Anti-Ulcerogenic Properties of Lycium chinense Mill Extracts against Ethanol-Induced Acute Gastric Lesion in Animal Models and Its Active Constituents
Abstract
:1. Introduction
2. Results
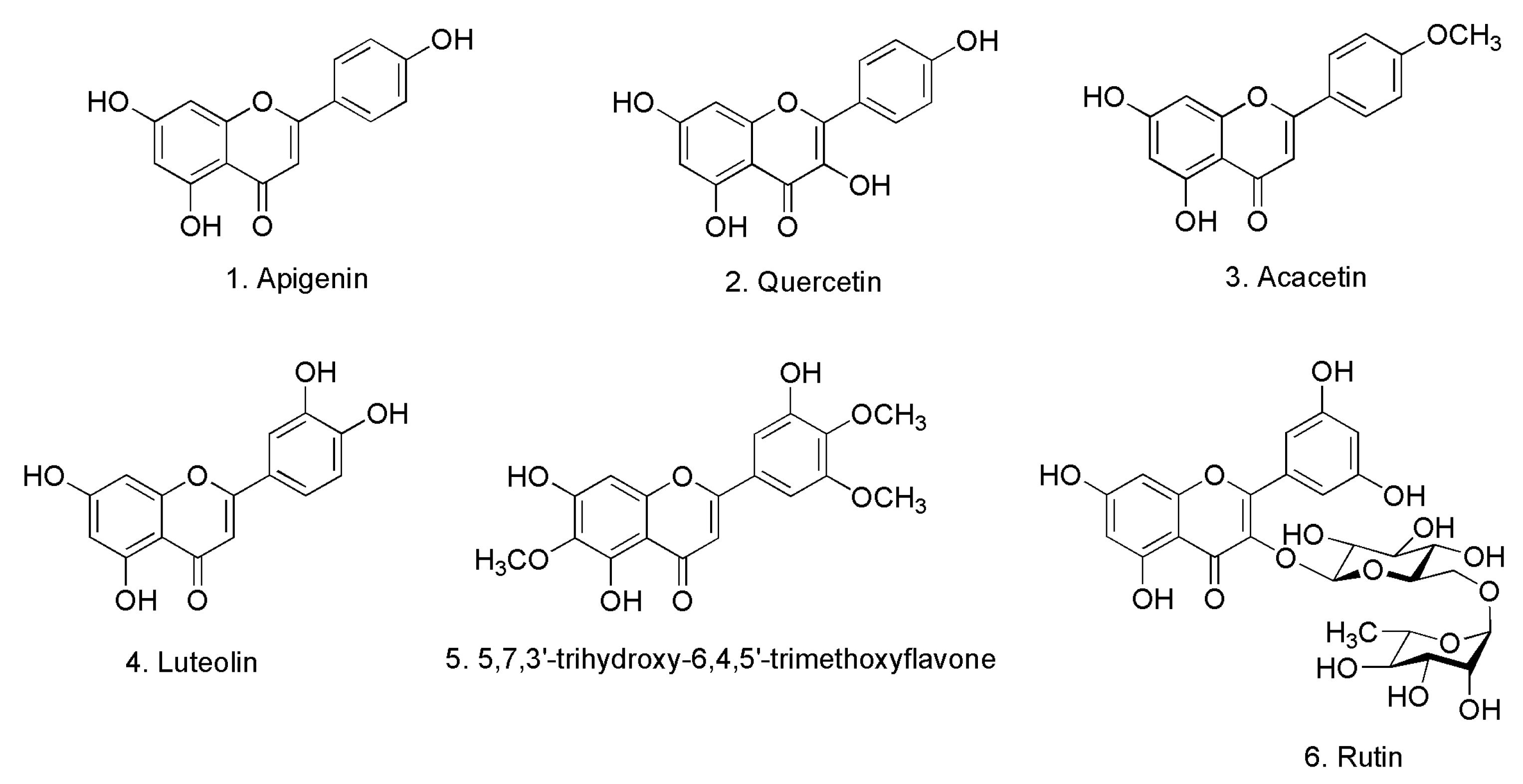
2.1. Identification of Isolated Compounds
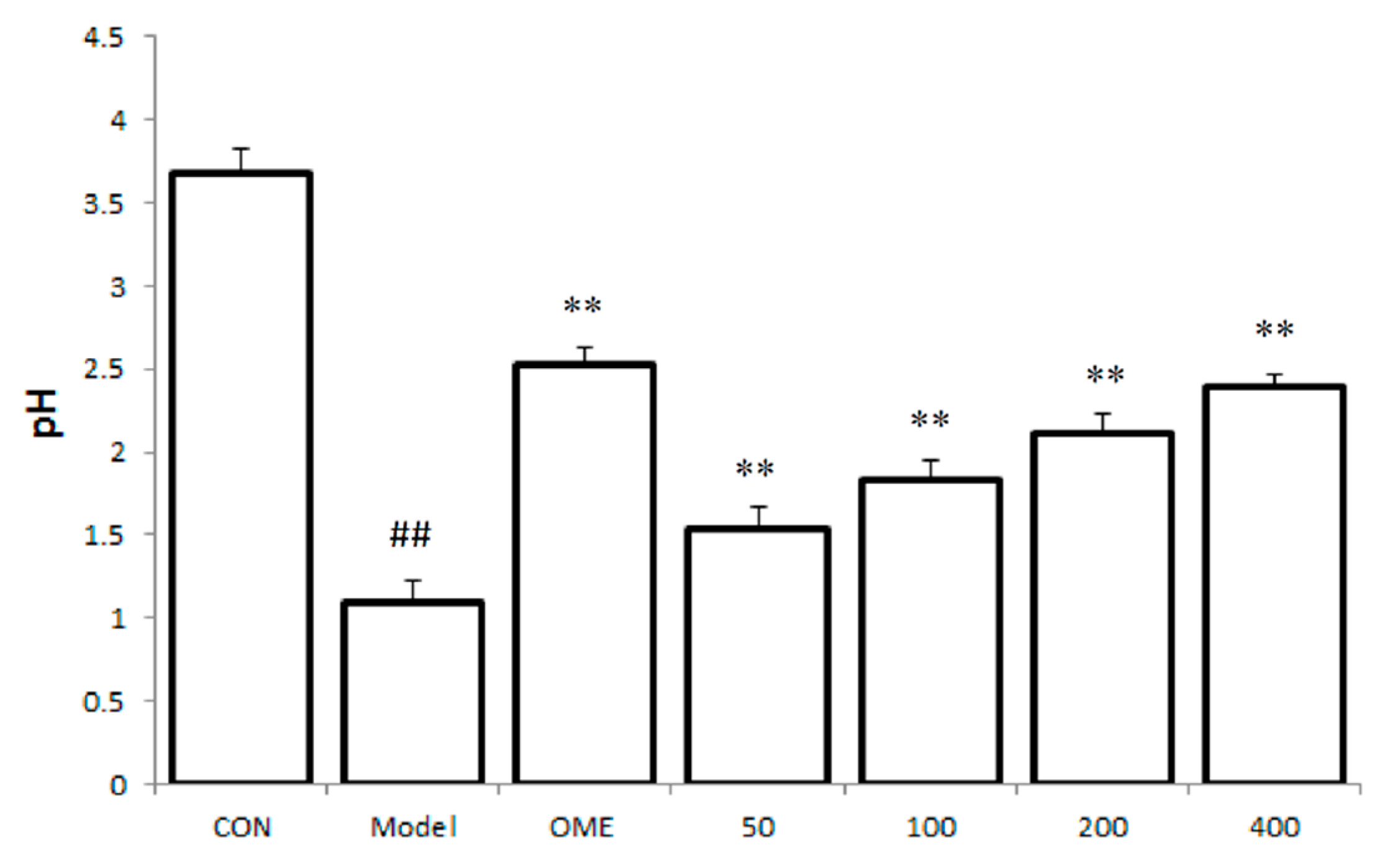
2.2. Effect of LCA on Gastric Juice Acid Content (pH)
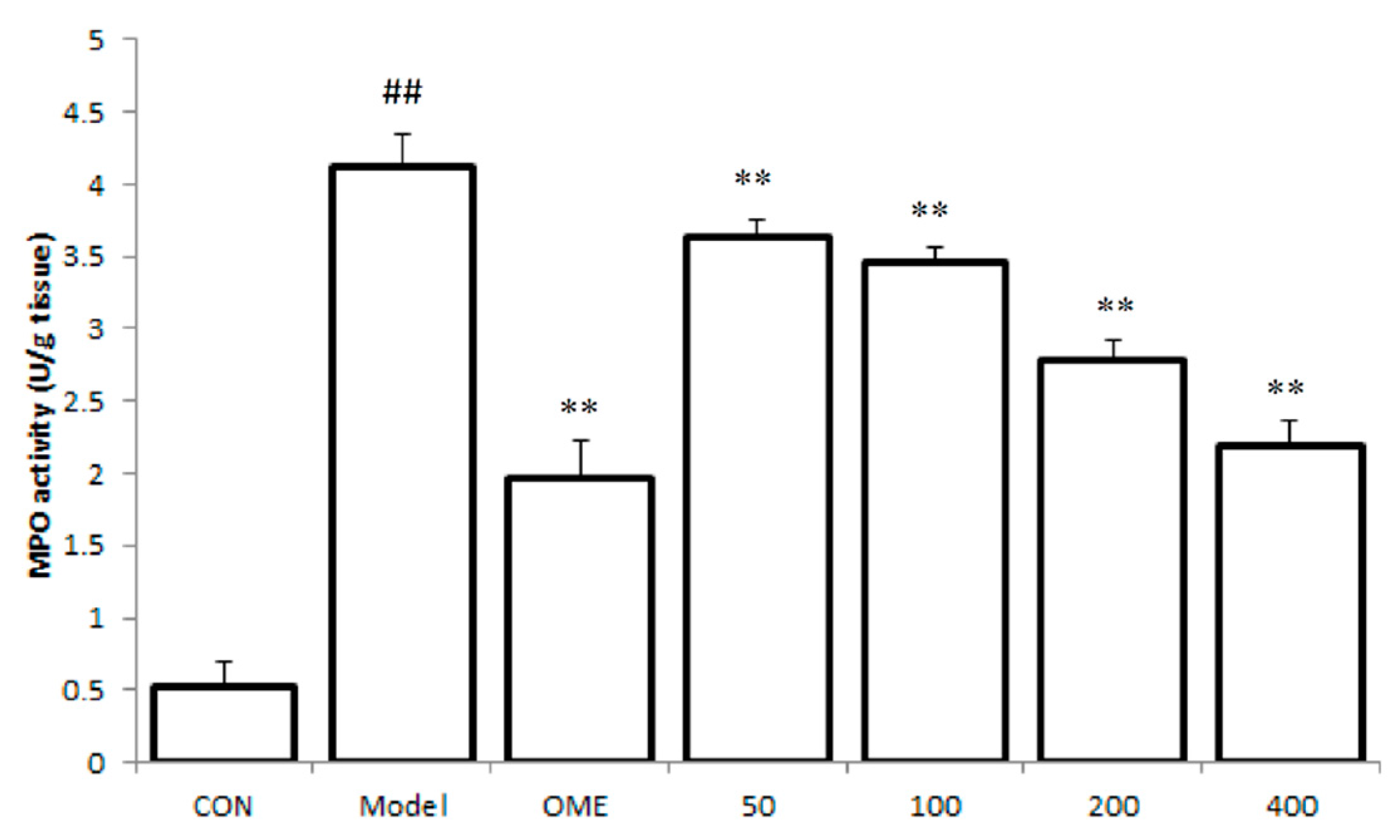
2.3. Effect of LCA on MPO Activity
2.4. Effect of LCA on MDA and SOD and GSH Levels
2.5. Effect of LCA on Cytokine Levels in the Serum
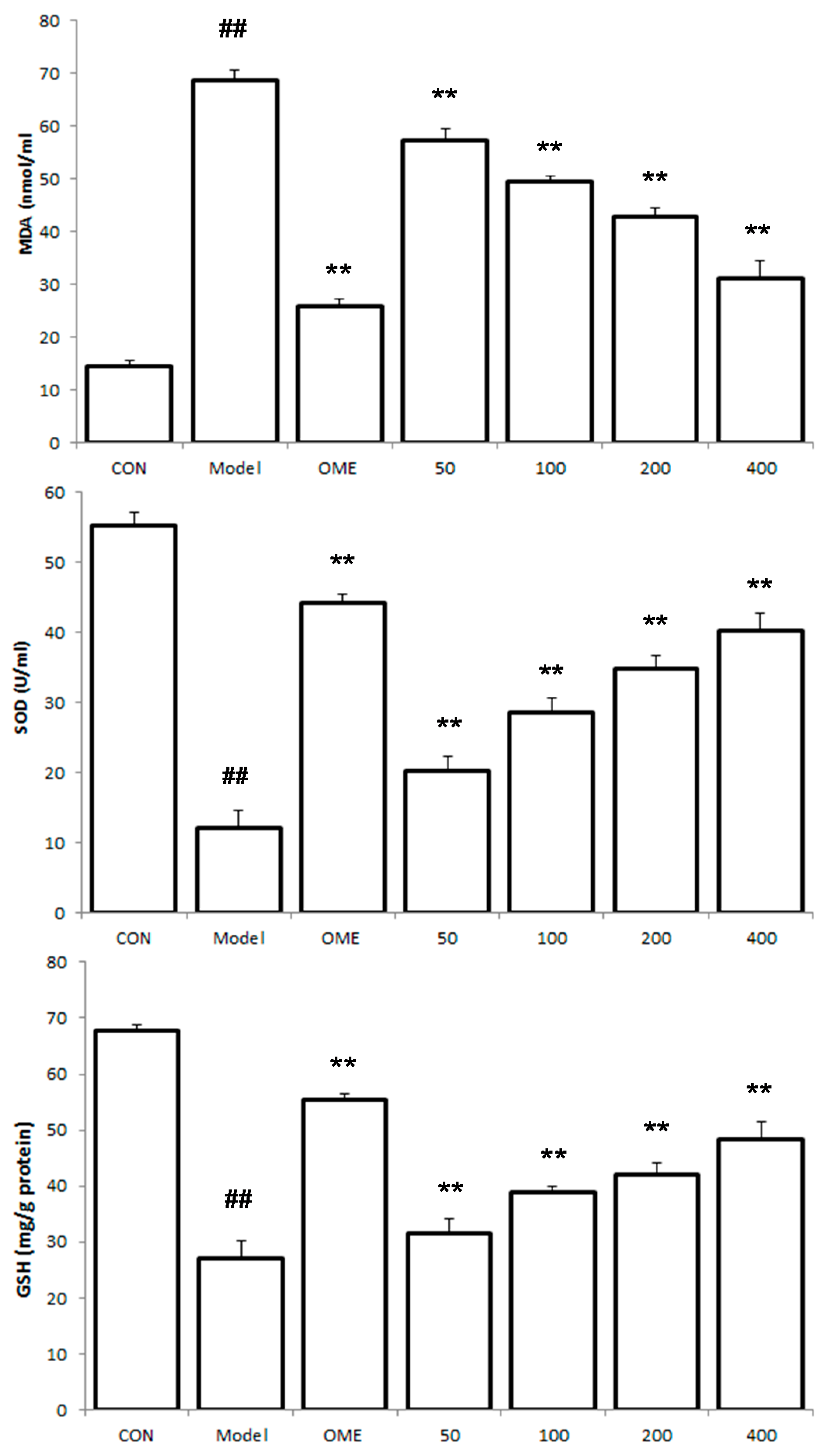
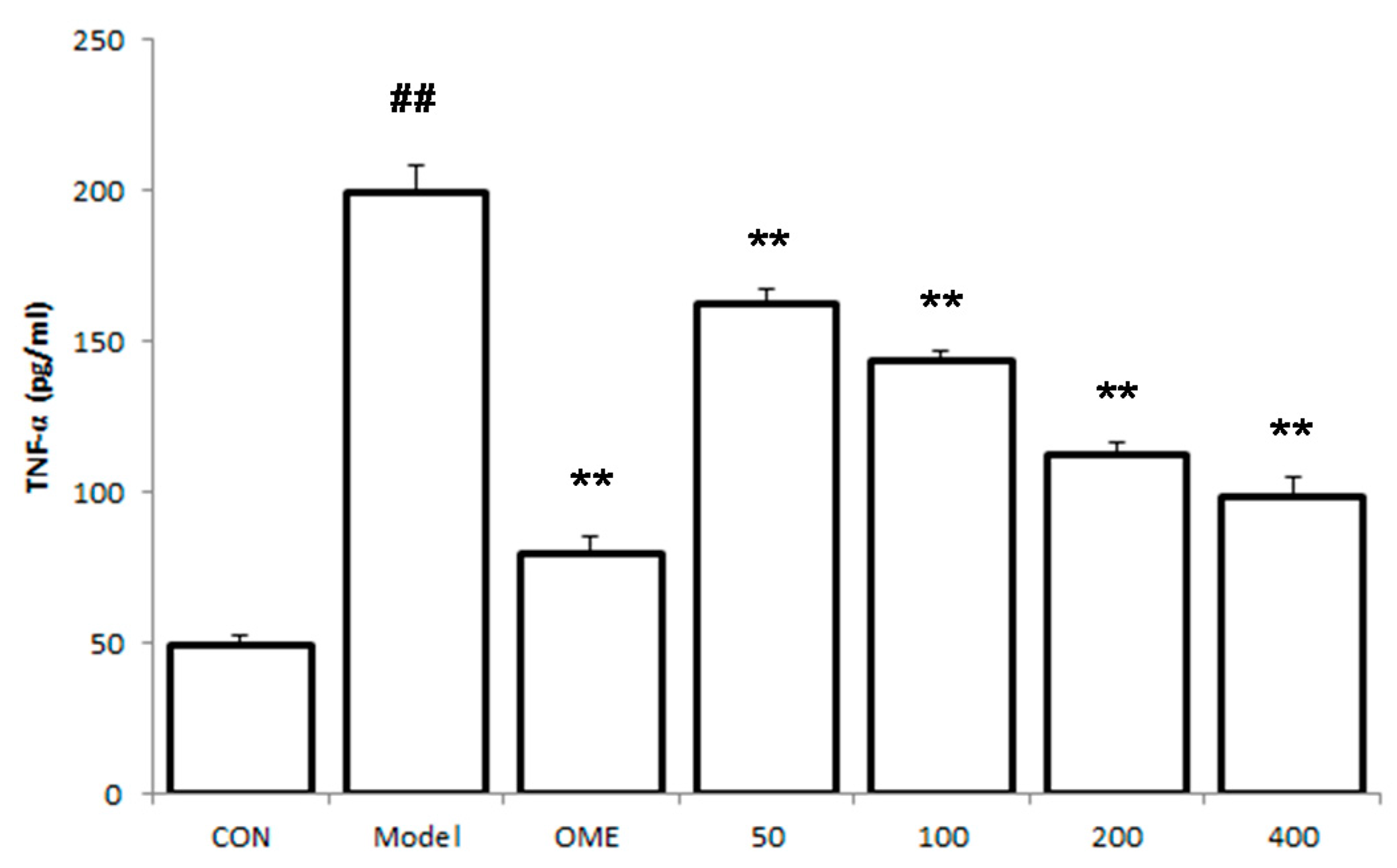
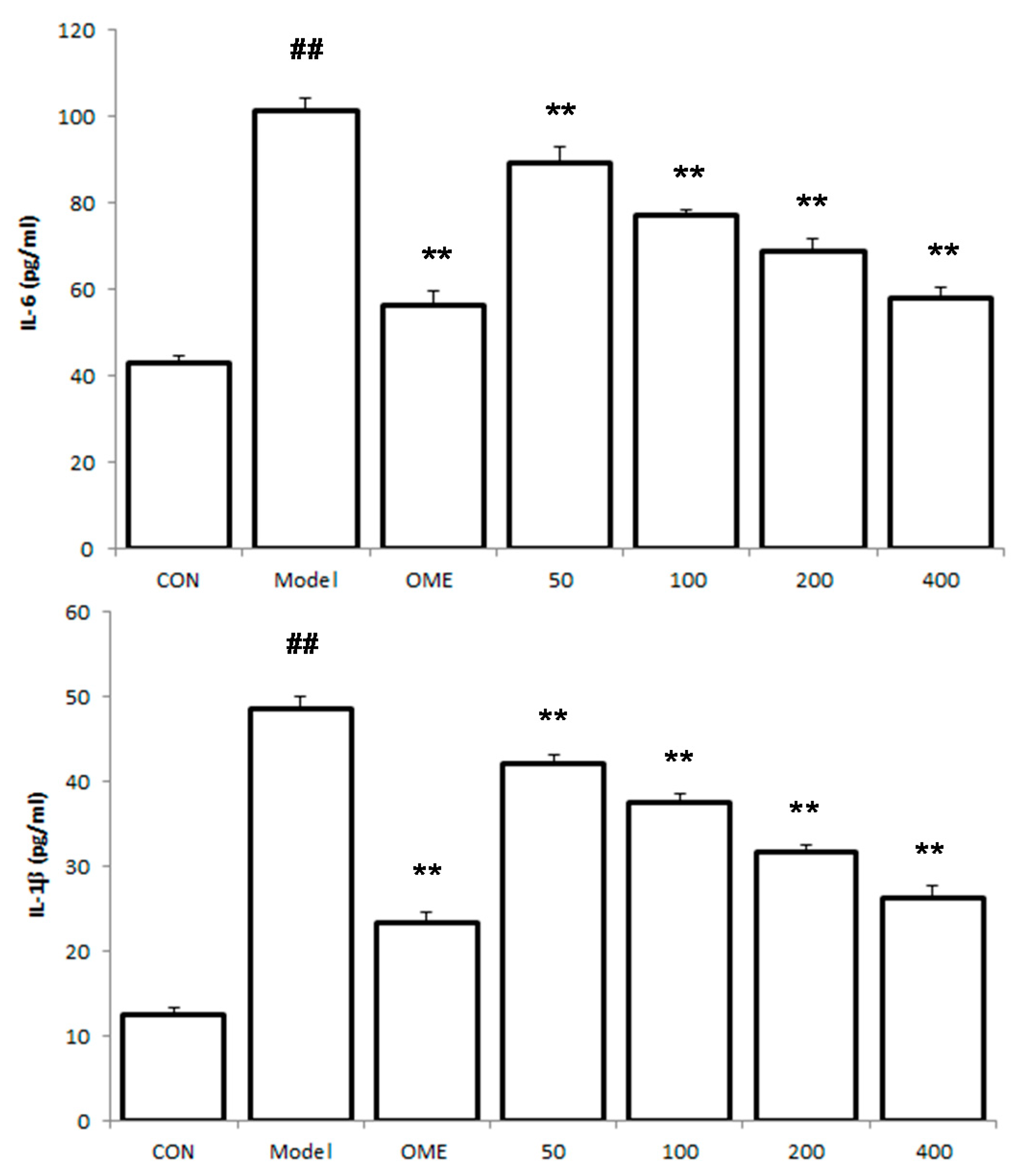
2.6. Histology of Gastric Lesions
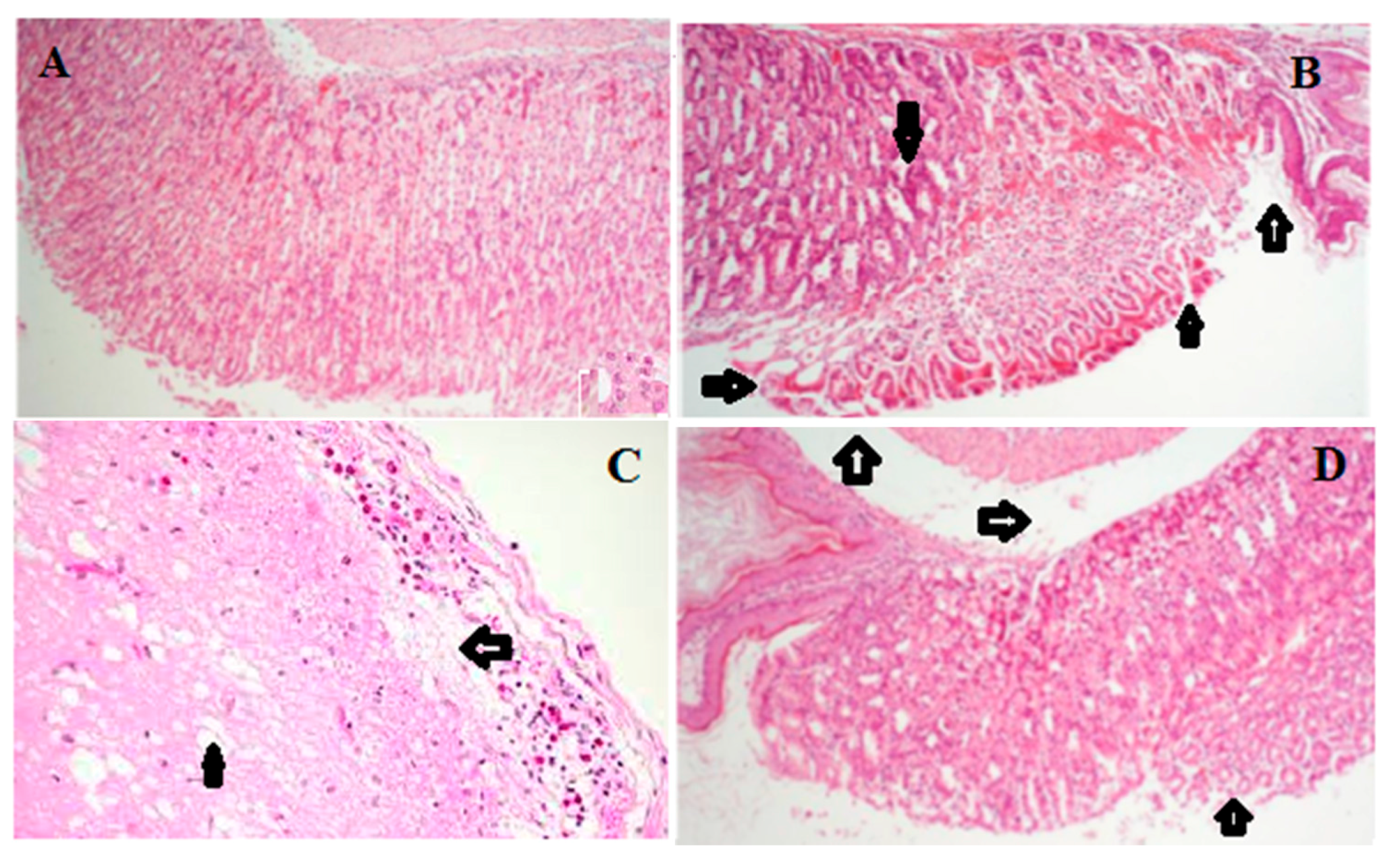
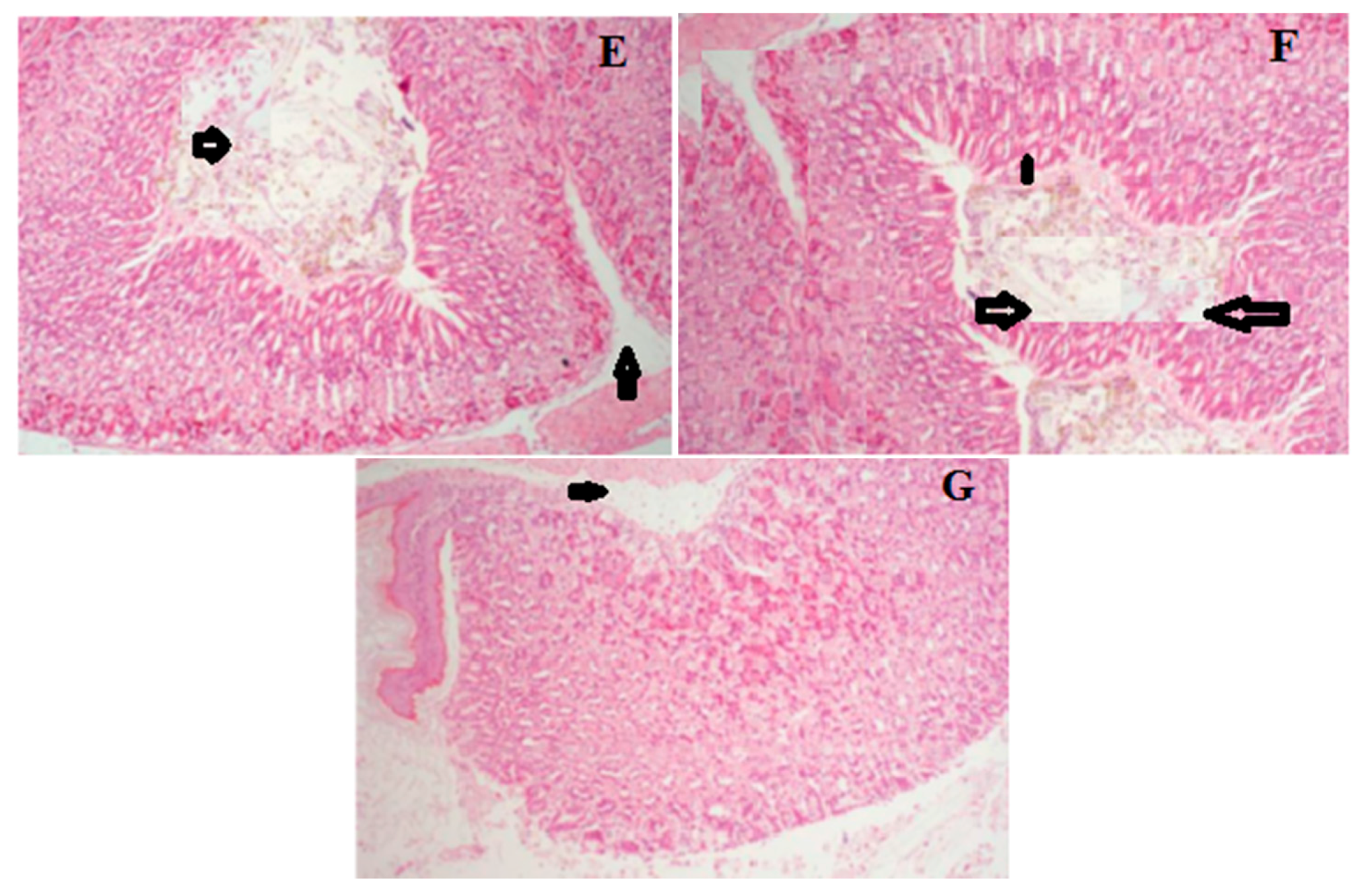
| Groups | Doses (mg/kg) | Hemorrhagic Damage | Edema | Epithelial Cell Loss | Inflammatory Cells |
|---|---|---|---|---|---|
| Control | - | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Model | - | 3.78 ± 0.32 ## | 3.83 ± 0.62 ## | 3.62 ± 0.17 ## | 2.54 ± 0.48 ## |
| OME | 20 | 1.31 ± 0.44 ** | 1.58 ± 0.36 ** | 1.47 ± 0.24 ** | 1.30 ± 0.33 ** |
| LCA | 50 | 3.42 ± 0.41 ** | 3.65 ± 0.17 ** | 3.34 ± 0.27 ** | 2.21 ± 0.28 ** |
| 100 | 2.97 ± 0.14 ** | 2. 87 ± 0.52 ** | 2.90 ± 0.38 ** | 1.96 ± 0.12 ** | |
| 200 | 2.48 ± 0.47 ** | 2.43 ± 0.12 ** | 2.62 ± 0.37 ** | 1.81 ± 0.23 ** | |
| 400 | 2.21 ± 0.29 ** | 1.82 ± 0.56 ** | 2.22 ± 0.35 ** | 1.50 ± 0.10 ** |
2.7. Effect of LCA on NF-κBp65 Expression
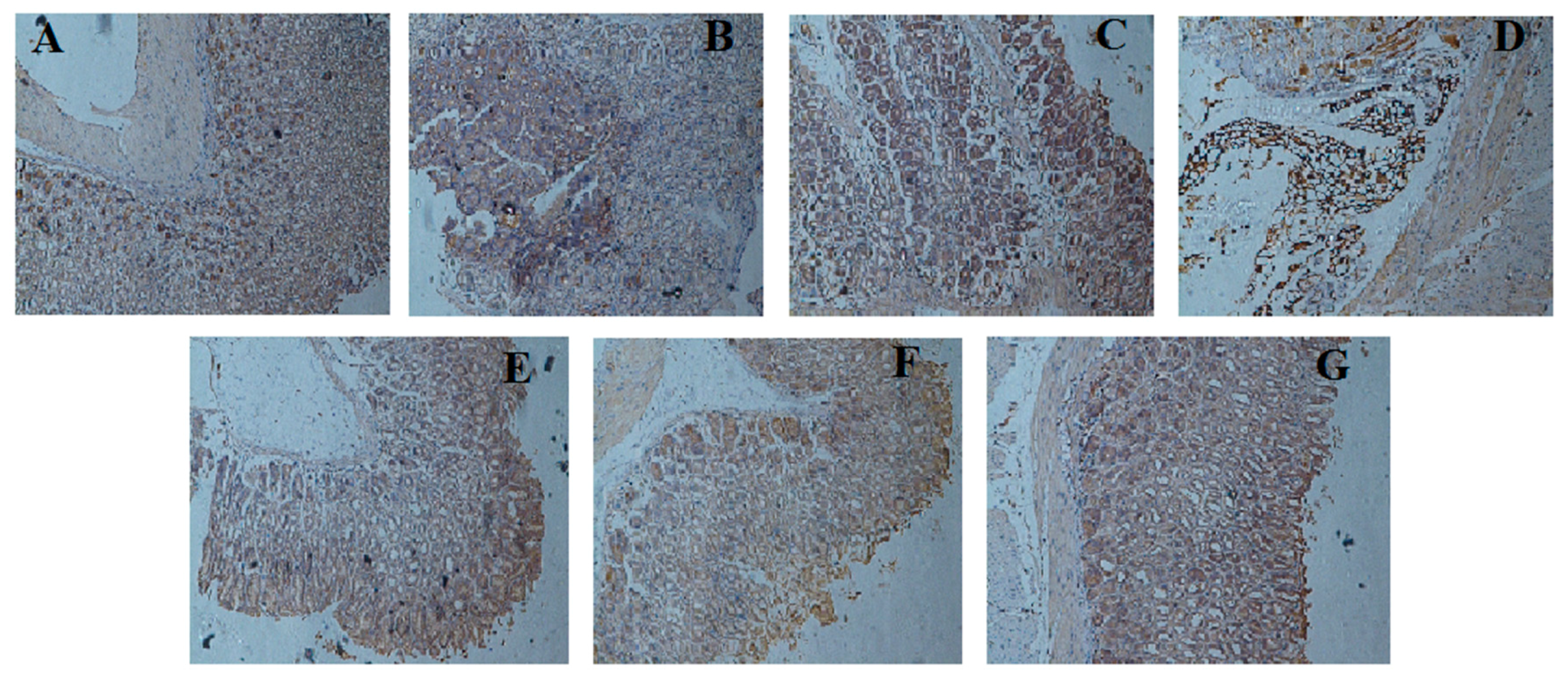
3. Discussion
4. Experimental Section
4.1. Chemical and Reagent
4.2. Plant Material
Extraction of Plant Material
4.3. Animals
4.3.1. Induction of Gastric Ulcer and Treatment
4.3.2. Measurement of Gastric Juice Acid Content (pH)
4.3.3. MPO, MDA, GSH and SOD Levels Determination
4.3.4. Cytokines Determination
4.3.5. Histological Procedure and Evaluation
4.3.6. Immunohistochemical Analysis
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Caldas, G.F.R.; do Amaral Costa, I.M.; da Silva, J.B.R.; da Nóbrega, R.F.; Rodrigues, F.F.G.; da Costa, J.G.M.; Wanderley, A.G. Antiulcerogenic activity of the essential oils of Hyptis martiusii Benth. (Lamiaceae). J. Ethnopharmacol. 2011, 137, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Viana, A.F.; Fernandes, H.B.; Silva, F.V.; Oliveira, I.S.; Freitas, F.F.; Machado, F.D.; Costa, C.L.; Arcanjo, D.D.; Chaves, M.H.; Oliveira, F.A.; et al. Gastroprotective activity of Cenostigma macrophyllum Tul. Var. acuminata Teles Freire leaves on experimental ulcer models. J. Ethnopharmacol. 2013, 150, 316–323. [Google Scholar]
- Behrman, S.W. Management of complicated peptic ulcer disease. Arch. Surg. 2005, 140, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Klein, L.C., Jr.; Gandolfi, R.B.; Santin, J.R.; Lemos, M.; Cechinel Filho, V.; de Andrade, S.F. Antiulcerogenic activity of extracts, fractions, and some compounds obtained from Polygala cyparissias St. Hillaire & Moquin (Poligalaceae). Naunyn Schmiedebergs Arch. Pharmacol. 2010, 381, 121–126. [Google Scholar] [PubMed]
- Tsukimi, Y.; Nakai, H.; Itoh, S.; Amagase, K.; Okabe, S. Involvement of heat shock proteins in the healing of acetic acid-induced gastric ulcers in rats. J. Physiol. Pharmacol. 2001, 52, 391–406. [Google Scholar] [PubMed]
- Gadekar, R.; Singour, P.K.; Chaurasiya, P.K.; Pawar, R.S.; Patil, U.K. A potential of some medicinal plants as an ulcer agents. Pharmacogn. Rev. 2010, 4, 136–146. [Google Scholar] [PubMed]
- Malfertheiner, P.; Chan, F.K.; Mccoll, K.E. Peptic ulcer disease. Lancet 2009, 374, 1449–1461. [Google Scholar] [CrossRef]
- Awaad, A.S.; El-Meligy, R.M.; Soliman, G.A. Natural products in treatment of ulcerative colitis and peptic ulcer. J. Saudi Chem. Soc. 2013, 17, 101–124. [Google Scholar] [CrossRef]
- Tuorkey, M.; Karoli, K. Anti-ulcer activity of curcumin on experimental gastric in rats and its effects on oxidative stress/antioxidant, IL-6 and enzyme activities. Biomed. Environ. Sci. 2009, 22, 488–495. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Bandyopadhyay, U.; Biswas, K.; Maity, P.; Banerjee, R.K. Indomethacin inactivates gastric peroxidase to induce reactive oxygen mediated gastric mucosal injury and curcumin protects it by preventing peroxidase inactivation and scavenging reactive oxygen. Free Radic. Biol. Med. 2006, 40, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.Y.; Pan, K.Y.; Nicholas, J.T. Flora of China; Science Press: Beijing, China, 2004; pp. 1–9. [Google Scholar]
- Yao, X.; Peng, Y.; Xu, L.J.; Li, L.; Wu, Q.L.; Xiao, P.G. Phytochemical and biological studies of Lycium medicinal plants. Chem. Biodivers. 2011, 8, 976–1010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.X.; Guan, S.H.; Feng, R.H.; Wang, W.; Wu, Z.Y.; Zhang, Y.B.; Chen, X.H.; Bi, K.S.; Guo, D.A. Neolignanamides, lignanamides and other phenolic compounds from the root back of Lycium chinense. J. Nat. Prod. 2013, 76, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Vlase, L.; Vodnar, D.C.; Bischin, C.; Hanganu, D.; Gheldiu, A.M.; Oprean, R.; Silaghi-Dumitrescu, R.; Crișan, G. Polyphenolic content, antioxidant and antimicrobial activities of Lycium barbarum L. and Lycium chinense Mill. Leaves. Molecules 2014, 19, 10056–10073. [Google Scholar] [CrossRef] [PubMed]
- Potterat, O. Goji (Lycium barbarum and L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010, 76, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Nashida, K.; Ohta, Y.; Ishiguro, I. Contribution of nitric oxide synthases to neutrophil infiltration in the gastric mucosal lesions in rats with water immersion restraint stress. FEBS Lett. 1998, 425, 243–248. [Google Scholar] [CrossRef]
- Martin, G.R.; Wallace, J.L. Gastrointestinal inflammation: A central component of mucosal defense and repair. Exp. Biol. Med. 2006, 231, 130–137. [Google Scholar]
- Faubion, W.A.; Gores, G.J. Death receptor in liver biology and pathobiology. Hepatology 1999, 29. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, M.; Watanabe, T.; Higuchi, K.; Tominaga, K.; Fujiwara, Y.; Arakawa, T. Mechanisms and roles of neutrophil infiltration in stress-induced gastric injury in rats. Dig. Dis. Sci. 2001, 46, 2708–2715. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.M.; Heywood, G.J.; di Girolamo, N.; Thomas, P.S. Nicorandil inhibits the release of TNF-alpha from a lymphocyte cell line and peripheral blood lymphocytes. Int. Immunopharmacol. 2003, 3, 1581–1588. [Google Scholar] [CrossRef]
- Kishimoto, T. Interleukin-6: From basic science to medicine—40 years in immunology. Annu. Rev. Immunol. 2005, 23, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Augusto, A.C.; Miguel, F.; Mendonca, S.; Pedrazzoli, J., Jr.; Gurgueira, S.A. Oxidative stress expression status associated to Helicobacter pylori virulence in gastric diseases. Clin. Biochem. 2007, 40, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Wassmann, S.; Stump, M.; Strehlow, K.; Schmid, A.; Schieffer, B.; Bohm, M.; Nickenig, G. Interleukin-6 induces oxidative stress and endothelial dysfunction by over expression of the angiotensin II type 1 receptor. Circ. Res. 2004, 94, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Rozza, A.L.; de Mello Moraes, T.; Kushima, H.; Tanimoto, A.; Marques, M.O.; Bauab, T.M.; Hiruma-Lima, C.A.; Pellizzon, C.H. Gastroprotective mechanisms of Citrus lemon (Rutaceae) essential oil and its majority compounds limonene and beta-pinene: Involvement of heat shock protein-70, vasoactive intestinal peptide, glutathione, sulfhydryl compounds, nitric oxide and postagladin E2. Chem. Biol. Interact. 2011, 189, 82–89. [Google Scholar] [PubMed]
- Sidahmed, H.M.; Hashim, N.M.; Amir, J.; Abdulla, M.A.; Hadi, A.H.; Abdelwahab, S.I.; Taha, M.M.; Hassandarvish, P.; Teh, X.; Loke, M.F.; et al. Pyranocycloartobiloxanthone A, a novel gastroprotective compound from Artocarpus obtusus Jarret against ethanol-induced acute gastric ulcer in vivo. Phytomedicine 2013, 20, 834–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, J.Y.; Abdulla, M.A.; Raman, J.; Phan, C.W.; Kuppusamy, U.R.; Golbabapour, S.; Sabaratnam, V. Gastroprotective effects of Lions Mane mushroom Hericium erinaceus (Bull.: Fr.) Pers. (Aphyllophoromycetideae) extract against ethanol induced ulcer in rats. Evid. Based Complement. Alternat. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Chen, N.; Chi, G.; Yuan, X.; Guan, S.; Li, H.; Zhong, W.; Guo, W.; Soromou, L.W.; Gao, R.; et al. Traditional medicine alpinetin inhibits the inflammatory response in RAW 264.7 cells and mouse models. Int. Immunopharmacol. 2012, 12, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Hacker, H.; Karin, M. Regulation and function of IKK and IKK-related kinases. Sci. STKE 2006. [Google Scholar] [CrossRef] [PubMed]
- Rogler, G.; Brand, K.; Vogl, D.; Page, S.; Hofmeister, R.; Andus, T. Nuclear factor κB is activated in macrophages and epithelial cells of inflaned intestinal mucosa. Gastroenterology 1998, 115, 357–369. [Google Scholar] [CrossRef]
- Baldwin, A.S., Jr. Series introduction. The transcription factor NF-κB and human diseases. J. Clin. Investig. 2001, 107, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Yang, Q.; Xiao, W.; Liu, F.; Chen, J. Anti-ulcer effects of Lycium barbarum polysaccharide in rats. J. Med. Coll. PLA 2005, 20, 161–164. [Google Scholar]
- Laine, L.; Weinstein, W.M. Histology of alcoholic hemorrhage gastritis a prospective evaluation. Gastroenterology 1998, 94, 1254–1262. [Google Scholar]
- Gomma, W.; Ke, Y.; Fujii, H.; Helliwell, T. Tissue microarray of head and neck squamous carcinoma: validation of the methodology for the study of cutaneous fatty acid binding protein, vascular endothelial growth factor, involucrin and Ki-67. Virchows Arch. 2005, 447, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olatunji, O.J.; Chen, H.; Zhou, Y. Anti-Ulcerogenic Properties of Lycium chinense Mill Extracts against Ethanol-Induced Acute Gastric Lesion in Animal Models and Its Active Constituents. Molecules 2015, 20, 22553-22564. https://doi.org/10.3390/molecules201219867
Olatunji OJ, Chen H, Zhou Y. Anti-Ulcerogenic Properties of Lycium chinense Mill Extracts against Ethanol-Induced Acute Gastric Lesion in Animal Models and Its Active Constituents. Molecules. 2015; 20(12):22553-22564. https://doi.org/10.3390/molecules201219867
Chicago/Turabian StyleOlatunji, Opeyemi J., Hongxia Chen, and Yifeng Zhou. 2015. "Anti-Ulcerogenic Properties of Lycium chinense Mill Extracts against Ethanol-Induced Acute Gastric Lesion in Animal Models and Its Active Constituents" Molecules 20, no. 12: 22553-22564. https://doi.org/10.3390/molecules201219867