Role of Plant Growth Promoting Rhizobacteria in Agricultural Sustainability—A Review
Abstract
:1. Introduction
2. Plant Growth Promoting Rhizobacteria
3. Role of Plant Growth Promoting Rhizobacteria for Plant Growth Enhancement
3.1. Abiotic Stress Tolerance in Plants
3.2. Nutrient Availability for Plant Uptake
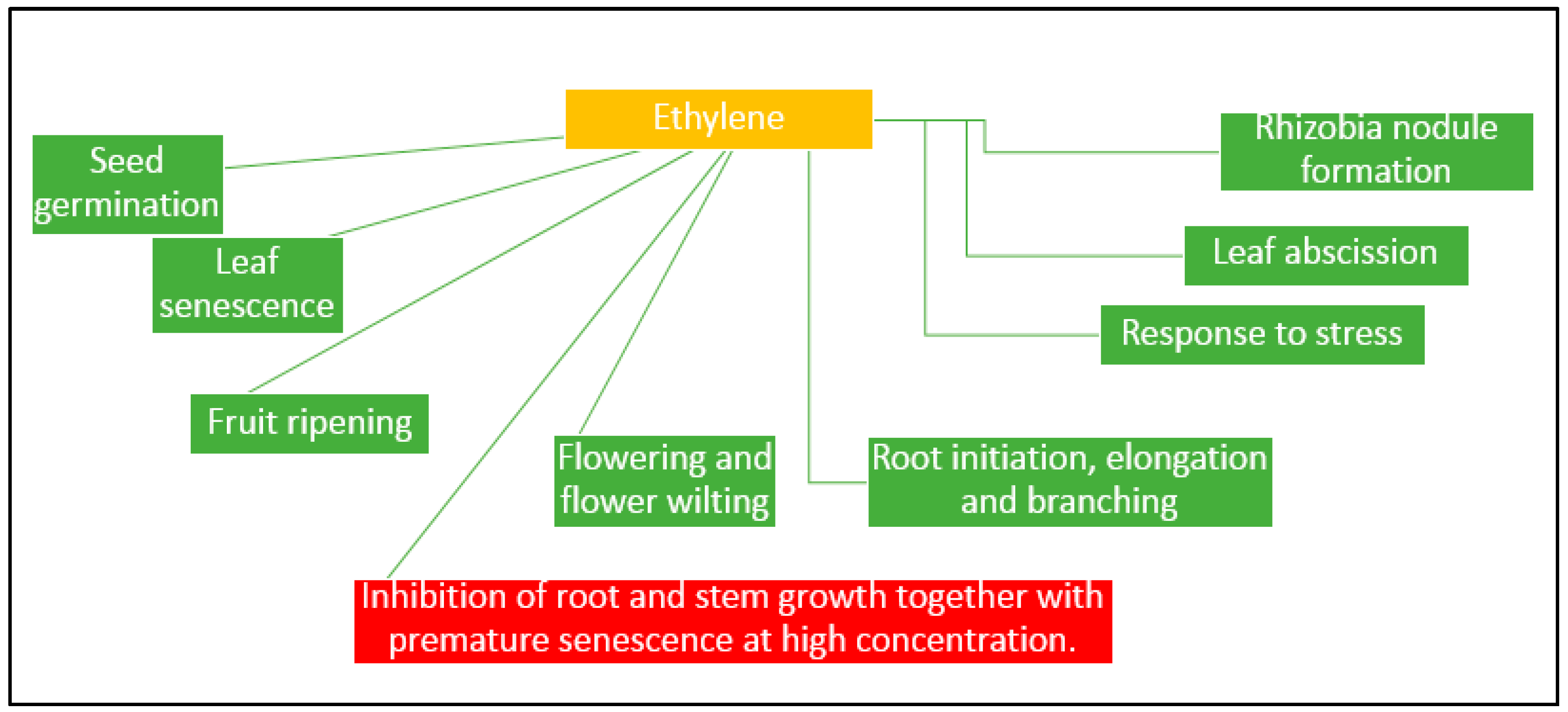
3.3. Plant Growth Regulators
3.4. Production of Hormones
3.5. Production of Siderophores
3.6. Production of Volatile Organic Compound
3.7. Production of Enzymes
4. Beneficial and Harmful Aspects of Plant Growth Promoting Rhizobacteria
5. Role of Plant Growth Promoting Rhizobacteria as a Biofertilizer
6. Role of Nanotechnology for Agricultural Sustainability
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Draft U.S. Greenhouse Gas Inventory Report: 1990–2014. Available online: https://www3.epa.gov/climatechange/ghgemissions/usinventoryreport.html (accessed on 23 March 2016).
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B 2013, 368. [Google Scholar] [CrossRef] [PubMed]
- Armada, E.; Portela, G.; Roldan, A.; Azcon, R. Combined use of beneficial soil microorganism and agrowaste residue to cope with plant water limitation under semiarid conditions. Geoderma 2014, 232, 640–648. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.M.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications; Hindawi Publishing Corporation, Scientifica: Waterloo, Canada, 2012. [Google Scholar]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.-P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). (A Marschner Review). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Martinez-Viveros, O.; Jorquera, M.A.; Crowley, D.E.; Gajardo, G.; Mora, M.L. Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J. Soil Sci. Plant Nutr. 2010, 10, 293–319. [Google Scholar] [CrossRef]
- Dey, R.; Pal, K.K.; Bhatt, D.M.; Chauhan, S.M. Growth promotion and yield enhancement of peanut (Arachis hypogeal L.) by application of plant growth-promoting rhizobacteria. Microbiol. Res. 2004, 159, 371–394. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, D.K.; Sharma, K.P.; Gaur, R.K. Biotechnological perspectives of microbes in agro-ecosystems. Biotechnol. Lett. 2011, 33, 1905–1910. [Google Scholar] [CrossRef] [PubMed]
- Nakkeeran, S.; Fernando, W.G.D.; Siddiqui, Z.A. Plant growth promoting rhizobacteria formulations and its scope in commercialization for the management of pests and dideases. In PGPR: Biocontrol and Biofertilization; Siddiqui, Z.A., Ed.; Springer: Dordrecht, The Netherlands, 2005; pp. 257–296. [Google Scholar]
- Ahmad, F.; Ahmad, I.; Khan, M.S. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol. Res. 2008, 163, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Burdman, S.; Jurkevitch, E.; Okon, Y. Recent advances in the use of plant growth promoting rhizobacteria (PGPR) in agriculture. In Microbial Interactions in Agriculture and Forestry; Subba Rao, N.S., Dommergues, Y.R., Eds.; Science Publishers: Enfield, NH, USA, 2000; pp. 229–250. [Google Scholar]
- Weller, D.M.; Thomashow, L.S. Current challenges in introducing beneficial microorganisms into the rhizosphere. In Molecular Ecology of Rhizosphere Microorganisms: Biotechnology and Release of GMOs; O’Gara, F., Dowling, D.N., Boesten, B., Eds.; VCH: New York, NY, USA, 1994; pp. 1–18. [Google Scholar]
- Kaymak, D.C. Potential of PGPR in agricultural innovations. In Plant Growth and Health Promoting Bacteria; Maheshwari, D.K., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Saharan, B.S.; Nehra, V. Plant growth promoting rhizobacteria: A critical review. Life Sci. Med. Res. 2011, 21, 1–30. [Google Scholar]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. Wood J. Microb. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.W.; Schroth, M.N. Plant growth-promoting rhizobacteria on radishes. In Station de Pathologie, Proceedings of the 4th International Conference on Plant Pathogenic Bacteria, Tours, France, 27 August–2 September 1978; Végétale et Phyto-Bactériologie, Ed.; pp. 879–882.
- Kloepper, J.W.; Leong, J.; Teintze, M.; Schroth, M.N. Enhanced plant growth by siderophores produced by plant growth promoting rhizobacteria. Nature 1980, 286, 885–886. [Google Scholar]
- Son, J.S.; Sumayo, M.; Hwang, Y.J.; Kim, B.S.; Ghim, S.Y. Screening of plant growth promoting rhizobacteria as elicitor of systemic resistance against grey leaf spot dieses in pepper. Appl. Soil Ecol. 2014, 73, 1–8. [Google Scholar] [CrossRef]
- Gray, E.J.; Smith, D.L. Intracellular and extracellular PGPR: Commonalities and distinctions in the plant-bacterium signaling processes. Soil Biol. Biochem. 2005, 37, 395–412. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Lugtenberg, B. Use of Plant Growth-Promoting Rhizobacteria to Alleviate Salinity Stress in Plants. In Use of Microbes for the Alleviation of Soil Stresses; Springer: New York, NY, USA, 2014; Volume 1, pp. 73–96. [Google Scholar]
- García-Fraile, P.; Menéndez, E.; Rivas, R. Role of bacterial biofertilizers in agriculture and forestry. AIMS Bioeng. 2015, 2, 183–205. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Zahir, Z.A.; Naveed, M.; Ashraf, M. Microbial ACC-deaminase; prospects and applications for inducing salt tolerance in plants. Crit. Rev Plant Sci. 2010, 29, 360–393. [Google Scholar] [CrossRef]
- Pishchik, V.N.; Vorobyev, N.J.; Chernyaeva, L.I.; Timofeeva, S.V.; Kazhemyakov, A.P.; Alexeev, Y.V. Experimental and mathematical simulation of plant growth promoting rhizobacteria and plant interaction under cadmium stress. Plant Soil 2002, 243, 173–186. [Google Scholar] [CrossRef]
- Nautiyal, C.S.; Govindarajan, R.; Lavania, M.; Pushpangadan, P. Novel mechanisms of modulating natural antioxidants in functional foods: Involvement of plant growth promoting rhizobacteria NRRL B-30488. J. Agric. Food Chem. 2008, 56, 4474–4481. [Google Scholar] [CrossRef] [PubMed]
- Reinhold-Hurek, B.; Hurek, T. Interactions of gramineous plants with Azoarcus spp. and other diazotrophs: Identification, localization, and perspectives to study their function. Crit. Rev. Plant Sci. 1998, 17, 29–54. [Google Scholar] [CrossRef]
- Aloni, R.; Aloni, E.; Langhans, M. Role of cytokinin and auxin in shaping root architecture: Regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann. Bot. 2006, 97, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Sabry, S.R.S.; Saleh, S.A.; Batchelor, C.A. Endophytic establishment of Azorhizobium caulinodans in wheat. Proc. Biol. Sci. 1997, 264, 341–346. [Google Scholar] [CrossRef]
- De Felipe, M.R. Fijación biológica de dinitrógeno atmosférico en vida libre. In Fijación de Nitrógeno: Fundamentos y Aplicaciones. Granada: Sociedad Española de Microbiología; Bedmar, E., Gonzálo, J., Lluch, C., et al., Eds.; Sociedad Española de Fijación de Nitrógeno: Granada, Spain, 2006; pp. 9–16. [Google Scholar]
- Tejera, N.; Lluch, C.; Martínez-Toledo, M.V. Isolation and characterization of Azotobacter and Azospirillum strains from the sugarcane rhizosphere. Plant Soil 2005, 270, 223–232. [Google Scholar] [CrossRef]
- Sahoo, R.K.; Ansari, M.W.; Pradhan, M. Phenotypic and molecular characterization of native Azospirillum strains from rice fields to improve crop productivity. Protoplasma 2014, 251, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Berg, R.H.; Tyler, M.E.; Novick, N.J. Biology of Azospirillum-sugarcane association: Enhancement of nitrogenase activity. Appl. Environ. Microbiol. 1980, 39, 642–649. [Google Scholar] [PubMed]
- Wani, S.A.; Chand, S.; Ali, T. Potential Use of Azotobacter Chroococcum in Crop Production: An Overview. Curr. Agric. Res. J. 2013, 1, 35–38. [Google Scholar] [CrossRef]
- Ahmed, A.; Hasnain, S. Auxin producing Bacillus sp.: Auxin quantification and effect on the growth Solanum tuberosum. Pure Appl. Chem. 2010, 82, 313–319. [Google Scholar] [CrossRef]
- Sokolova, M.G.; Akimova, G.P.; Vaishlia, O.B. Effect of phytohormones synthesized by rhizosphere bacteria on plants. Prikl Biokhim Mikrobiol 2011, 47, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Joo, G.J.; Kim, Y.M.; Kim, J.T. Gibberellins-producing rhizobacteria increase endogenous gibberellins content and promote growth of red peppers. J. Microbiol. 2005, 43, 510–515. [Google Scholar] [PubMed]
- Han, H.S.; Lee, K.D. Phosphate and Potassium Solubilizing Bacteria Effect on Mineral Uptake, Soil Availability and Growth of Eggplant. Res. J. Agric. Biol. Sci. 2005, 1, 176–180. [Google Scholar]
- Han, H.S.; Supanjani, S.; Lee, K.D. Effect of co-inoculation with phosphate and potassium solubilizing bacteria on mineral uptake and growth of pepper and cucumber. Plant Soil Environ. 2006, 52, 130–136. [Google Scholar]
- Egamberdiyeva, D. The effect of plant growth promoting bacteria on growth and nutrient uptake of maize in two different soils. Appl. Soil Ecol. 2007, 36, 184–189. [Google Scholar] [CrossRef]
- El-Akhal, M.R.; Rincon, A.; Coba de la Peña, T.; Lucas, M.M.; El Mourabit, N.; Barrijal, S.; Pueyo, J.J. Effects of salt stress and rhizobial inoculation on growth and nitrogen fixation of three peanut cultivars. Plant Biol. 2013, 15, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Silo-Suh, L.A.; Lethbridge, B.J.; Raffel, S.J. Biological activities of two fungistatic antibiotics produced by Bacillus cereus UW85. Appl. Environ. Microbiol. 1994, 60, 2023–2030. [Google Scholar] [PubMed]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Dobereiner, J. Nitrogen-fixing bacteria of the genus Beijerinckia Derx in the rhizosphere of sugar cane. Plant Soil 1961, 15, 211–216. [Google Scholar] [CrossRef]
- Govindarajan, M.; Balandreau, J.; Kwon, S.W. Effects of the inoculation of Burkholderia vietnamensis and related endophytic diazotrophic bacteria on grain yield of rice. Microb. Ecol. 2007, 55, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.M.; Chen, S.C.; Chen, Y.S. Detection of Burkholderia pseudomallei in rice fields with PCR-based technique. Folia Microbiol. 2003, 48, 521–524. [Google Scholar] [CrossRef]
- Radzki, W.; Gutierrez Manero, F.J.; Algar, E. Bacterial siderophores efficiently provide iron to iron-starved tomato plants in hydroponics culture. Antonie Van Leeuwenhoek 2013, 104, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Simonet, P.; Normand, P.; Moiroud, A. Identification of Frankia strains in nodules by hybridization of polymerase chain reaction products with strain-specific oligonucleotide probes. Arch. Microb. 1990, 153, 235–240. [Google Scholar] [CrossRef]
- Muñoz-Rojas, J.; Caballero-Mellado, J. Population dynamics of Gluconacetobacter diazotrophicus in sugarcane cultivars and its effect on plant growth. Microb. Ecol. 2003, 46, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Elbeltagy, A.; Nishioka, K.; Sato, T. Endophytic colonization and in planta nitrogen fixation by a Herbaspirillum sp. isolated from wild rice species. Appl. Environ. Microbiol. 2001, 67, 5285–5293. [Google Scholar] [CrossRef] [PubMed]
- Bent, E.; Tuzun, S.; Chanway, C.P. Alterations in plant growth and in root hormone levels of lodgepole pines inoculated with rhizobacteria. Can. J. Microbiol. 2001, 47, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Sangeeth, K.P.; Bhai, R.S.; Srinivasan, V. Paenibacillus glucanolyticus, a promising potassium solubilizing bacterium isolated from black pepper (Piper nigrum L.) rhizosphere. J. Spices Aromat. Crops 2012, 21, 118–124. [Google Scholar]
- Flores-Felix, J.D.; Silva, L.R.; Rivera, L.P. Plants probiotics as a tool to produce highly functional fruits: The case of Phyllobacterium and vitamin C in strawberries. PLoS ONE 2015, 10, e0122281. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.K.; Khare, E.; Oh, J.H. Diverse mechanisms adopted by Pseudomonas fluorescent PGC2 during the inhibition of Rhizoctonia solani and Phytophthora capsici. World J. Microbiol. Biotechnol. 2008, 24, 581–585. [Google Scholar] [CrossRef]
- Ahmad, M.; Zahir, Z.A.; Khalid, M. Efficacy of Rhizobium and Pseudomonas strains to improve physiology, ionic balance and quality of mung bean under salt-affected conditions on farmer’s fields. Plant Physiol. Biochem. 2013, 63, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Shaharoona, B.; Naveed, M.; Arshad, M. Fertilizer-dependent efficiency of Pseudomonas for improving growth, yield, and nutrient use efficiency of wheat (Triticum aestivum L.). Appl. Microbiol. Biotechnol. 2008, 79, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Wu, Z.; Zheng, Y. Growth promotion and protection against salt stress by Pseudomonas putida Rs-198 on cotton. Eur. J. Soil Biol. 2010, 46, 49–54. [Google Scholar] [CrossRef]
- Mazzola, M.; Fujimoto, D.K.; Thomashow, L.S. Variation in Sensitivity of Gaeumannomyces graminis to Antibiotics Produced by Fluorescent Pseudomonas spp. and Effect on Biological Control of Take-All of Wheat. Appl. Environ. Microbiol. 1995, 61, 2554–2559. [Google Scholar] [PubMed]
- Kumar, H.; Bajpai, V.K.; Dubey, R.C. Wilt disease management and enhancement of growth and yield of Cajanus cajan (L) var. Manak by bacterial combinations amended with chemical fertilizer. Crop Protect. 2010, 29, 591–598. [Google Scholar] [CrossRef]
- Young, J.P.W.; Haukka, K.E. Diversity and phylogeny of rhizobia. New Phytol. 1996, 133, 87–94. [Google Scholar]
- Thamer, S.; Schädler, M.; Bonte, D. Dual benefit from a belowground symbiosis: Nitrogen fixing rhizobia promote growth and defense against a specialist herbivore in a cyanogenic plant. Plant Soil 2011, 341, 209–219. [Google Scholar] [CrossRef]
- Yanni, Y.; Rizk, R.; Abd-El Fattah, F. The beneficial plant growth-promoting association of Rhizobium leguminosarum bv. trifolii with rice roots. Aust. J. Plant Physiol. 2001, 28, 845–870. [Google Scholar]
- Garcia-Fraile, P.; Carro, L.; Robledo, M. Rhizobium promotes non-legumes growth and quality in several production steps: Towards a biofertilization of edible raw vegetables healthy for humans. PLoS ONE 2012, 7, e38122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores-Felix, J.D.; Menendez, E.; Rivera, L.P. Use of Rhizobium leguminosarum as a otential biofertilizer for Lactuca sativa and Daucus carota crops. J. Plant Nutr. Soil Sci. 2013, 176, 876–882. [Google Scholar] [CrossRef]
- Khan, A.L.; Waqas, M.; Kang, S.M. Bacterial endophyte Sphingomonas sp. LK11 produces gibberellins and IAA and promotes tomato plant growth. J. Microbiol. 2014, 52, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.C.; Singh, S.K.; Prakash, S. Bio-control and plant growth promotion potential of siderophore producing endophytic Streptomyces from Azadirachta indica A. Juss. J. Basic Microb. 2011, 51, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hussain, M.B.; Zahir, Z.A.; Mitter, B.; Sessitsch, A. Drought stress amelioration in wheat through inoculation with Burkholderia phytofirmans strain PsJN. Plant Growth Regul. 2014, 73, 121–131. [Google Scholar] [CrossRef]
- Sarma, R.K.; Saikia, R.R. Alleviation of drought stress in mung bean by strain Pseudomonas aeruginosa GGRK21. Plant Soils 2014, 377, 111–126. [Google Scholar] [CrossRef]
- Marulanda, A.; Azcón, R.; Chaumont, F.; Ruiz-Lozano, J.M.; Aroca, R. Regulation of plasma membrane aquaporins by inoculation with a Bacillus megaterium strain in maize (Zea mays L.) plants under unstressed and salt-stressed conditions. Planta 2010, 232, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Gond, S.K.; Bergen, M.S.; Torres, M.S.; White, J.F.; Kharwar, R.N. Effect of bacterial endophyte on expression of defense genes in Indian popcorn against Fusarium moniliforme. Symbiobosis 2015. [Google Scholar] [CrossRef]
- Gonzalez, A.J.; Larraburu, E.E.; Llorente, B.E. Azospirillum brasilense increased salt tolerance of jojoba during in vitro rooting. Ind. Crop Prod. 2015, 76, 41–48. [Google Scholar] [CrossRef]
- Gabriela, F.; Casanovas, E.M.; Quillehauquy, V.; Yommi, A.K.; Goni, M.G.; Roura, S.I.; Barassi, C.A. Azospirillum inoculation effects on growth, product quality and storage life of lettuce plants grown under salt stress. Sci. Hortic. 2015, 195, 154–162. [Google Scholar]
- Lloret, L.; Martinez-Romero, E. Evolution and phylogeny of rhizobia. Rev. Latinoam. Microbiol. 2005, 47, 43–60. [Google Scholar] [PubMed]
- Raymond, J.; Siefert, J.L.; Staples, C.R. The natural history of nitrogen fixation. Mol. Biol. Evol. 2004, 21, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Wani, P.A.; Khan, M.S.; Zaidi, A. Synergistic effect of the inoculation with nitrogen-fixing and phosphate-solubilizing rhizobacteria on performance of field-grown chickpea. J. Plant Nutr. Soil Sci. 2007, 170, 283–287. [Google Scholar] [CrossRef]
- Goswami, D.; Pithwa, S.; Dhandhukia, P.; Thakker, J.N. Delineating Kocuria turfanensis 2M4 as a credible PGPR: A novel IAA producing bacteria isolated from saline desert. J. Plant Interact. 2014, 9, 566–576. [Google Scholar] [CrossRef]
- Lavakush, Y.J.; Verma, J.P.; Jaiswal, D.K.; Kumar, A. Evaluation of PGPR and different concentration of phosphorous level on plant growth, yield and nutrient content of rice (Oryza sativa). Ecol. Eng. 2014, 62, 123–128. [Google Scholar] [CrossRef]
- Lugtenberg, B.J.; Chin A-Woeng, T.F.; Bloemberg, G.V. Microbeeplant interactions: Principles and mechanisms. Antonie Van Leeuwenhoek 2002, 81, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Somers, E.; Vanderleyden, J.; Srinivasan, M. Rhizosphere bacterial signalling: A love parade beneath our feet. Crit. Rev. Microbiol. 2004, 30, 205–240. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, E. Regulation and root growth by plant hormones-roles for auxins and gibberellins. Crit. Rev. Plant Sci. 2005, 24, 249–265. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-acetic acid in microbial and microorganism-plant signaling. In FEMS Microbiology Reviews; Unden, F., Ed.; Blackwell Publishing Ltd.: New York, NY, USA, 2007; pp. 425–448. [Google Scholar]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.-L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D. Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottini, R.; Cassan, F.; Piccoli, P. Gibberellin production by bacteria and its involvement in plant growth promotion and yield increase. Appl. Microbiol. Biotechnol. 2004, 65, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Spaepen, S.; Vanderleyden, J. Auxin and plant-microbe interactions. Cold Spring Harbor Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Riefler, M.; Novak, O.; Strnad, M.; Schm€ulling, T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, rootdevelopment, and cytokinin metabolism. Plant Cell 2006, 18, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xing, S.; Ma, H. Cytokinin-producing, plant growth-promoting rhizobacteria that confer resistance to drought stress in Platycladus orientalis container seedlings. Appl. Microbiol. Biotechnol. 2013, 97, 9155–9164. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.S. The role of ethylene in flower senescene. Acta Hortic. 1981, 261, 157–169. [Google Scholar]
- Li, Q.; Saleh-Lakha, S.; Glick, B.R. The effect of native and ACC deaminasecontaining Azospirillum brasilense Cdl843 on the rooting of carnation cuttings. Can. J. Microbiol. 2005, 51, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Plant growth promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.B. Ethylene in root growth and development. In The Plant Hormone Ethylene; Matoo, A.K., Suttle, J.C., Eds.; CRC Press: Boca Raton, FL, USA, 1991; pp. 159–181. [Google Scholar]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Davies, P. (Ed.) Plant Hormones: Physiology, Biochemistry and Molecular Biology; Springer Science & Business Media: New York, NY, USA, 2013.
- Porcel, R.; Zamarreño, Á.M.; García-Mina, J.M.; Aroca, R. Involvement of plant endogenous ABA in Bacillus megaterium PGPR activity in tomato plants. BMC Plant Biol. 2014, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Kende, H.; Zeevaart, J. The Five “Classical” Plant Hormones. Plant Cell 1997, 9, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E. Photosynthesis: Physiological and ecological considerations. Plant Physiol. 2002, 9, 172–174. [Google Scholar]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Wei, H.X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef] [PubMed]
- Effmert, U.; Kalderás, J.; Warnke, R.; Piechulla, B. Volatilemediated interactions between bacteria and fungi in the soil. J. Chem. Ecol. 2012, 38, 665–703. [Google Scholar] [CrossRef] [PubMed]
- Kanchiswamy, C.N.; Malnoy, M.; Maffei, M.E. Chemical diversity of microbial volatiles and their potential for plant growth and productivity. Front. Plant Sci. 2015, 6, 151. [Google Scholar] [CrossRef] [PubMed]
- Bakker, A.W.; Schippers, P. Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp.-mediated plant growth-stimulation. Soil Biol. Biochem. 1987, 19, 451–457. [Google Scholar] [CrossRef]
- Xie, H.; Pasternak, J.J.; Glick, B.R. Isolation and characterization of mutants of the plant growth-promoting rhizobacterium Pseudomonas putida CR12–2 that overproduce indoleacetic acid. Curr. Microbiol 1996, 32, 67–71. [Google Scholar] [CrossRef]
- Vijayan, R.; Palaniappan, P.; Tongmin, S.A.; Padmanaban, E.; Natesan, M. Rhizobitoxine enhances nodulation by inhibiting Ethylene synthesis of Bradyrhizobium elkanii from Lespedeza species: Validation by homology modeling and molecular docking study. World J. Pharm. Pharm. Sci. 2013, 2, 4079–4094. [Google Scholar]
- Xiong, K.; Fuhrmann, J.J. Comparison of rhizobitoxine-induced inhibition of β-cystathionase from different bradyrhizobia and soybean genotypes. Plant Soil 1996, 186, 53–61. [Google Scholar] [CrossRef]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Mishra, D.; Rajvir, S.; Mishra, U.; Kumar, S.S. Role of bio-fertilizer in organic agriculture: A review. Res. J. Recent Sci. 2013, 2, 39–41. [Google Scholar]
- Malusá, E.; Vassilev, N. A contribution to set a legal framework for biofertilisers. Appl. Microbial. Biotechnol. 2014, 98, 6599–6607. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, P.; Gianinazzi, S.; Perotto, S.; Turnau, K.; Barea, J.M. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol. Fertile. Soils 2003, 37, 1–16. [Google Scholar]
- Podile, A.R.; Kishore, G.K. Plant growth-promoting rhizobacteria. In Plant-Associated Bacteria; Springer: Dordrecht, The Netherlands, 2006; pp. 195–230. [Google Scholar]
- Franche, C.; Lindström, K.; Elmerich, C. Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant Soil 2009, 321, 35–59. [Google Scholar] [CrossRef]
- Khalid, A.; Arshad, M.; Shaharoona, B.; Mahmood, T. Plant growth promoting rhizobacteria and sustainable agriculture. In Microbial Strategies for Crop Improvement; Springer: Berlin/Heidelberg, Germany, 2009; pp. 133–160. [Google Scholar]
- Chen, L.H.; Tang, X.M.; Raze, W.; Li, J.H.; Liu, Y.X.; Qiu, M.H.; Zhang, F.G.; Shen, Q.R. Trichoderma harzianum SQR-T037 rapidly degrades allelochemicals in rhizospheres continuously cropped cucumbers. Appl. Microbiol. Biotechnol. 2011, 89, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Wang, J.D.; Zhang, J.; Yang, H. Study of the antifungal ability of Bacillus subtilis strain PY-1 in vitro and identification of its antifungal substance (Iturin A). Acta Biochim Biophys Sin 2006, 38, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Leonardo, D.; Blanca, L.F.; Landa, B.; Weller, D.M. Host crop affects rhizosphere colonization and competitiveness of 2,4-diacetylphloroglucinol-producing Pseudomonas fluoresens. Phytopathology 2006, 96, 751–762. [Google Scholar]
- Francis, I.; Holsters, M.; Vereecke, D. The gram-positive side of plant-microbe interaction. Environ. Microbial. 2010, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garcia, A.; Romero, D.; de Vicente, A. Plant protection and growth simulation by microorganism: Biotechnological applications of Bacillus in agriculture. Curr. Open. Biotechnol. 2011, 22, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Waddington, S.R. Organic matter management: From science to practice. Soil Fertil. 1998, 62, 24–25. [Google Scholar]
- Yang, X.; Chen, L.; Yong, X.; Shen, Q. Formulations can affect rhizosphere colonization and biocontrol efficiency of Trichoderma harzianum SQR-T037 againts Fusartium wilt of cucumbers. NBiol. Fertil. Soils 2011, 47, 239–248. [Google Scholar] [CrossRef]
- Tarafdar, A.; Raliya, R.; Wang, W.N.; Biswas, P.; Tarafdar, J.C. Green synthesis of TiO2 nanoparticle using Aspergillus tubingensis. Adv. Sci. Eng. Med. 2013, 5, 943–949. [Google Scholar] [CrossRef]
- Liu, X.M.; Feng, Z.B.; Zhang, F.D.; Zhang, S.Q.; He, X.S. Preparation and testing of cementing and coating nano-subnanocomposites of slow/controlled-release fertilizer. Agric. Sci. China 2006, 5, 700–706. [Google Scholar] [CrossRef]
- Suman, P.R.; Jain, V.K.; VArman, A. Role of nanomaterilas in symbiotic fungus growth enhancement. Curr. Sci. 2010, 99, 1189–1191. [Google Scholar]

| PGPR | PGPR Mechanisms | Crops | Application Mode | Observation/Findings | Ref. |
|---|---|---|---|---|---|
| Azoarcus | Nitrogen fixation | rice | Plants were grown gnotobiotically with a mutant of strain BH72 expressing the b-glucuronidase gene constitutively. | The presence of Azoarcus in the stele, especially in the stelar tissue of culms, suggests that these bacteria might spread systemically in situ, and underline their endophytic life style. | [27] |
| Azobacter | Cytokinin synthesis | Cucumber | - | - | [28] |
| Azorhizobium | Nitrogen fixation | Wheat | 2 mL of rhizobial culture were added four times to each wheat plant, once during the planting of the seeds, and subsequently three times at one-week intervals. | Five weeks after inoculation with A. caulinodans IRBG314, there were approximately five times more short lateral roots, each up to 3 mm in length, present on inoculated wheat. | [29] |
| Azospirillum | Nitrogen fixation | sugar cane | - | - | [30,31,32,33] |
| Azotobacter | Nitrogen fixation | Wheat, barley, oats, rice, sunflowers, maize, line, beetroot, tobacco, tea, coffee and coconuts | - | - | [34] |
| Bacillus | Auxin synthesis | Potato | Seed-dipping (108 mL−1 cfu) | Both the strains enhanced the auxin content of inoculated plants up to 71.4% and 433%, respectively, as compared to non-inoculated plants. | [35] |
| Bacillus | Cytokinin synthesis | Cucumber | Seed-dipping 106 cells/mL (106 CFU/mL) | Cucumber seedlings subjected to bacterization had well developed lateral roots. | [36] |
| Bacillus | Gibberelin synthesis | Pepper | - | - | [37] |
| Bacillus | Potassium solubilization | pepper, cucumber | Seedling was inoculated with 1 mL of inoculum containing around 108 cells. | The results showed that there was a relatively higher availability of P and K in soils planted with pepper than with cucumber. | [38,39] |
| Bacillus | Induction of plant stress resistance | Peanuts Maize | Plants were inoculated with 1 mL of a 108 cfu suspension Seed-dipping for 30 min | Increasing salt concentrations, biological N fixation may be competitive, becoming a more economic and sustainable alternative to chemical fertilization. The bacterial inoculants increased the total N, P, and K contents of the shoot and root of maize in calcisol soil from 16% to 85% significantly as compared to the control counterpart. | [40,41] |
| Bacillus | Antibiotic production | Alfalfa | Seedling was inoculated | Filtrates of cultures suppressed alfalfa disease caused by P. medicaginis and inhibited the growth of the pathogen in an agar plate assay. | [42] |
| Bacillus | Siderophore production | Maize, pepper | - | - | [43] |
| Beijerinckia | Nitrogen fixation | Sugar cane | - | - | [30,44] |
| Burkholderia | Nitrogen fixation | Rice | - | - | [45,46] |
| Chryseobacterium | Siderophore production | Tomato | Soil drenched | Siderophore production increased as bacterial biomass increased after 16 h of culture | [47] |
| Frankia | Nitrogen fixation | Alnus | - | - | [48] |
| Gluconacetobacter | Nitrogen fixation | Sugar cane | Root-dipping of seedlings for 1 h | The endophytic establishment of G. diazotrophicus within stems of sugarcane was confirmed by the scanning electron microscopy. | [49] |
| Herbaspirillum | Nitrogen fixation | rice | Seed was inoculated | GFP-tagged cells of Herbaspirillum sp. strain B501gfp1 were apparently localized in intercellular spaces of shoot tissues of 7-day-old seedlings of O. officinalis W0012. | [50] |
| Mycobacterium | Induction of plant stress resistance | Maize | - | - | [40] |
| Paenibacillus | Indole acetic acid synthesis | Lodgepole pine | - | - | [51] |
| Paenibacillus | Potassium solubilization | Black pepper | - | - | [52] |
| Phyllobacterium | Phosphate solubilization | Strawberries | The strawberry seedlings were inoculated with 1 mL of 108 CFU/mL suspensions. | Strain PEPV15 was able to solubilize moderate amounts of phosphate (5mm radius around the colonies). | [53] |
| Phyllobacterium | Siderophore production | Strawberries | The strawberry seedlings were inoculated with 1 mL of 108 CFU/mL suspensions. | The strain grew on the CAS indicator medium where the colonies were surrounded by a yellow-orange halo (3.5 mm radius around colonies) indicative of the siderophore production. | [53] |
| Pseudomonas | Chitinase and β-glucanases production | Several crops | - | - | [54] |
| Pseudomonas | ACC deaminase synthesis | Mung beans, wheat | - | - | [55,56] |
| Pseudomonas | Induction of plant stress resistance | Cotton, Maize | - | - | [40,57] |
| Pseudomonas | Antibiotic production | Wheat | - | - | [58] |
| Pseudomonas | Chitinase and β-glucanases production | Pigeon pea | The method of Weller and Cook (1983) was adopted for seed bacterization | P. fluorescens LPK2 and S. fredii KCC5 showed chitinase activity on chitinase minimal medium. b-1,3-glucanase activity was more pronounced in the fluorescent pseudomonads strains. | [59] |
| Pseudomonas | Siderophore production | Potato, maize | - | - | [43] |
| Rhizobia | Nitrogen fixation | Legumes | - | - | [60] |
| Rhizobia | Induction of plant stress resistance | Peanuts | - | - | [41] |
| Rhizobia | Hydrogen Cyanide Production | Legumes | - | - | [61] |
| Rhizobium | Nitrogen fixation | Rice | - | - | [62] |
| Rhizobium | Indole acetic acid synthesis | Pepper, tomato, lettuce, carrot | Seed Inoculation Seedlings were inoculated with 250 µL plant−1 of a bacterial suspension with a turbidity of 5 in McFarland standards (1.5 × 109 CFUmL−1). | The dry weight of the inoculated seedlings (shoots and roots) was more than twice with respect to the un-inoculated seedlings. Concentrations of N, P, and Ca were significantly higher in inoculated plants, indicating that they had higher potential for nutrient uptake than control plants. | [63,64] |
| Rhizobium | ACC deaminase synthesis | Pepper, tomato mung beans, | - | - | [55,63] |
| Rhizobium | Siderophore production | Tomato, pepper, Carrot, lettuce, | Seed Inoculation Seedlings were inoculated with 250 lL plant−1 of a bacterial suspension with a turbidity of 5 in McFarland standards (1.5 × 109 CFU/mL−1). | The colonies of strain TPV08 were surrounded by a yellow-orange halo (3.5 mm radium around colonies) indicative of siderophore production. | [63,64] |
| Sinorhizobium | Chitinase and β-glucanases production | Pigeon pea | - | - | [59] |
| Sphingomonas | Gibberelin synthesis | Tomato | - | - | [65] |
| Streptomyces | Indole acetic acid synthesis | Indian lilac | - | - | [66] |
| Streptomyces | Siderophore production | Indian lilac | - | - | [66] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of Plant Growth Promoting Rhizobacteria in Agricultural Sustainability—A Review. Molecules 2016, 21, 573. https://doi.org/10.3390/molecules21050573
Vejan P, Abdullah R, Khadiran T, Ismail S, Nasrulhaq Boyce A. Role of Plant Growth Promoting Rhizobacteria in Agricultural Sustainability—A Review. Molecules. 2016; 21(5):573. https://doi.org/10.3390/molecules21050573
Chicago/Turabian StyleVejan, Pravin, Rosazlin Abdullah, Tumirah Khadiran, Salmah Ismail, and Amru Nasrulhaq Boyce. 2016. "Role of Plant Growth Promoting Rhizobacteria in Agricultural Sustainability—A Review" Molecules 21, no. 5: 573. https://doi.org/10.3390/molecules21050573






