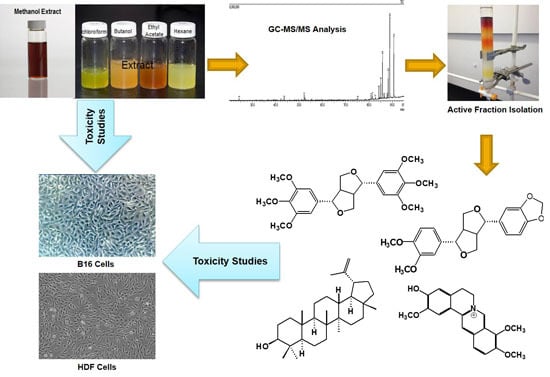
Bioactive Constituents of Zanthoxylum rhetsa Bark and Its Cytotoxic Potential against B16-F10 Melanoma Cancer and Normal Human Dermal Fibroblast (HDF) Cell Lines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Bioactive Compounds Through GC-MS Analysis
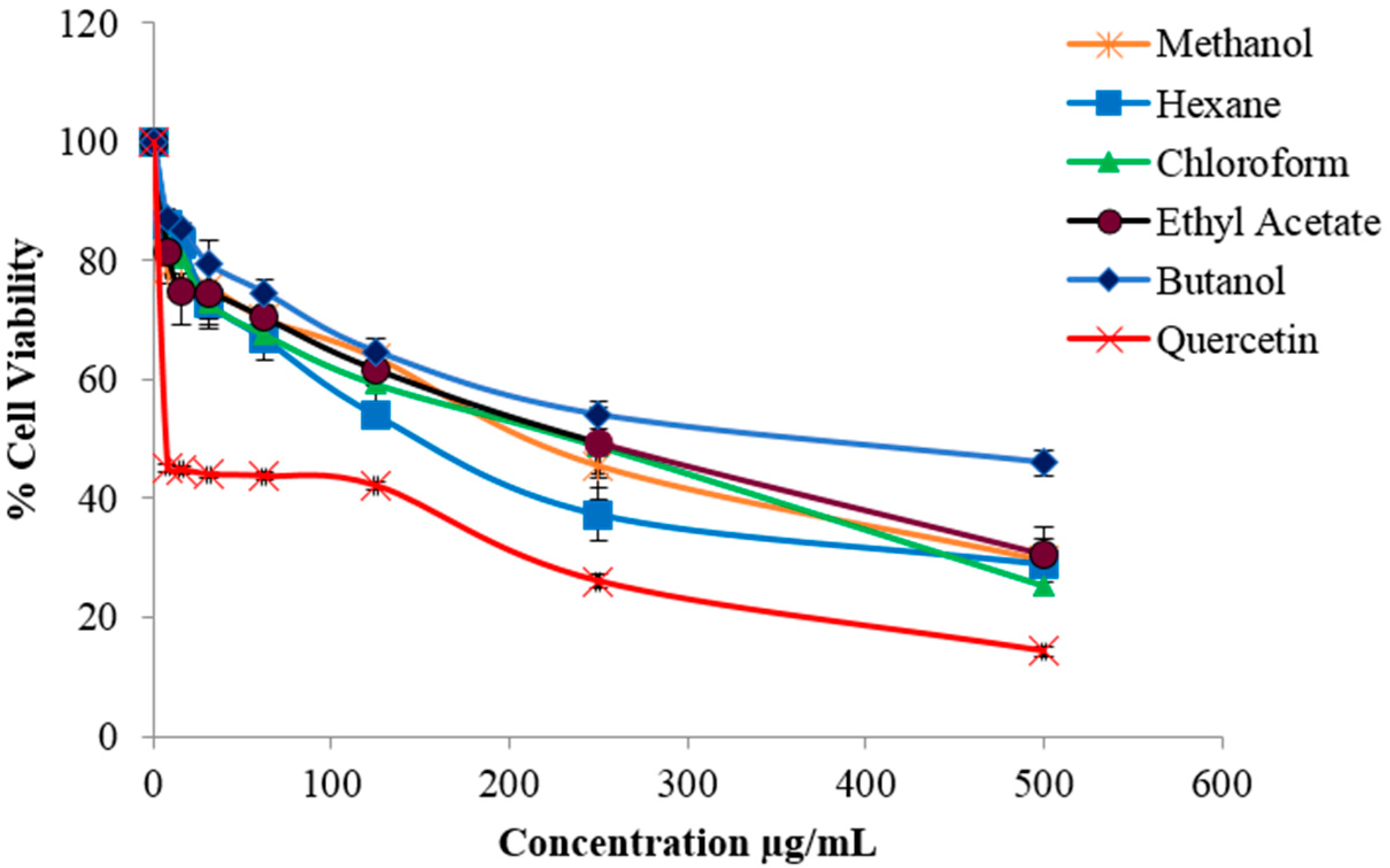
2.2. Evaluation of Cytotoxic Activity
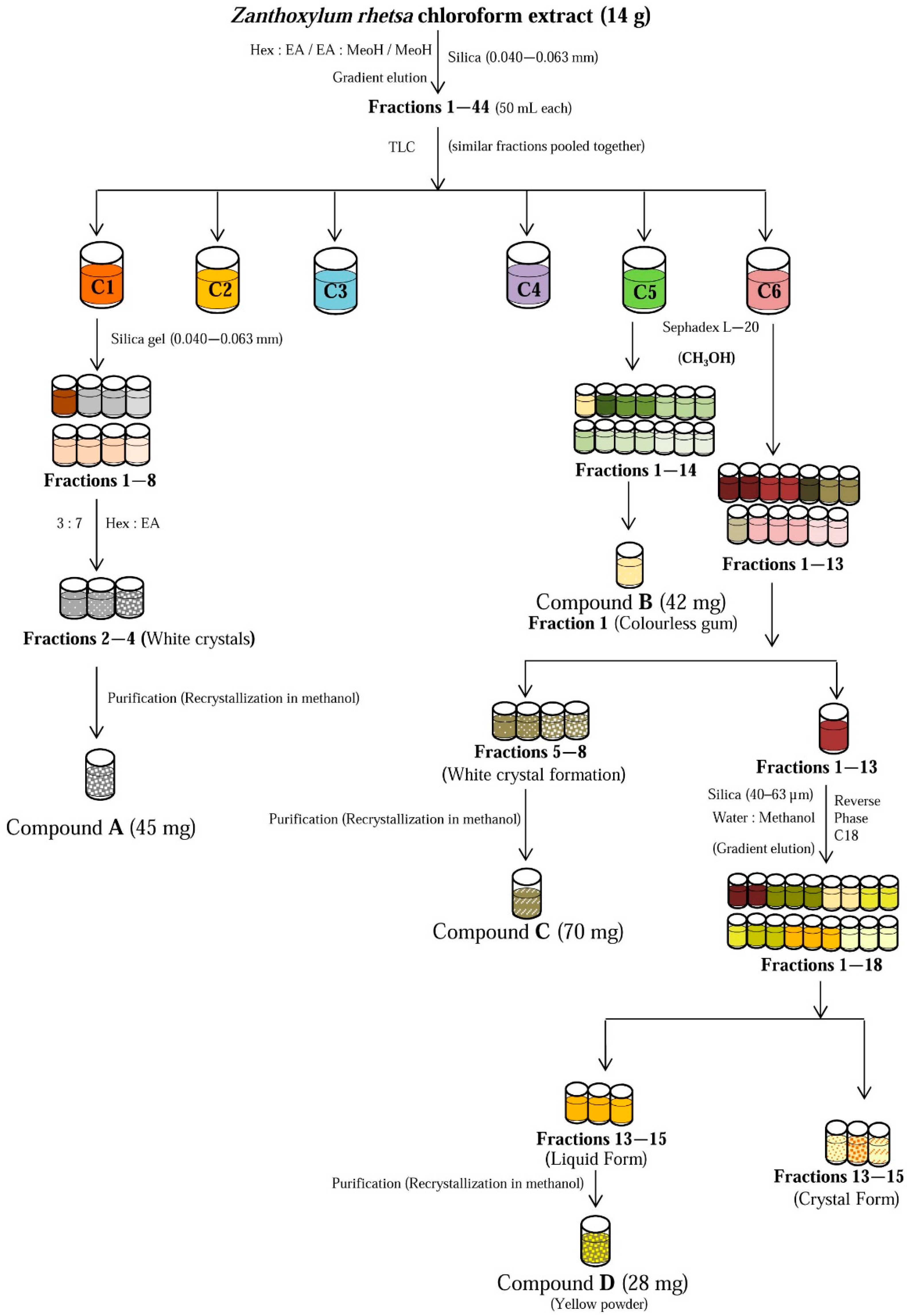
2.3. Structural Identification of the Isolated Compounds
2.4. Cytotoxicity Evaluation of Isolated Compounds
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Sample Collection
3.3. Extraction
3.4. Cell Culture
3.5. MTT Proliferation Assay
3.6. GC-MS Analysis of Solvent Fractions
3.7. Isolation of Chemical Constituents from the Bioactive Chloroform Fraction
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kolarsick, P.A.J.; Kolarsick, M.A.; Goodwin, C. Anatomy and physiology of the skin. J. Dermatol. Nurses Assoc. 2011, 3, 203–213. [Google Scholar] [CrossRef]
- The Skin Cancer Foundation. Available online: http://www.skincancer.org/skin-cancer-information/skin-cancer-facts#melanoma (accessed on 29 March 2016).
- Melanoma Skin Cancer. Available online: http://www.cancer.org/acs/groups/cid/documents/webcontent/003120-pdf.pdf/ (accessed on 1 November 2015).
- Skin Cancer Facts. Available online: http://www.cancer.org/cancer/cancercauses/sunanduvexposure/skin-cancer-facts/ (accessed on 18 May 2015).
- Melanoma of the skin statistics. Available online: http://canceraustralia.gov.au/affected-cancer/cancer-types/melanoma-skin/melanoma-skin-statistics/ (accessed on 10 October 2015).
- Hartley, T.G. A revision of the Malaysian species of Zanthoxylum (Rutaceae). J. Arnold Arboretum. 1970, 51, 423–426. [Google Scholar]
- Payum, T.; Das, A.K.; Shankar, R.; Tamuly, C.; Hazarika, M. Folk use and antioxidant potential determination of Zanthoxylum rhetsa DC. shoot-a highly utilized hot spice folk vegetable of Arunachal Pradesh, India. Int. J. Pharm. Sci. Res. 2013, 4, 1000–1005. [Google Scholar]
- Singh, A.; Singh, R.K.; Bharadwaj, R.; Singh, A.K. Adaptations of culturally and nutritionally important foods in Eastern Himalaya: A case study with Adi women of Arunachal Pradesh. Indian J. Tradit. Knowl. 2014, 11, 623–633. [Google Scholar]
- Nimachow, G.; Rawar, J.S.; Arunachalam, A.; Oyi, D. Ethno-medicines of Aka tribe, West Kameng District, Arunachal Pradesh (India). Sci. Cult. 2011, 77, 149–155. [Google Scholar]
- Patiño, L.O.J.; Prieto, R.J.A.; Cuca, S.L.E. Zanthoxylum Genus as Potential Source of Bioactive Compounds. In Bioactive Compounds in Phytomedicine; Rasooli, I., Ed.; InTech: Rijeka, Croatia, 2008; pp. 185–218. [Google Scholar]
- Ahsan, M.; Zaman, T.A.; Hasan, C.M.; Ito, C.; Islam, S.K.N. Constituents and cytotoxicity of Zanthoxylum rhesta stem bark. Fitoterapia 2000, 71, 697–700. [Google Scholar] [CrossRef]
- Tantapakul, C.; Phakhodee, W.; Ritthiwigrom, T.; Yossathera, K.; Deachathai, S.; Laphookhieo, S. Antibacterial compounds from Zanthoxylum rhetsa. Arch. Pharm. Res. 2012, 35, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.; Haque, M.R.; Hossain, M.B.; Islam, S.N.; Gray, A.I.; Hasan, C.M. Cytotoxic dimeric quinolone-terpene alkaloids from the root bark of Zanthoxylum rhetsa. Phytochemistry 2014, 103, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Lalitharani, S.; Kalpanadevi, V.; Mohan, V.R. Pharmacognostic studies on the spine of Zanthoxylum rhetsa (Roxb.) DC. Biosci. Discov. 2013, 4, 5–11. [Google Scholar]
- Shankaracharya, N.B.; Naik, J.P.; Nagalakshmi, S.; Rao, L.J.M. Chemical composition and flavour quality of tirphal Zanthoxylum rhetsa. PAFAI J. 1994, 16, 15–21. [Google Scholar]
- Shafi, P.M.; Saidutty, A.; Clery, R.A. Volatile Constituents of Zanthoxylum rhetsa Leaves and Seeds. J. Essent. Oil Res. 2000, 12, 179–182. [Google Scholar]
- Rana, V.S.; Blazquez, M.A. Volatile Constituents of the Seed Coat of Zanthoxylum rhetsa (Roxb.) DC. J. Essent. Oil Res. 2010, 22, 430–432. [Google Scholar] [CrossRef]
- Thu, N.B.; Trung, T.N.; Ha, D.T. Zanthoxylum rhetsa Stem bark extract inhibits LPS-induced COX-2 and iNOS expression in RAW 264.7 cells via the NF-κB inactivation. Nat. Prod. Sci. 2010, 16, 265–270. [Google Scholar]
- Kale, S.S.; Rajmane, A.H.; Urunkar, V.C.; Gaikwad, M.K.; Bhandare, S.B. Formulation and In-vitro Evaluation of Sun Protection Factor of Methanolic Extract of Zanthoxylum rhetsa DC Sunscreen lotion. Res. J. Pharmacogn. Phytochem. 2011, 3, 206–210. [Google Scholar]
- Santhanam, R.; Ahmad, S.; Abas, F.; Ismail, I.S.; Rukayadi, Y.; Shaari, K. Photoprotective properties of Zanthoxylum rhetsa: An in vitro analysis. J. Chem. Pharm. Res. 2013, 5, 1512–1520. [Google Scholar]
- Yang, C.H.; Cheng, M.J.; Lee, S.J.; Yang, C.W.; Chang, H.S.; Chen, I.S. Secondary metabolites and cytotoxic activities from the stem bark of Zanthoxylum nitidum. Chem. Biodivers. 2009, 6, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, Y.N.; Yan, X.T.; Yang, S.Y.; Kim, E.J.; Kang, H.K.; Kim, Y.H. Coumarins and lignans from Zanthoxylum schinifolium and their anticancer activities. J. Agric. Food Chem. 2013, 61, 10730–10740. [Google Scholar] [CrossRef] [PubMed]
- Gaya, C.H.; Kawaka, J.F.; Muchugi, A.; Ngeranwa, J.J. Variation of alkaloids in the Kenyan Zanthoxylum gilletii (De Wild Waterman). Afr. J. Plant Sci. 2013, 7, 438–444. [Google Scholar] [CrossRef]
- Mukhija, M.; Dhar, K.L.; Kalia, A.N. Bioactive Lignans from Zanthoxylum alatum Roxb. stem bark with cytotoxic potential. J. Ethnopharmacol. 2014, 152, 106–112. [Google Scholar] [PubMed]
- Kumar, V.; Kumar, S.; Singh, B.; Kumar, N. Quantitative and structural analysis of amides and lignans in Zanthoxylum armatum by UPLC-DAD-ESI-QTOF-MS/MS. J. Pharm. Biomed. Anal. 2014, 94, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Pelter, A. The mass spectra of oxygen heterocycles. Part IV. The mass spectra of some complex lignans. J. Chem. Soc. C Org. 1967. [Google Scholar] [CrossRef]
- Tonelli, F.M.P.; de Siqueira, J.M.; Mala, G.A.S.; Soares, L.F.; da Silva, D.B.; Carolla, C.A.; Sartori, A.L.B. Bioautography as a search tool to identify the allelopathic compounds in Virola sebifera. Allelopath J. 2014, 33, 277–288. [Google Scholar]
- Paik, S.Y.; Koh, K.H.; Beak, S.M.; Paek, S.H.; Kim, J.A. The essential oils from Zanthoxylum schinifolium pericarp induce apoptosis of HepG2 human hepatoma cells through increased production of reactive oxygen species. Biol. Pharm. Bull. 2005, 28, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Li, H.; Chen, Y.Y.; Zhang, Y.W.; Liu, N.; Zhang, Q.; Wu, Q. Essential oil from Zanthoxylum bungeanum Maxim. and its main components used as transdermal penetration enhancers: A comparative study. J. Zhejiang Univ. Sci B 2014, 15, 940–952. [Google Scholar] [PubMed]
- Su, G.Y.; Wang, K.W.; Wang, X.Y.; Wu, B. Bioactive lignans from Zanthoxylum planispinum with cytotoxic potential. Phytochem. Lett. 2015, 11, 120–126. [Google Scholar] [CrossRef]
- Jain, P.S.; Bari, S.B. Isolation of lupeol, stigmasterol and campesterol from petroleum ether extract of woody stem of Wrightia tinctoria. Asian J. Plant Sci. 2010, 9, 163–167. [Google Scholar] [CrossRef]
- Chang, S.W.; Kim, K.H.; Lee, I.K.; Choi, S.U.; Lee, K.R. Phytochemical Constituents of Geranium eriostemon. Nat. Prod. Sci. 2009, 15, 151–155. [Google Scholar]
- Kim, J.Y.; Lim, H.J.; Lee, D.Y.; Kim, J.S.; Kim, D.H.; Lee, H.J.; Kim, H.D.; Jeon, R.; Ryu, J.H. In vitro anti-inflammatory activity of lignans isolated from Magnolia fargesii. Bioorg. Med. Chem. Lett. 2009, 19, 937–940. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.J.; Chia, Y.C.; Wu, Y.C.; Chen, C.Y. Chemical Constituents from the Stems of Mahonia Japonica. J. Chin. Chem. Soc. 2004, 51, 443–446. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.A.; Khondkar, P.; Gray, A.I. Alkaloids and lignans from Zanthoxylum budrunga (Rutaceae). Biochem. Syst. Ecol. 2005, 33, 91–96. [Google Scholar] [CrossRef]
- Stermitz, F.R.; Sharifi, I.A. Alkaloids of Zanthoxylum monophyllum and Zanthoxylum punctatum. Phytochemistry 1977, 16, 2003–2006. [Google Scholar] [CrossRef]
- Sreelekha, M.; Anto, N.P.; Anto, R.J.; Shafi, R.J. Cytotoxicity of 6-acetonyldihydro-chelerythrin, arnottianamide and 6-(2-hydroxypropyl)-dihydrochelerythrine towards human cancer cell lines. Indian J. Chem. Sect. B 2014, 53, 647–651. [Google Scholar]
- Zhang, L.L.; Ma, L.N.; Yan, D.; Zhang, C.E.; Gao, D.; Xiong, Y.; Sheng, F.Y.; Dong, X.P.; Xiao, X.H. Dynamic monitoring of the cytotoxic effects of protoberberine alkaloids from Rhizoma coptidis on HepG2 cells using the xCELLigence system. Chin. J. Nat. Med. 2014, 12, 428–435. [Google Scholar] [CrossRef]
- Badami, S.; Vijayan, P.; Mathew, N.; Chandrasekhar, R.; Godavarthi, A.; Dhanaraj, S.A.; Suresh, B. In vitro cytotoxic properties of Grewia tiliaefolia bark and lupeol. Indian J. Pharmacol. 2003, 35, 250–251. [Google Scholar]
- Saleem, M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009, 285, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Park, S.H.; Seo, D.H.; Lee, J.H.; Hong, S.W.; Hong, S.S. Antioxidative and skin-whitening effect of an aqueous extract of Salicornia herbacea. Biosci. Biotechnol. Biochem. 2009, 73, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Iwashita, K.; Kobori, M.; Yamaki, K.; Tsushida, T. Flavonoids inhibit cell growth and induce apoptosis in B16 melanoma 4A5 cells. Biosci. Biotechnol. Biochem. 2000, 64, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Chinembirim, T.N.; du Plessis, L.H.; Gerber, M.; Hamman, J.H.; du Plessis, J. Review of natural compounds for potential skin cancer treatment. Molecules 2014, 19, 11679–11721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albert, A. Fundamental aspects of selective toxicity. Ann. N. Y. Acad. Sci. 1965, 123, 5–18. [Google Scholar] [CrossRef]
- Milajerdi, A.; Djafarian, K.; Hosseini, B. The toxicity of saffron (Crocus sativus L.) and its constituents against normal and cancer cells. J. Nutr. Intermed. Metab. 2016, 3, 23–32. [Google Scholar] [CrossRef]
- Sandjo, L.P.; Kuete, V.; Tchangna, R.S.; Efferth, T.; Ngadjui, B.T. Cytotoxic Benzophenanthridine and Furoquinoline Alkaloids from Zanthoxylum buesgenii (Rutaceae). Chem. Cent. J. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Jang, Y.-O.; Kim, B.-T.; Hwang, K.-J.; Lee, J.-C. Induction of caspase-dependent apoptosis in melanoma cells by the synthetic compound (E)-1-(3,4-dihydroxyphenethyl)-3-styrylurea. BMB Rep. 2009, 42, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Pure compounds not available, solvent fractions are available from the authors.


| Sample | Compound Name | Retention Time | MW | m/z Fragments | Area (%) |
|---|---|---|---|---|---|
| Methanol crude extract | 2-Undecanone | 24.33 | 170 | 58, 38, 43, 71 | 1.984 |
| Coniferyl alcohol | 43.672 | 180 | 137, 124, 91 | 0.936 | |
| Scoparone | 52.2 | 206 | 191, 178, 163, 131 | 4.104 | |
| Reticuline (+) | 75.2 | 329 | 192,177 | 5.111 | |
| Allocryptopine | 80.9 | 369 | 283, 206, 164 | 2.038 | |
| Unknown | 81.38 | 343 | 58 | 13.87 | |
| epi-Eudesmin | 82.787 | 386 | 177, 165, 151, 107 | 2.018 | |
| Sesamin | 84.25 | 354 | 323, 203, 161, 149 | 3.731 | |
| Kobusin | 85.052 | 370 | 339, 219, 203, 165, 149 | 3.488 | |
| Eudesmin | 85.823 | 386 | 355, 219, 165,151 | 17.57 | |
| 8-Hydroxy-4′-methoxy-pinoresinol | 86.502 | 388 | 357, 339, 208, 165,151 | 0.642 | |
| β-Amyrin | 87.8 | 426 | 218, 203, 189 | 1.197 | |
| Mangnolin | 88.25 | 416 | 219, 207, 195, 165, 151 | 5.337 | |
| Lupeol | 88.91 | 426 | 411, 315, 218, 207,189 | 13.74 | |
| Yangambin | 90.65 | 446 | 415, 224,195,181 | 24.24 | |
| Hexane | 2-Undecanone | 24.35 | 170 | 71, 38, 43 | 13.11 |
| 2-Tridecanone | 33.373 | 198 | 71, 58, 43 | 2.662 | |
| Farnesol | 42.26 | 222 | 136, 93, 81, 69 | 9.583 | |
| Hexadecanoic acid | 49.7 | 270 | 227, 143, 87, 74, 57 | 2.1 | |
| 9-Octadecenoic acid-(Z)-methyl ester | 55.47 | 296 | 264, 222, 180, 97, 55 | 1.349 | |
| Germacrene | 83.66 | 204 | 189, 161, 121, 107, 69 | 1.33 | |
| Sesamin | 84.25 | 354 | 323, 219, 203, 149 | 3.622 | |
| Kobusin | 85.065 | 370 | 339, 219, 203, 165, 149 | 2.342 | |
| Eudesmin | 85.825 | 386 | 355, 219, 165, 151 | 8.488 | |
| Stigmast-5-en-3-ol | 86.75 | 414 | 396, 381, 329, 213, 107 | 0.735 | |
| 9,12-Octadecadienoic acid | 87.4 | 279 | 204, 189, 161, 136, 121, 69 | 4.182 | |
| β-Amyrin | 87.82 | 426 | 218, 203, 189 | 5.597 | |
| Mangnolin | 88.26 | 416 | 219, 207, 195, 177, 165 | 2.304 | |
| Lupeol | 89.03 | 426 | 411, 315, 218, 207, 189 | 36.94 | |
| Yangambin | 90.65 | 446 | 415, 224, 195, 181 | 5.659 | |
| Chloroform | Scoparone | 52.27 | 206 | 191, 178, 163 , 135 | 7.343 |
| Sinapyl alcohol | 52.69 | 210 | 182, 167, 149,107 | 1.468 | |
| Allocryptopine | 80.922 | 369 | 283, 206, 164, 149 | 1.717 | |
| Usambanoline | 82.85 | 386 | 204, 189, 151 | 3.822 | |
| Sesamin | 84.31 | 354 | 323, 203, 161, 149 | 5.913 | |
| Unknown | 84.51 | 506 | 181,182, 151 | 2.13 | |
| Dihydronitidine | 84.67 | 349 | 333, 304, 290, 204, 149 | 0.401 | |
| Kobusin | 85.14 | 370 | 339, 219, 203, 165, 149 | 6.04 | |
| Epi-eudesmin | 85.52 | 386 | 372, 219, 194, 165, 151 | 0.356 | |
| Eudesmin | 85.96 | 386 | 355, 219, 194, 177, 165, 151 | 26.75 | |
| 8-Hydroxy-4′-methoxy-pinoresinol | 86.56 | 388 | 357, 339, 208, 194, 165, 151 | 1.398 | |
| Mangnolin | 88.37 | 416 | 385, 219 , 207, 195, 177, 165, 151 | 8.118 | |
| Lupeol | 88.91 | 426 | 411, 315, 218, 207,189 | 3.55 | |
| Yangambin | 90.82 | 446 | 415, 224,195,181 | 30.38 | |
| Ethyl acetate | 2,2-Dimethoxybutane | 3.475 | 118 | 103. 87, 55 | 1.091 |
| 4-vinylsyringol | 36.521 | 180 | 165, 137 122 | 6.444 | |
| Unknown | 38.86 | - | 143, 95,59 | 1.258 | |
| Coniferyl alcohol | 43.645 | 180 | 137, 124, 91 | 24.172 | |
| Butyl 4-hydroxybenzoate | 48.66 | 194 | 138, 121 | 2.818 | |
| Benzeneacetic acid | 51.42 | 166 | 107, 69 | 1.745 | |
| Unknown | 51.88 | 223 | 179, 123, 81 | 0.797 | |
| Sinapyl alcohol | 52.6 | 210 | 182, 167, 149, 121 | 7.073 | |
| 4-[(6,7-Dimethoxy-1,2,3,4-tetrahydro-1-isoquinolinyl)methyl]phenol | 53.52 | 299 | 213 | 1.88 | |
| Quinic acid | 59.44 | 191 | 123,107, 69 | 1.249 | |
| Hesperetin | 80.54 | 302 | 179, 150, 137 | 1.374 | |
| Allocryptopine | 80.79 | 369 | 283, 206, 164, 149 | 5.706 | |
| Sesamin | 84.1 | 354 | 203,149,161, 121, 103 | 1.63 | |
| Dihydronitidine | 84.52 | 349 | 332, 290, 102, 204, 167 | 6.43 | |
| Kobusin | 84.89 | 370 | 219, 203,177, 165, 149 | 3.041 | |
| Eudesmin | 85.59 | 386 | 189,165, 151, | 10.778 | |
| Mangnolin | 88.07 | 416 | 219, 207, 195, 165, 151 | 5.125 | |
| Lupeol | 88.65 | 426 | 412, 315, 218, 207,189 | 3.187 | |
| Unknown | 90.01 | 388 | 226, 207, 193, 181 | 1.819 | |
| Yangambin | 90.38 | 446 | 415, 224,195,181 | 12.383 | |
| Butanol | 1-Butanol | 2.62 | 74 | 56, 43 | 41.59 |
| 2-Butoxyethanol | 7.02 | 118 | 87, 57 | 1.394 | |
| 4-vinylsyringol | 36.588 | 180 | 165, 137, 122 | 1.007 | |
| Coniferyl alcohol | 43.69 | 180 | 137, 124, 109 | 0.815 | |
| Sinapyl alcohol | 52.673 | 210 | 182, 167, 121, 107 | 0.565 | |
| (−)-1,2,3,4-Tetrahydro-isoquinolin-6-ol-1-carboxylic acid | 72.31 | 192 | 177, 148, | 2.142 | |
| Unknown | 74.678 | 343 | 58 | 3.548 | |
| (+)-Reticuline | 75.27 | 329 | 192, 177 | 14.04 | |
| Unknown | 76.69 | 327 | 58 | 1.993 | |
| Unknown | 78.26 | 313 | 58 | 3.885 | |
| Allocryptopine | 80.94 | 369 | 283, 206, 164, 149 | 3.029 | |
| N-Methyllaurotetanine | 81.165 | 341 | 326, 310, 206, 164 | 0.152 | |
| Unknown | 91.6 | 343 | - | 25.01 | |
| Nitidine | 84.653 | 349 | 332,304, 290 | 0.208 | |
| Chelerythrine | 85.687 | 350 | 349, 332, 304 | 0.618 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santhanam, R.K.; Ahmad, S.; Abas, F.; Safinar Ismail, I.; Rukayadi, Y.; Tayyab Akhtar, M.; Shaari, K. Bioactive Constituents of Zanthoxylum rhetsa Bark and Its Cytotoxic Potential against B16-F10 Melanoma Cancer and Normal Human Dermal Fibroblast (HDF) Cell Lines. Molecules 2016, 21, 652. https://doi.org/10.3390/molecules21060652
Santhanam RK, Ahmad S, Abas F, Safinar Ismail I, Rukayadi Y, Tayyab Akhtar M, Shaari K. Bioactive Constituents of Zanthoxylum rhetsa Bark and Its Cytotoxic Potential against B16-F10 Melanoma Cancer and Normal Human Dermal Fibroblast (HDF) Cell Lines. Molecules. 2016; 21(6):652. https://doi.org/10.3390/molecules21060652
Chicago/Turabian StyleSanthanam, Ramesh Kumar, Syahida Ahmad, Faridah Abas, Intan Safinar Ismail, Yaya Rukayadi, Muhammad Tayyab Akhtar, and Khozirah Shaari. 2016. "Bioactive Constituents of Zanthoxylum rhetsa Bark and Its Cytotoxic Potential against B16-F10 Melanoma Cancer and Normal Human Dermal Fibroblast (HDF) Cell Lines" Molecules 21, no. 6: 652. https://doi.org/10.3390/molecules21060652