation at 12000 rpm for 5 min, the plasma was collected immediately and stored at −20 °C until sample analysis.
3.6. Plasma Sample Preparation
Before the experiment, the plasma samples were thawed naturally at room temperature. As a liquid-liquid extraction method, 100 μL of methanol and 50 μL of I.S. (2000 ng/mL) were added to a rat plasma sample (100 μL), and then the content was mixed by a vortex mixer for 30 s. After this step, a 3 mL aliquot of ethyl acetate (extraction agent) was accurately added and the mixture vortexed for 60 s. Then the samples were put into a centrifuge. The organic supernatant was pipetted into clean glass tubes individually after centrifugation 4000 rpm for 5 min. The upper organic layer was removed and evaporated to dryness at 40 °C under a stream of nitrogen. The residue was then reconstituted with 100 μL of mobile phase (5 mM ammonium acetate in water/methanol, 10:90, v/v), vortexed for 30 s and filtered by a 0.22 μm membrane. A 5 μL aliquot of the solution was injected into the UHPLC-MS/MS system for analysis.
3.7. Method Validation
The method was fully validated according to the Food and Drug Administration (FDA) guidance for biological method validation (FDA 2001 [
28]) in terms of specificity and selectivity, linearity, intra- and inter-day precisions, accuracy, stability, matrix effects, and extraction recovery. The validation runs were conducted on three consecutive days. Each validation run consisted of two sets of calibration standards and six replicates of QC samples at three different concentrations.
3.7.1. Specificity and Selectivity
Selectivity is the ability of an analytical method to differentiate and quantify the analytes in the presence of other components in the sample and endogenous substances in plasma. Comparing and analyzing blank samples from six rats, blank plasma spiked with the three analytes and the I.S., and the plasma sample from rats fed the extract of R. cordifolia, there were no detectable interfering peaks at the respective retention times of quantification of the three analytes (LLOQ).
3.7.2. Linearity and Lower Limits of Quantification (LLOQ)
Seven different concentrations of standard plasma samples were used to establish calibration curves on three consecutive days. The linearity of each calibration curve was determined by plotting the peak area ratio (y) of the analyte to the I.S. against the nominal concentration (x) of the analyte with weighted (1/x2) least square linear regression. The LLOQ was determined as the lowest concentration point of the calibration curve at which the measured precision, expressed as the relative standard deviation (RSD), was required to be within ±20%, and the accuracy, expressed as relative error (RE%), was required to be within ±20%.
3.7.3. Precision and Accuracy
Four different concentration levels of QC samples were selected to verify the intra- and inter- day accuracy and precision. Four levels of QC samples (LLOQ, LQC, MQC, and HQC) in six replicates were analyzed on the same day to determine the intra-day precision and on three consecutive days to evaluate the inter-day precision. The precision was expressed as the RSD with a criterion of less than 15% and the accuracy was assessed by comparing the measured concentration with its nominal value calculated as the RE with requirement of within ±15%.
3.7.4. Extraction Recovery and Matrix Effect
The extraction efficiency of the three analytes were determined by analyzing six replicates of the plasma samples at their respective LQCs, MQCs and HQCs. To investigate recovery and matrix effect, extracted samples (A), unextracted samples (B), and post-extracted spiked samples (C) were analyzed in the same assay. The extraction recovery was expressed as (mean observed peak areas of A)/(peak areas of C) × 100% and the matrix effect was acquired by calculating (mean observed peak areas of A)/(peak areas of B) × 100%. The extraction recovery and the matrix effect were similarly evaluated for the I.S. at one concentration.
3.7.5. Stability Experiments
To study the stability, QC samples of the three analytes at three QC levels (LQC, MQC, and HQC) were added into drug-free plasma samples and each levels contained six replicates. Four different manipulations were designed to assess the stability involving short-term stability (storage for 4 h at ambient temperature), long-term stability (storage for 2 weeks at −20 °C), freeze-thaw stability (three freeze at −20 °C and thaw cycles), and post-preparative stability (storage for 12 h after sample preparation at 4 °C). A series of concentrations of stability testing QC samples was obtained by the calibration curve which was established from freshly prepared standard samples.
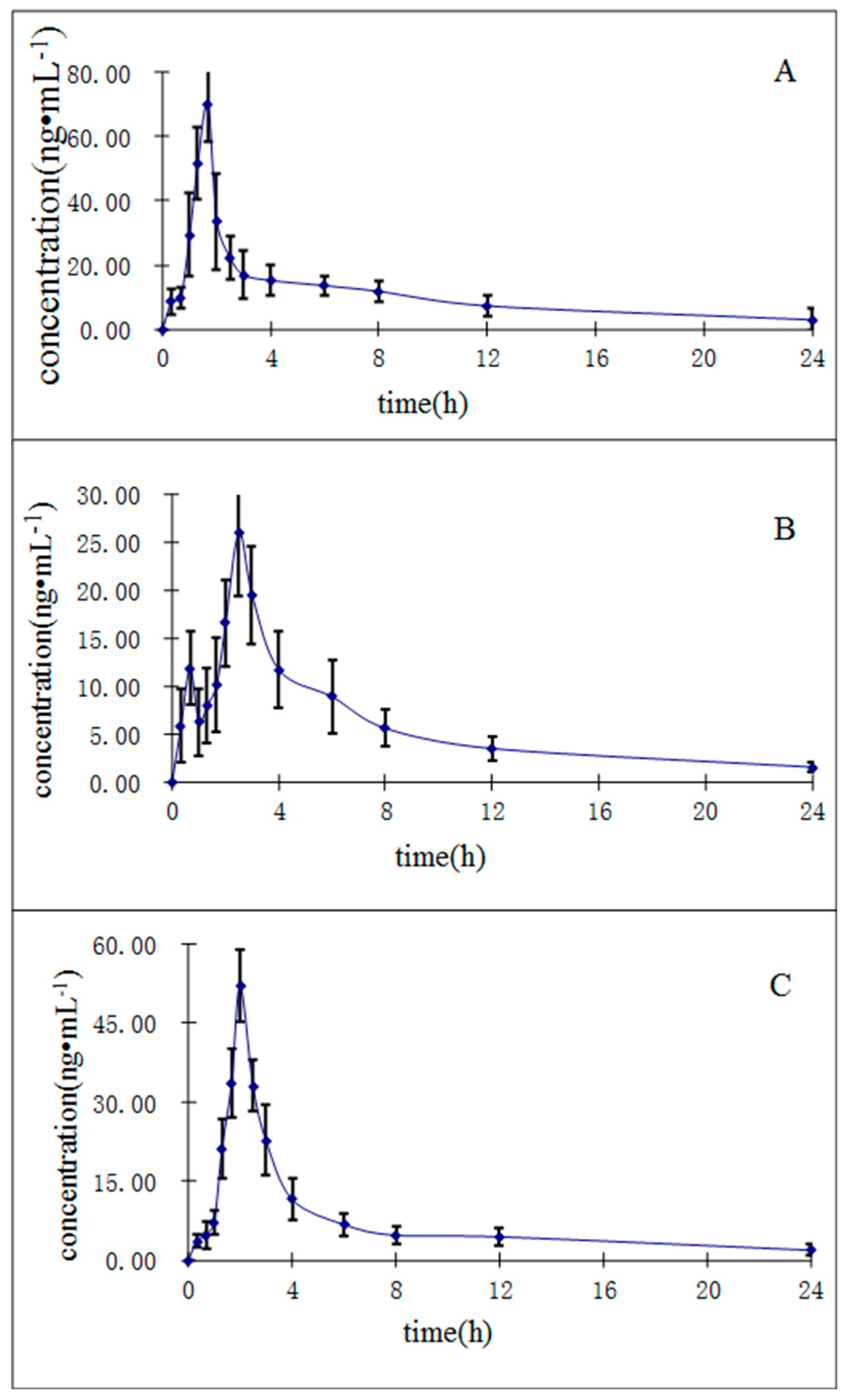
3.8. Application to Pharmacokinetic Study
Noncompartmental methods were applied to calculate the following pharmacokinetic parameters. The peak plasma concentration (
Cmax) and the time of maximum plasma concentration (
Tmax) were observed directly from the measured data. The elimination rate constant (
Ke) was calculated using the linear regression of the terminal points in a semi-log plot of the plasma concentration against time. The elimination half-life (
t1/2) was calculated using the formula:
The area under the peak plasma concentration-time curve (AUC
0→t) to the last measurable plasma concentration (
Ct) was estimated using the linear trapezoidal rule. The area under the plasma concentration-time curve to time infinity (AUC
0→∞) was calculated as following:
4. Conclusions
A specific, simple and sensitive UHPLC-MS/MS method has been developed and validated for the quantification of purpurin, munjistin, and mollugin in rat plasma using liquid-liquid extraction as sample preparation procedure for the first time. This validated method was found to be precise, fast and highly accurate, and it met all bio-analysis requirements. The method has been applied to the pharmacokinetic study of purpurin, munjistin, and mollugin in rat plasma. It is expected that the results of this study will provide some useful references to the further pharmacological study of R. cordifolia.