Bioactive Compound Content and Cytotoxic Effect on Human Cancer Cells of Fresh and Processed Yellow Tomatoes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Content of Antioxidant Compounds
| Genotype | Solid Content | Total Carotenoids | Lycopene | β-Carotene | ||||
|---|---|---|---|---|---|---|---|---|
| NP | P | NP | P | NP | P | NP | P | |
| E40 | 7.20 ± 0.05 | 7.60 ± 0.06 | 0.68 ± 0.01 | 0.66 ± 0.03 | n.d | n.d | 0.68 ± 0.1 | 0.42 ± 0.01 ** |
| E87 | 7.03 ± 0.03 | 6.75 ± 0.05 | 0.53 ± 0.1 | 0.62 ± 0.03 | n.d | n.d | 0.53 ± 0.1 | 0.42 ± 0.02 |
| E92 | 5.61 ± 0.04 | 6.25 ± 0.07 | 0.57 ± 0.2 | 0.93 ± 0.06 ** | n.d | n.d | 0.57 ± 0.2 | 0.61 ± 0.03 |
2.1.1. Carotenoids
2.1.2. Ascorbic Acid and Total Phenolics
| Sample | Ascorbic Acid | Total Phenolics | Total Flavonoids | Flavonoids/Phenolics | ||||
|---|---|---|---|---|---|---|---|---|
| NP | P | NP | P | NP | P | NP | P | |
| E40 | 54.09 ± 1.12 | 21.76 ± 0.97 *** | 50.86 ± 1.74 | 44.73 ± 0.63 *** | 22.52 ± 0.73 | 23.03 ± 1.22 | 0.44 | 0.51 |
| E87 | 64.00 ± 0.91 | 28.93 ± 1.14 *** | 52.86 ± 1.76 | 44.10 ± 1.05 *** | 17.69 ± 0.24 | 21.31 ± 0.12 *** | 0.33 | 0.48 |
| E92 | 63.84 ± 0.86 | 32.95 ± 1.27 *** | 53.48 ± 1.22 | 39.54 ± 0.61 *** | 13.21 ± 0.24 | 23.38 ± 0.12 *** | 0.24 | 0.59 |
2.2. Identification of Individual Phenolic Compounds
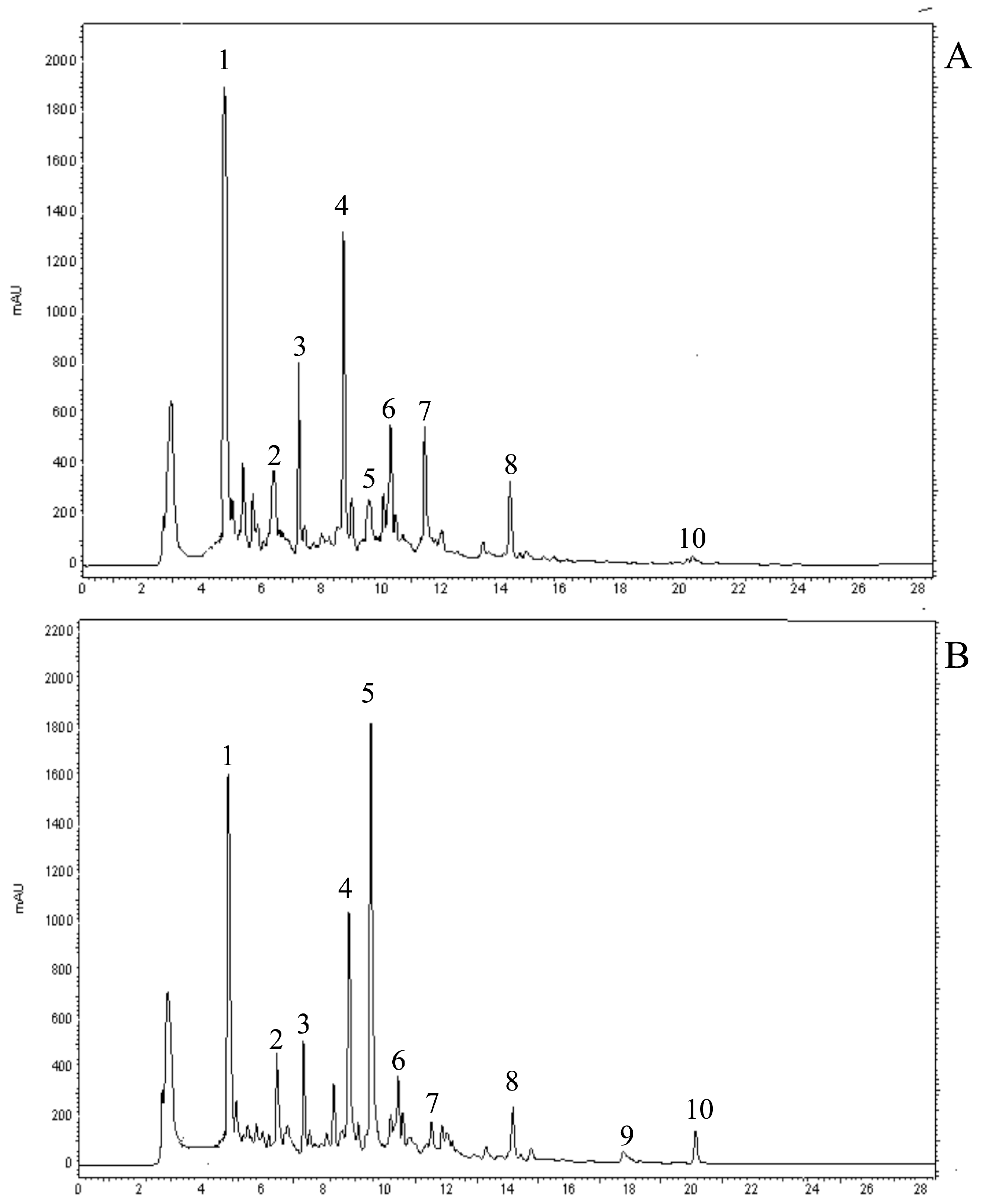
| Phenolic acid | Treatment | E40 | E87 | E92 |
|---|---|---|---|---|
| Chlorogenic Acid | NP | 23.16 ± 0.38 | 15.63 ± 0.63 | 16.07 ± 0.51 |
| P | 10.10 ± 0.13 *** | 8.08 ± 1.21 *** | 6.67 ± 0.35 *** | |
| Caffeic Acid | NP | 1.80 ± 0.16 | 1.14 ± 0.11 | 0.92 ± 0.13 |
| P | 1.41 ± 0.15 ** | 0.74 ± 0.10 ** | 0.69 ± 0.08 | |
| 5-p-CQA | NP | 7.53 ± 0.71 | 2.17 ± 0.11 | 2.92 ± 0.11 |
| P | 4.66 ± 0.30 *** | 1.56 ± 0.04 *** | 1.69 ± 0.10 *** | |
| Sum of phenolic acids | NP | 32.49 ± 1.25 | 18.94 ± 0.85 | 19.91 ± 0.75 |
| P | 16.17 ± 0.58 *** | 10.38 ± 1.35 *** | 9.05 ± 0.53 *** |
| Flavonoid | Treatment | E40 | E87 | E92 |
|---|---|---|---|---|
| Rutin | NP | 29.37 ± 0.76 | 10.66 ± 0.02 | 10.78 ± 0.34 |
| P | 21.90 ± 3.78 ** | 11.26 ± 0.83 | 8.64 ± 0.61 *** | |
| quercetin | NP | 1.17 ± 0.07 | 0.32 ± 0.08 | 0.25 ± 0.01 |
| P | 1.55 ± 0.07 *** | 0.48 ± 0.04 ** | 0.75 ± 0.02 *** | |
| Quercetin3-β-D-glucoside | NP | 2.34 ± 0.14 | 1.39 ± 0.21 | 1.15 ± 0.17 |
| P | 0.96 ± 0.09 *** | 0.98 ± 0.06 ** | 7.42 ± 0.23 *** | |
| Naringin | NP | n.d. | 0.22 ± 0.03 | 0.51 ± 0.02 |
| P | n.d. | 0.20 ± 0.01 | 0.22 ± 0.03 *** | |
| Naringeninchalcone | NP | 0.69 ± 0.13 | 0.05 ± 0.01 | 0.08 ± 0.02 |
| P | n.d. | n.d. | n.d. | |
| Naringenin | NP | n.d. | n.d. | n.d. |
| P | 1.08 ± 0.05 | 1.58 ± 0.11 | 1.84 ± 0.02 | |
| Kampferol-3-rutinoside | NP | 1.66 ± 0.01 | 0.44 ± 0.01 | 0.53 ± 0.01 |
| P | 0.51 ± 0.04 *** | 0.23 ± 0.01 *** | 0.12 ± 0.01 *** | |
| Chalconaringenin-exoside | NP | 0.62 ± 0.09 | 0.30 ± 0.03 | 0.39 ± 0.01 |
| P | 0.21 ± 0.02 ** | 0.22 ± 0.02 ** | 0.28 ± 0.01 *** | |
| Sum of flavonoids | NP | 35.85 ± 1.19 | 13.38 ± 0.37 | 13.69 ± 0.58 |
| P | 26.21 ± 4.05 * | 14.95 ± 1.07 | 19.27 ± 0.90 *** |
2.3. Antioxidant Activity
| Genotype | LAA | HAA (ABTS) | HAA (FRAP) | |||
|---|---|---|---|---|---|---|
| NP | P | NP | P | NP | P | |
| E40 | 39.49 ± 2.50 | 44.70 ± 0.91 * | 299.23 ± 4.83 | 241.28 ± 4.26 *** | 387.14 ± 23.71 | 279.57 ± 3.82 *** |
| E87 | 52.25 ± 1.20 | 30.36 ± 1.44 *** | 258.81 ± 6.06 | 293.50 ± 12.21 ** | 427.38 ± 11.27 | 372.86 ± 17.91 * |
| E92 | 39.28 ± 0.85 | 38.69 ± 0.81 | 308.46 ± 8.22 | 253.57 ± 11.35 ** | 493.00 ± 12.56 | 269.14 ± 11.41 *** |
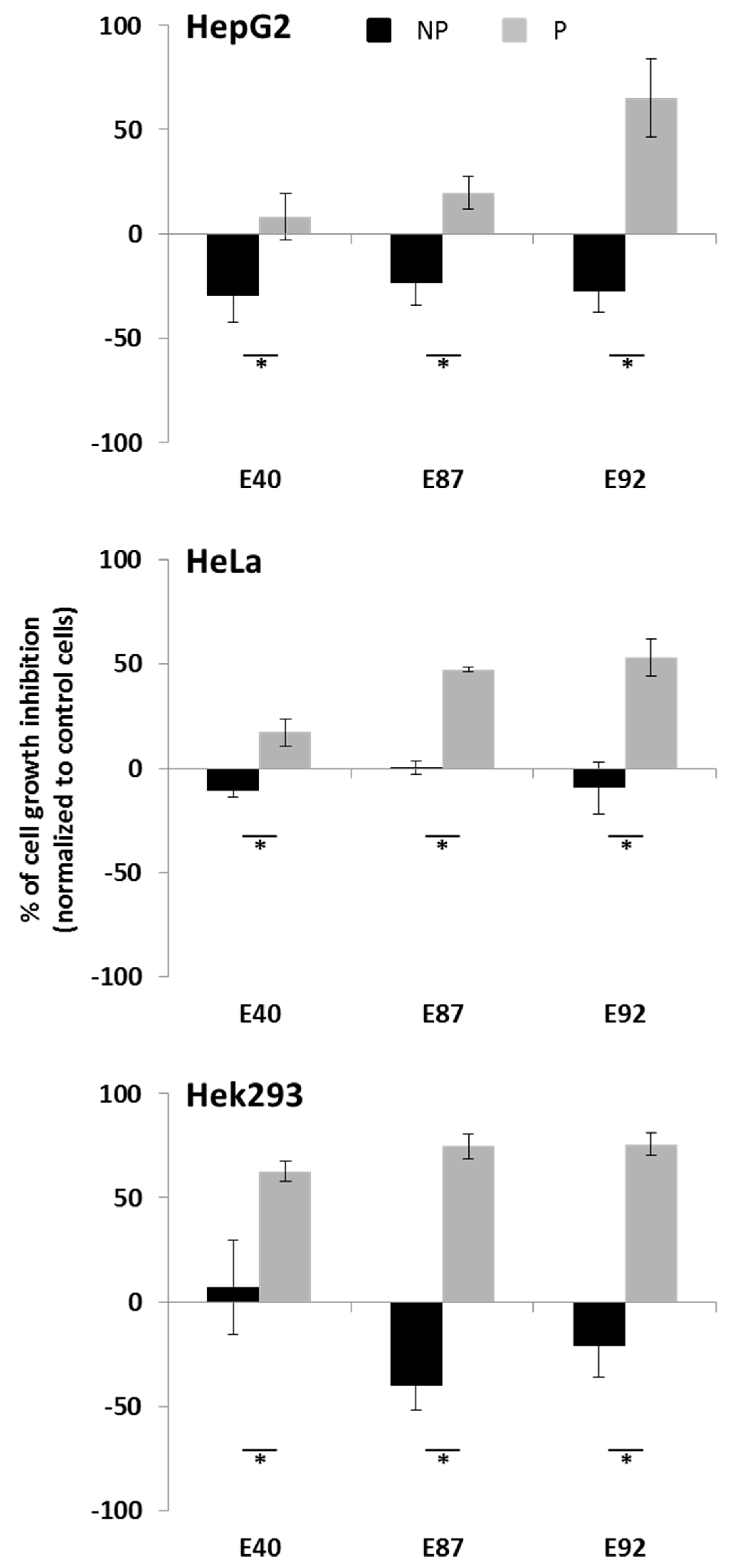
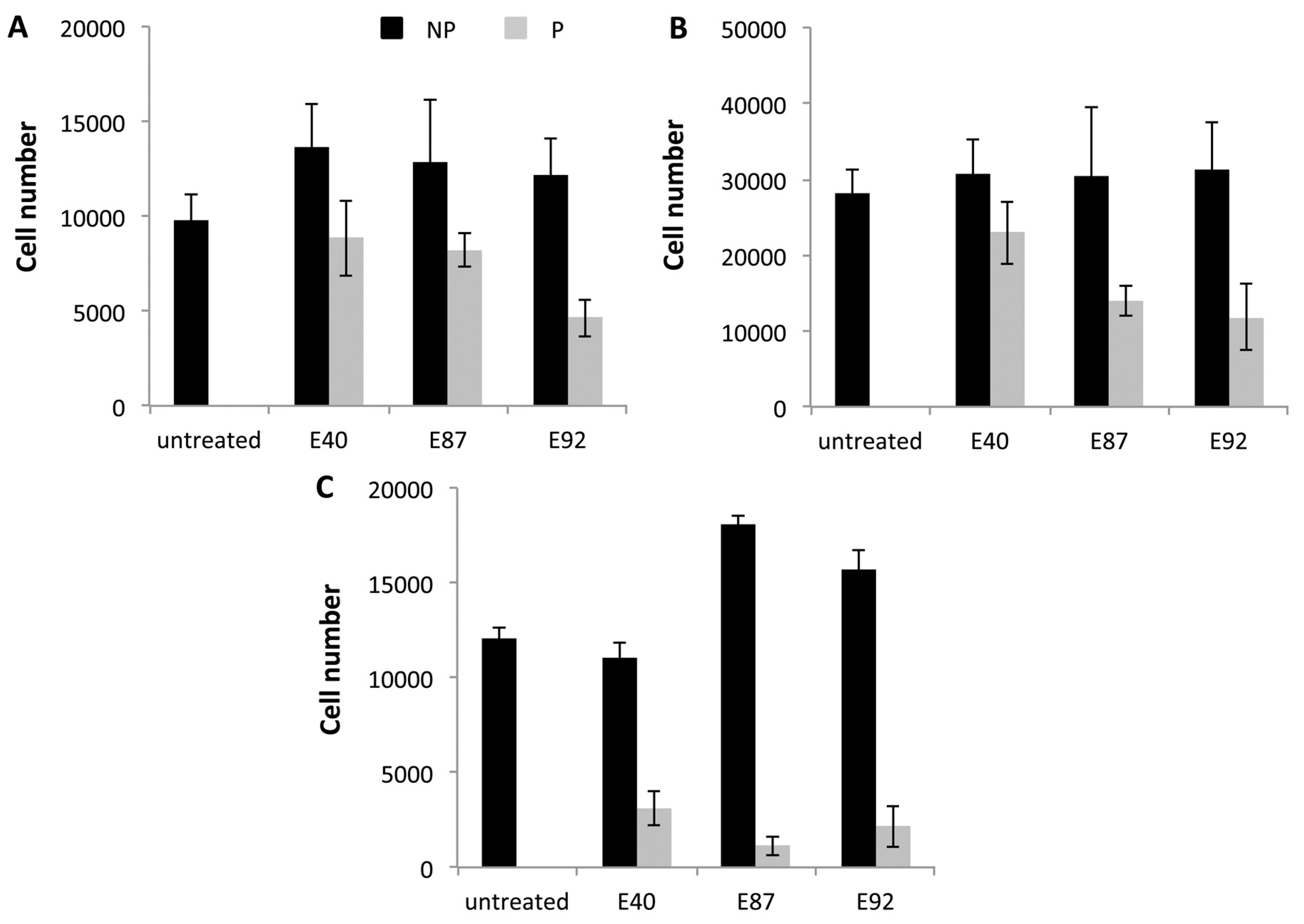
2.4. Effects of Tomato Extracts on Cell Viability
3. Experimental Section
3.1. Chemicals and Reagents
3.2. Plant Material and Processing Material
3.3. Chemical Extractions
3.4. Carotenoid Determination
3.5. Ascorbic Acid Determination
3.6. Total Phenolics and Total Flavonoids Determination
3.7. Individual Phenolics Compounds Determination
3.8. Antioxidant Activity Determination
3.9. Cell viability assays
3.10. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
References
- Faostat. Available online: http://faostat.fao.org (accessed on 20 November 2015).
- Canene-Adams, K.; Campbell, J.K.; Zaripheh, S.; Jeffery, E.H.; Erdman, J.W., Jr. The tomato as a functional food. J. Nutr. 2005, 135, 1226–1230. [Google Scholar] [PubMed]
- Raiola, A.; Rigano, M.M.; Calafiore, R.; Frusciante, L.; Barone, A. Enhancing the health promoting effects of tomato fruit for biofortified food. Mediat. Inflamm. 2014. [Google Scholar] [CrossRef] [PubMed]
- Periago, M.J.; Garcia-Alonso, J. Bioactive compounds, folates and antioxidant properties of tomatoes (Lycopersicum esculentum) during vine ripening. Int. J. Food Sci. Nutr. 2009, 60, 694–708. [Google Scholar] [CrossRef] [PubMed]
- Rigano, M.M.; Raiola, A.; Tenore, G.C.; Monti, D.M.; Del Giudice, R.; Frusciante, L.; Barone, A. Quantitative trait loci pyramiding can improve the nutritionalpotential of tomato (Solanumlycopersicum) fruits. J. Agric. Food. Chem. 2014, 62, 11519–11527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcìa-Valverde, V.; Navarro-Gonzales, I.; Garcia-Alonso, J.; Periago, M.J. Antioxidant bioactive compounds in selected industrial processing and fresh consumption tomato cultivars. Food Bioprocess. Technol. 2013, 6, 391–402. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Shishodia, S. Suppression of the nuclear factor-κB activation pathway by spice-derived phytochemicals: reasoning for seasoning. Ann. N. Y. Acad. Sci. 2004, 1030, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Biswas, K.; Kirkham, P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef] [PubMed]
- Murcia, M.A.; Martínez-Tomé, M. Antioxidant activity of resveratrol compared with common food additives. J. Food. Prot. 2001, 64, 379–384. [Google Scholar] [PubMed]
- D’Introno, A.; Paradiso, A.; Scoditti, E.; D’Amico, L.; de Paolis, A.; Carluccio, M.A.; Nicoletti, I.; DeGara, L.; Santino, A.; Giovinazzo, G. Antioxidant and anti-inflammatory properties of tomato fruits synthesizing different amounts of stilbenes. Plant Biotechnol. J. 2009, 7, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Kauss, T.; Moynet, D.; Rambert, J. Rutoside decreases human macrophage-derived inflammatory mediators and improves clinical signs in adjuvant-induced arthritis. Arthritis Res. Ther. 2008, 10, R19. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xia, M.; Liu, C.; Guo, H.; Ye, Q.; Hu, Y.; Zhang, Y.; Hou, M.; Zhu, H.; Ma, J.; Ling, W. Cyanidin-3-O-β-glucoside inhibits iNOS and COX-2 expression by inducing liver X receptor alpha activation in THP-1 macrophages. Life Sci. 2008, 83, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Martín, A.R.; Villegas, I.; Sánchez-Hidalgo, M.; de la Lastra, C.A. The effects of resveratrol, a phytoalexin derived from red wines, on chronic inflammation induced in an experimentally induced colitis model. Br. J. Pharmacol. 2006, 147, 873–885. [Google Scholar] [CrossRef] [PubMed]
- González, R.; Ballester, I.; López-Posadas, R.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; de Medina, S.F. Effects of flavonoids and other polyphenols on inflammation. Crit. Rev. Food Sci. Nutr. 2011, 51, 331–362. [Google Scholar] [CrossRef] [PubMed]
- Sak, K. Site-specific anticancer effects of dietary flavonoid quercetin. Nutr. Cancer 2014, 66, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Abushita, A.A.; Daood, H.G.; Biacs, P.A. Change in carotenoids and antioxidant vitamins in tomato as a function of varietal and technological factors. J. Agric. Food Chem. 2000, 48, 2075–2081. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, G.R.; Dao, L.; Flessa, S.; Gillespie, D.M.; Jewell, W.T.; Huebner, B.; Bertow, D.; Eleber, S.E. Processing effects on lycopene content and antioxidant activity of tomatoes. J. Agric. Food. Chem. 2001, 49, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Capanoglu, E.; Beekwilder, J.; Boyacioglu, D.; Hall, R.; de Vos, R. Changes in antioxidant and metabolite profiles during production of tomato paste. J. Agric. Food Chem. 2008, 56, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Lamuela-Raventós, R.M.; Elez-Martínez, P.; Martín-Belloso, O. Changes in the polyphenol profile of tomato juices processed by pulsed electric fields. J. Agric. Food. Chem. 2012, 60, 9667–9672. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, V.; Francese, G.; Sacco, A.; D’Alessandro, A.; Rigano, M.M.; Parisi, M.; Milone, M.; Cardi, T.; Mennella, G.; Barone, A. An association mapping approach to identify favourable alleles for tomato fruit quality breeding. BMC Plant Biol. 2014, 14, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgé, S.; Tourniaire, F.; Gautier, H.; Goupy, P.; Rock, E.; Caris-Veyrat, C. Changes in the contents of carotenoids, phenolic compounds and vitamin C during technical processing and lyophilisation of red and yellow tomatoes. Food Chem. 2011, 124, 1603–1611. [Google Scholar] [CrossRef]
- Graziani, G.; Pernice, R.; Lanzuise, S.; Vitaglione, P.; Anese, M.; Fogliano, V. Effect of peeling and heating on carotenoid content and antioxidant activity of tomato and tomato-virgin olive oil systems. Eur. Food Res. Technol. 2003, 216, 116–121. [Google Scholar]
- Sánchez-Moreno, C.; Plaza, L.; de Ancos, B.; Cano, M.P. Impact of highpressure and traditional thermal processing of tomato puree on carotenoids, vitamin C and antioxidant activity. J. Sci. Food Agric. 2006, 86, 171–179. [Google Scholar] [CrossRef]
- Re, R.; Bramley, P.M.; Rice-Evans, C. Effects of food processing on flavonoids and lycopene status in a Mediterranean tomato variety. Free Radic. Res. 2002, 36, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Conesa, D.; García-Alonso, J.; García-Valverde, V.; Iniesta, M.D.; Jacob, K.; Sánchez-Siles, L.M.; Ros, G.; Periago, M.J. Changes in bioactive compounds and antioxidant activity during homogenization and thermal processing of tomato puree. Innov. Food Sci. Emerg. Technol. 2009, 10, 179–188. [Google Scholar] [CrossRef]
- Gahler, S.; Otto, K.; Böhm, V. Alterations of vitamin C, total phenolics, and antioxidant capacity as affected by processing tomatoes to different products. J. Agric. Food Chem. 2003, 51, 7962–7968. [Google Scholar] [CrossRef] [PubMed]
- Dewanto, V.; Wu, X.Z.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric Food Chem 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.W.; Montagu, V.M.; Inzé, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.J.; Strain, J.J.; Favell, D.; Fletcher, J. Plant l-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and total flavonoids in bulagrian fruits and vegetables. J. Univ. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Scaltber, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130 (Suppl. S8), 2072S–2085S. [Google Scholar]
- Fleuriet, A.; Macheix, J.J. Quinyl esters and glucose derivates of hydroxycinnamic acids during growth ripening of tomato fruits. Phytochemistry 1981, 20, 667–671. [Google Scholar] [CrossRef]
- Slimestad, R.; Verheul, M. Review of flavonoids and other phenolics from fruits of different tomato (Lycopersicon esculentum Mill.) cultivars. J. Sci. Food Agric. 2009, 89, 1255–1270. [Google Scholar] [CrossRef]
- Bugianesi, R.; Salucci, M.; Leonardi, C.; Ferracane, R.; Catasta, G.; Azzini, E.; Maiani, G. Effect of domestic cooking on human bioavailability of naringenin, chlorogenic acid, licopene and β-carotene in cherry tomatoes. Eur J. Nutr. 2004, 43, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Sahlin, E.; Savage, G.P.; Lister, C.E. Investigation of the antioxidant properties of tomatoes after processing. J. Food Compos. Anal. 2004, 17, 635–647. [Google Scholar]
- Krause, M.; Galensa, R. Determination of naringenin and naringeninchalcone in tomato skins by reversed phase HPLC after solid-phase extraction. Z. Lebensm. Unters Forsch. 1992, 194, 29–32. [Google Scholar] [CrossRef]
- Moco, S.; Bino, R.J.; Vorst, O.; Verhoeven, H.A.; de Groot, J.; van Beek, T.A.; Vervoort, J.; de Vos, R.C.H. A Liquid Chromatography-Mass Spectrometry-Based Metabolome Database for Tomato. Plant Physiol. 2006, 141, 1205–1218. [Google Scholar] [CrossRef] [PubMed]
- Seybold, D.C.; Fröhlich, K.; Bitsch, R.; Otto, K.; Böhm, V. Changes in contents of carotenoids and vitamin E during tomato processing. J. Agric. Food Chem. 2004, 52, 7005–7010. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Kim, H.; Kim, H.; Lee, I.; Kozukue, N.; Levin, C.E.; Friedman, M. Free amino acid and phenolic contents and antioxidative and cancer cell-inhibiting activities of extracts of 11 greenhouse-grown tomato varieties and 13 tomato-based foods. J. Agric. Food Chem. 2011, 59, 12801–12814. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Kim, D.S.; Kozukue, N.; Kim, H.J.; Nishitani, Y.; Mizuno, M.; Levin, C.E.; Friedman, M. Protein, free amino acid, phenolic, β-carotene, and lycopene content, and antioxidative and cancer cell inhibitory effects of 12 greenhouse-grown commercial cherry tomato varieties. J. Food Comp. Anal. 2014, 34, 115–127. [Google Scholar] [CrossRef]
- Mazzucato, A.; Willems, D.; Bernini, R.; Picarella, M.E.; Santangelo, E.; Ruiu, F.; Tilesi, F.; Soressi, G.P. Novel phenotypes related to the breeding of purple-fruited tomatoes and effect of peel extracts on human cancer cell proliferation. Plant Physiol. Biochem. 2013, 72, 125–133. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, M.E.; Franco, M.Y.E.; Alberto, T.G.; Sobreiro, M.A.; Conrado, M.A.; Priolli, D.G.; Sawaya, F.A.C.; Ruiz, A.L.; de Carvalho, J.E.; de Carvalho, P.O. Enzymatic de-glycosylation of rutin improves its antioxidant and antiproliferative activities. Food Chem. 2013, 141, 266–273. [Google Scholar] [CrossRef] [PubMed]
- You, H.J.; Ahn, H.J.; Ji, G.E. Transformation of rutin to antiproliferative quercetin-3-glucoside by Aspergillus niger. J. Agric. Food Chem. 2010, 58, 10886–10892. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, R.H. Synegistic effect of apple extracts and quercetin 3-β-d-glucoside combination on antiproliferative activity in MCF-7 human breast cancer cells in vitro. J. Agric. Food Chem. 2009, 57, 8581–8586. [Google Scholar] [CrossRef] [PubMed]
- Granado-Serrano, A.B.; Martín, M.A.; Izquierdo-Pulido, M.; Goya, L.; Bravo, L.; Ramos, S. Molecular mechanisms of (−)-Epicatechin and chlorogenic acid on the regulation of the apoptotic and survival/proliferation pathways in a human hepatoma cell line. J. Agric. Food Chem. 2007, 55, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Kanno, S.; Tomizawa, A.; Ohtake, T.; Koiwai, K.; Ujibe, M.; Ishikawa, M. Naringenin-induced apoptosis via activation of NF-Kappa B and necrosis involving the loss of ATP in human promyeloleukemia HL-60 cells. Toxicol. Lett. 2006, 166, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.D.; Yang, Z.Y.; Wang, Q.; Cai, T.K.; Crewdson, P. Synthesis, characterization, cytotoxic activities and DNA-binding properties of the La(III) complex with naringenin schiff-base. Bioorg. Med. Chem. 2006, 14, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, N.; Sulfikkarali, N.; RajendraPrasad, N.; Karthikeyan, S. Enhanced anticancer activity of naringenin-loaded nanoparticles in human cervical (HeLa) cancer cells. Biomed. Prev. Nutr. 2011, 1, 223–231. [Google Scholar] [CrossRef]
- Zouari, I.; Salvioli, A.; Chialva, M.; Novero, M.; Miozzi, L.; Tenore, G.C.; Bagnaresi, P.; Bonfante, P. From root to fruit: RNA-Seq analysis shows that arbuscularmycorrhizal symbiosis may affect tomato fruit metabolism. BMC Genomics 2014, 15, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Stevens, R.; Buret, M.; Garchery, C.; Carretero, Y.; Causse, M. Technique for rapid small-scale analysis of vitamin C levels in fruit and application to a tomato mutant collection. J. Agric. Food Chem. 2006, 54, 6159–6165. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdicphosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.N.; Rice-Evans, C.A. Factors influencing the antioxidant activity determined by the ABTS·+ radical cation assay. Free Radic. Res. 1997, 26, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, R.; Raiola, A.; Tenore, G.; Frusciante, L.; Barone, A.; Monti, D.M.; Rigano, M.M. Antioxidant bioactive compounds in tomato fruits at different ripening stages and their effects on normal and cancer cells. J. Funct. Foods 2015, 18, 83–94. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raiola, A.; Del Giudice, R.; Monti, D.M.; Tenore, G.C.; Barone, A.; Rigano, M.M. Bioactive Compound Content and Cytotoxic Effect on Human Cancer Cells of Fresh and Processed Yellow Tomatoes. Molecules 2016, 21, 33. https://doi.org/10.3390/molecules21010033
Raiola A, Del Giudice R, Monti DM, Tenore GC, Barone A, Rigano MM. Bioactive Compound Content and Cytotoxic Effect on Human Cancer Cells of Fresh and Processed Yellow Tomatoes. Molecules. 2016; 21(1):33. https://doi.org/10.3390/molecules21010033
Chicago/Turabian StyleRaiola, Assunta, Rita Del Giudice, Daria Maria Monti, Gian Carlo Tenore, Amalia Barone, and Maria Manuela Rigano. 2016. "Bioactive Compound Content and Cytotoxic Effect on Human Cancer Cells of Fresh and Processed Yellow Tomatoes" Molecules 21, no. 1: 33. https://doi.org/10.3390/molecules21010033







