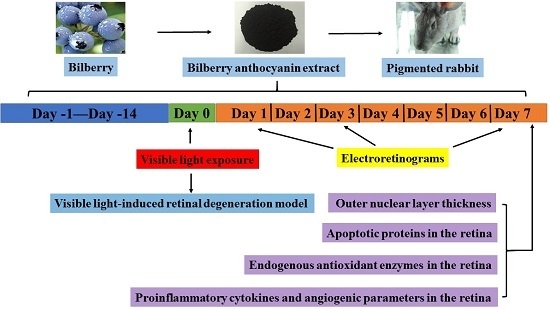
Retinoprotective Effects of Bilberry Anthocyanins via Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Mechanisms in a Visible Light-Induced Retinal Degeneration Model in Pigmented Rabbits
Abstract
:1. Introduction
2. Results and Discussion
2.1. Concentrations of Anthocyanins in Bilberry Anthocyanin Extract (BAE)

| Identification | Structure | [M+] m/z Total/Aglycon | mg of ACN/g of BAE | ||
|---|---|---|---|---|---|
| R1 | R2 | R3 | |||
| Delphinidin-3-galactoside | H | OH | Gal | 465/303 | 36.67 |
| Delphinidin-3 -glucoside | H | OH | Glu | 465/303 | 44.92 |
| Cyanidin-3-galactoside | H | H | Gal | 449/287 | 43.36 |
| Delphinidin-3-arabinoside | H | OH | Ara | 435/303 | 54.60 |
| Cyanidin-3-glucoside | H | H | Glu | 449/287 | 52.30 |
| Petunidin-3- galactoside | H | CH3O | Gal | 479/317 | 15.42 |
| Cyanidin-3-arabinoside | H | H | Ara | 419/287 | 44.18 |
| Petunidin-3-glucoside | H | CH3O | Glu | 479/317 | 35.81 |
| Peonidin-3-galactoside | CH3 | H | Gal | 463/301 | 7.14 |
| Petunidin-3-arabinoside | H | CH3O | Ara | 449/317 | 10.79 |
| Peonidin-3 -glucoside | CH3 | H | Glu | 463/301 | 26.25 |
| Malvidin-3-galactoside | CH3 | CH3O | Gal | 493/331 | 10.62 |
| Malvidin-3-glucoside | CH3 | CH3O | Glu | 493/331 | 26.79 |
| Malvidin-3-arabinoside | CH3 | CH3O | Ara | 463/331 | 1.31 |
| Total anthocyanins | 410.16 | ||||
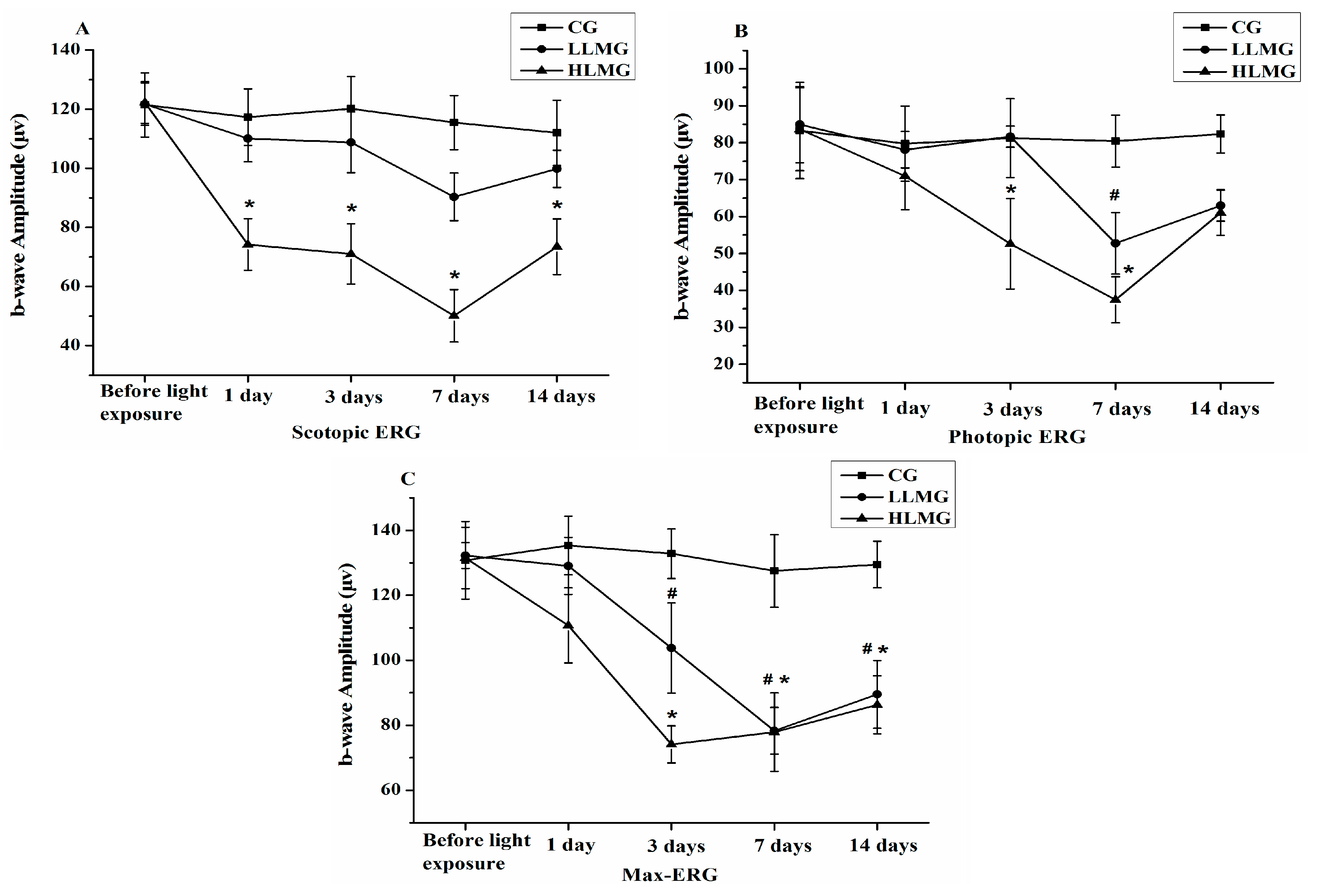
2.2. Effect of Visible Light Exposure on Retinal Function
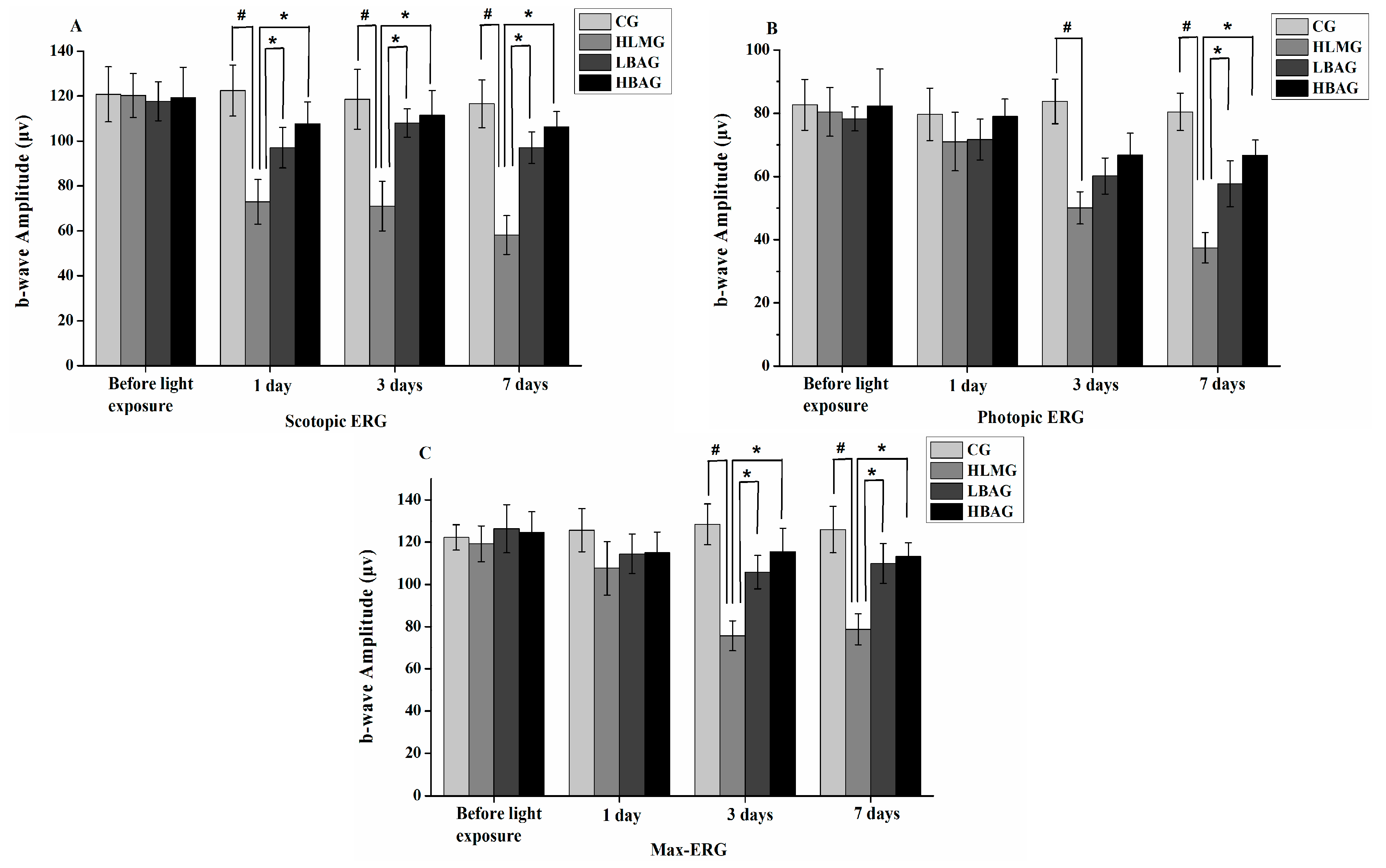
2.3. Protective Effect of Bilberry Anthocyanin Extract (BAE) on Visual Function
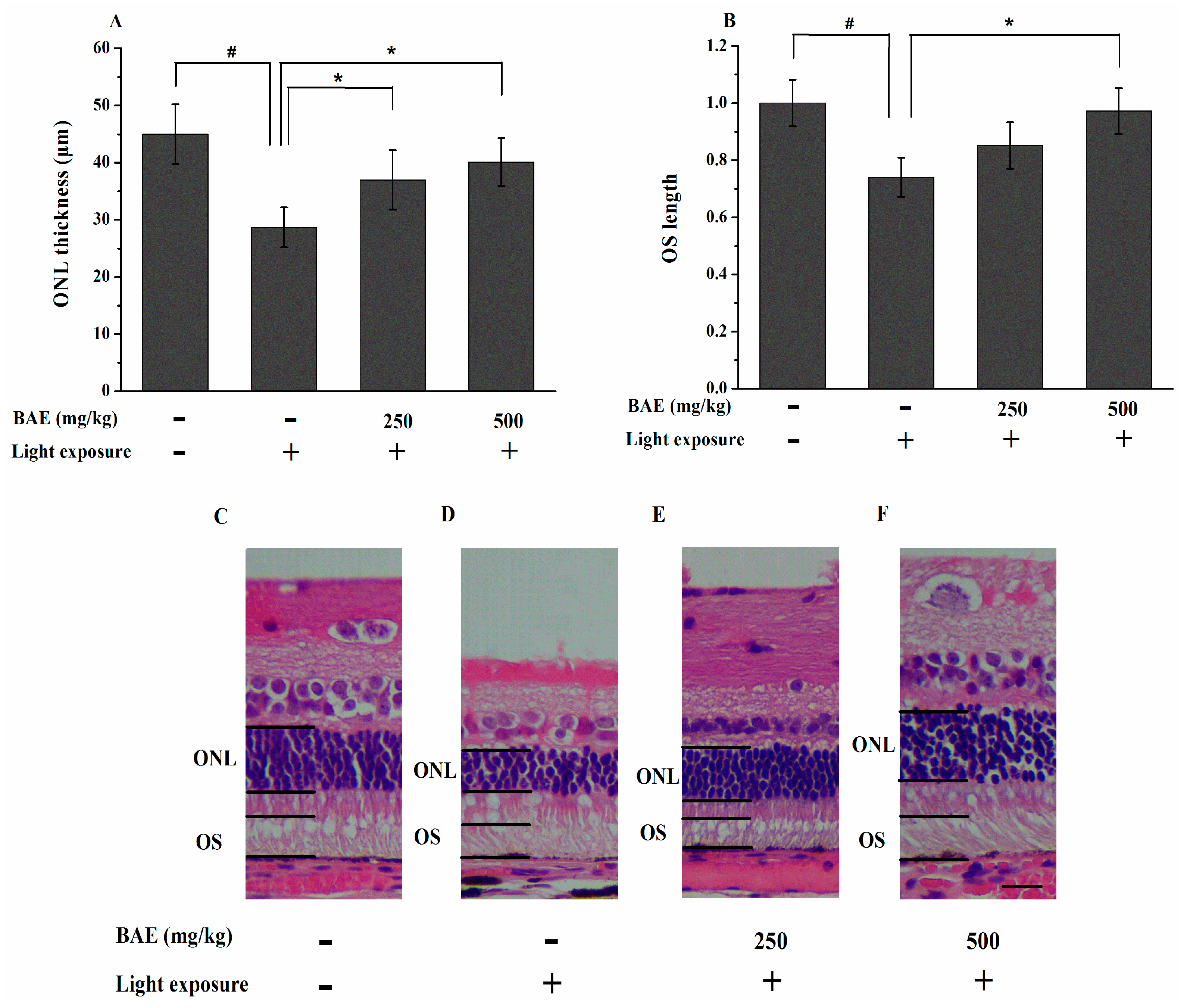
2.4. Protective Effect of Bilberry Anthocyanin Extract (BAE) on the Outer Nuclear Layer (ONL) Thickness and Outer Segment (OS) Length
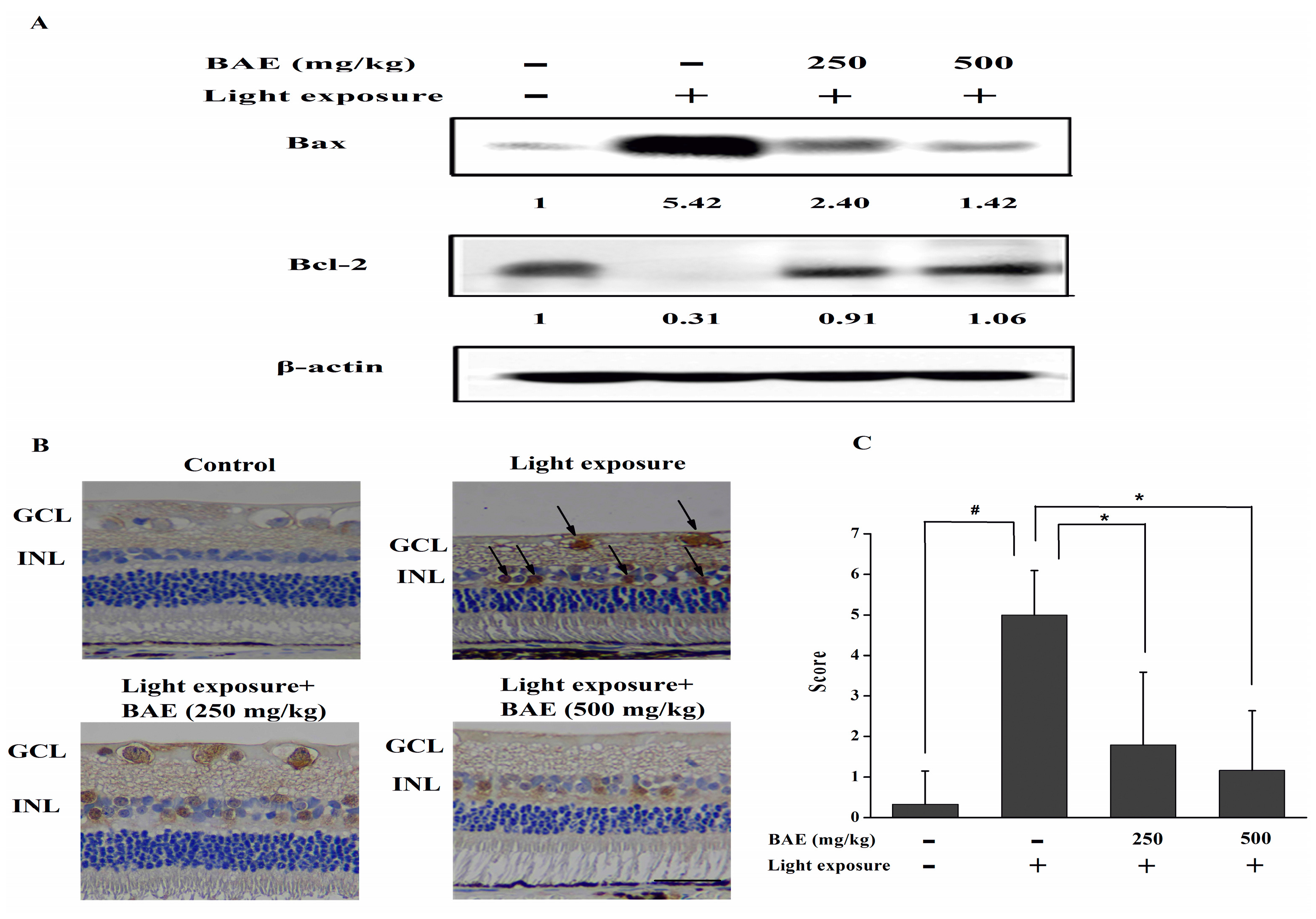
2.5. Bilberry Anthocyanin Extract (BAE) Protected Rabbits from Light-Induced Apoptosis in Retina
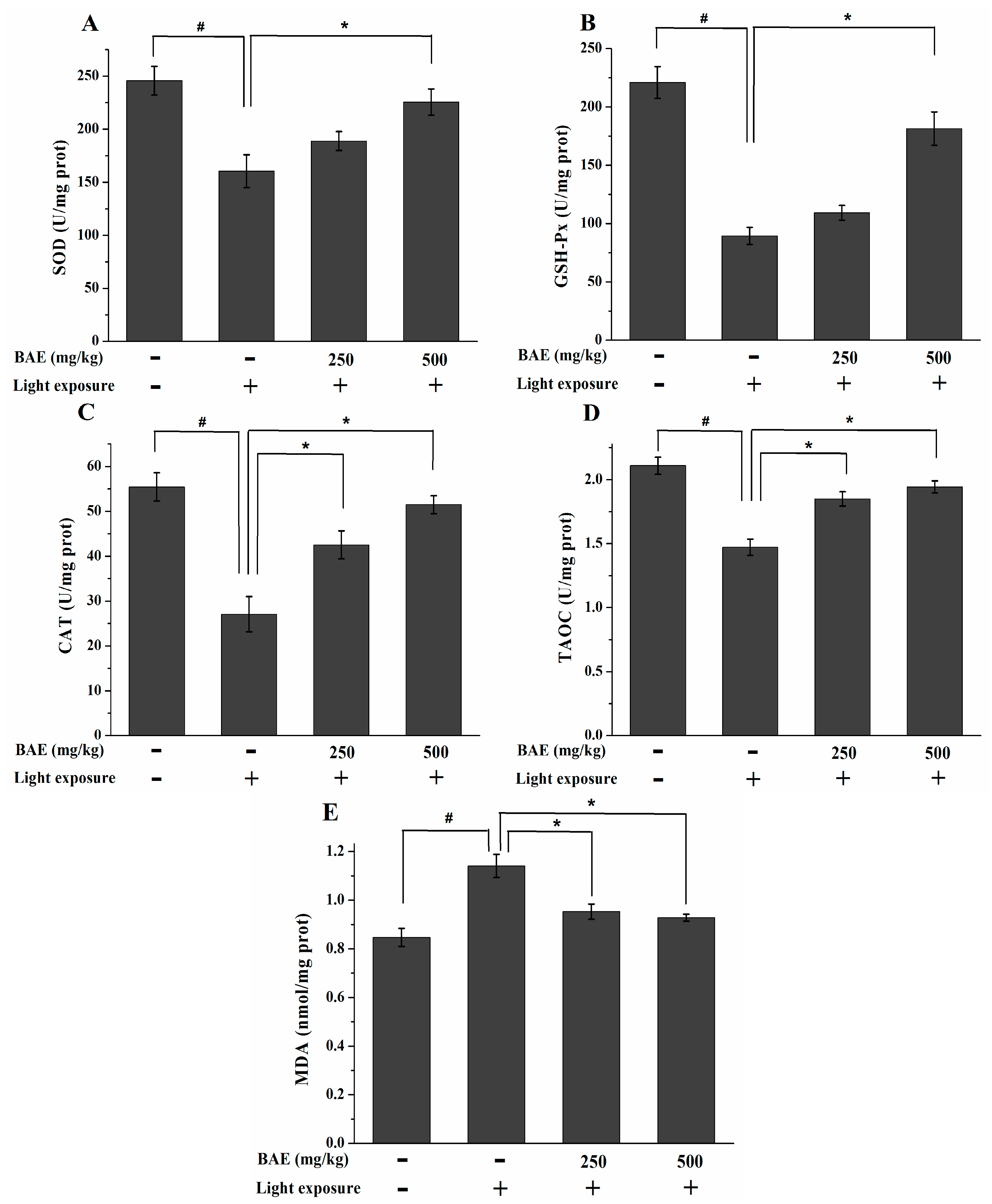
2.6. The Activities of SOD, GSH-Px and CAT, Levels of MDA and TAOC
2.7. The IL-1β and VEGF Levels

3. Experimental Section
3.1. Chemicals and Reagents
3.2. Liquid Chromatography-Electrospray Ionization Tandem Mass Spectrometry (LC-ESI-MS/MS) Analysis of Bilberry Anthocyanin Extract (BAE)
3.3. Animal Care
3.4. Treatment with Bilberry Anthocyanin Extract (BAE) and Exposure to Light
3.5. Electroretinograms (ERGs)
3.6. Hematoxylin and Eosin (HE) Staining
3.7. Measurement of the Outer Nuclear Layer (ONL) Thickness and Outer Segment (OS) Length
3.8. Western Blot Analysis
3.9. Immunohistochemistry for Caspase-3
3.10. Determination of the Activities of SOD, GSH-Px and CAT, Levels of MDA and TAOC
3.11. Determination of the IL-1β and VEGF Levels
3.12. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
| Abbreviation | Original Word |
|---|---|
| BAE | Bilberry anthocyanin extract |
| RPE | Retinal pigment epithelial |
| RP | Retinitis pigmentosa |
| ERG | Electroretinogram |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| MDA | Malondialdehyde |
| HLMG | High light-induced retinal damage model group |
| HBAG | High dosage of BAE group |
| ONL | Outer nuclear layer |
| HE | Hematoxylin and eosin |
| NF-κB | Nuclear factor-κB |
| VEGF | Vascular endothelial growth factor |
| AMD | Age-related macular degeneration |
| OS | Outer segment |
| max-ERG | Maximal response ERG |
| GSH-Px | Glutathione peroxidase |
| TAOC | Total antioxidant capacity |
| CG | Control group |
| LLMG | Low light-induced retinal damage model group |
| LBAG | Low dosage of BAE group |
| ONH | Optic nerve head |
| ROS | Reactive oxygen species |
| PUFAs | Polyunsaturated fatty acids |
References
- Wenzel, A.; Grimm, C.; Samardzija, M.; Reme, C.E. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog. Retin. Eye Res. 2005, 24, 275–306. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.J.; Morgan, J.I.W.; Merigan, W.H.; Sliney, D.H.; Sparrow, J.R.; Williams, D.R. The susceptibility of the retina to photochemical damage from visible light. Prog. Retin. Eye Res. 2012, 31, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Gehrs, K.M.; Anderson, D.H.; Johnson, L.V.; Hageman, G.S. Age-related macular degeneration—Emerging pathogenetic and therapeutic concepts. Ann. Med. 2006, 38, 450–471. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Chou, C.-F.; Klein, B.E.K.; Zhang, X.; Meuer, S.M.; Saaddine, J.B. Prevalence of age-related macular degeneration in the US population. Arch. Ophthalmol. 2011, 129, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Patel, M.; Chan, C.-C. Molecular pathology of age-related macular degeneration. Prog. Retin. Eye Res. 2009, 28, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, J.R.; Nakanishi, K.; Parish, C.A. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1981–1989. [Google Scholar]
- Jang, Y.P.; Zhou, J.L.; Nakanishi, K.; Sparrow, J.R. Anthocyanins protect against A2E photooxidation and membrane permeabilization in retinal pigment epithelial cells. Photochem. Photobiol. 2005, 81, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Sanchez, L.; Lax, P.; Noailles, A.; Angulo, A.; Maneu, V.; Cuenca, N. Natural compounds from saffron and bear bile prevent vision loss and retinal degeneration. Molecules 2015, 20, 13875–13893. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Kumar, B.; Srinivasan, B.P.; Nag, T.C.; Srivastava, S.; Saxena, R.; Aggarwal, A. Retinoprotective effects of moringa oleifera via antioxidant, anti-inflammatory, and anti-angiogenic mechanisms in streptozotocin-induced diabetic rats. J. Ocul. Pharmacol. Ther. 2013, 29, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, H.L.; Tuo, J.; Shen, D.F.; Zhang, J.; Cao, X.; Chew, E.Y.; Chan, C.-C. Nutrient supplementation with n3 polyunsaturated fatty acids, lutein, and zeaxanthin decrease A2E accumulation and VEGF expression in the retinas of Ccl2/Cx3cr1-deficient mice on Crb1(rd8) background. J. Nutr. 2013, 143, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; Hanneken, A.; Milbury, P.; Tremblay, F. Recent Research on polyphenolics in vision and eye health. J. Agric. Food Chem. 2010, 58, 4001–4007. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Wang, B.; Chen, F.; Huang, Z.; Wang, X.; Luo, P.G. Comparison of anthocyanins and phenolics in organically and conventionally grown blueberries in selected cultivars. Food Chem. 2011, 125, 201–208. [Google Scholar] [CrossRef]
- Tsuda, T. Dietary anthocyanin-rich plants: Biochemical basis and recent progress in health benefits studies. Mol. Nutr. Food Res. 2012, 56, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.E.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Miyake, S.; Takahashi, N.; Sasaki, M.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Vision preservation during retinal inflammation by anthocyanin-rich bilberry extract: Cellular and molecular mechanism. Labor. Investig. 2012, 92, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Nakamura, Y.; Tachibanaki, S.; Kawamura, S.; Hirayama, M. Stimulatory effect of cyanidin 3-glycosides on the regeneration of rhodopsin. J. Agric. Food Chem. 2003, 51, 3560–3563. [Google Scholar] [CrossRef] [PubMed]
- Yanamala, N.; Tirupula, K.C.; Balem, F.; Klein-Seetharaman, J. pH-dependent interaction of rhodopsin with cyanidin-3-glucoside. 1. Structural aspects. Photochem. Photobiol. 2009, 85, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Werner, L.; Chang, W.; Haymore, J.; Haugen, B.; Romaniv, N.; Sandstedt, C.; Chang, S.; Mamalis, N. Retinal safety of the irradiation delivered to light-adjustable intraocular lenses evaluated in a rabbit model. J. Cataract Refract. Surg. 2010, 36, 1392–1397. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yasumura, D.; Ma, A.A.K.; Matthes, M.T.; Yang, H.; Nielson, G.; Huang, Y.; Szoka, F.C.; LaVail, M.M.; Diamond, M.I. Intravitreal administration of HA-1077, a ROCK inhibitor, improves retinal function in a mouse model of huntington disease. PLoS ONE 2013, 8, e56026. [Google Scholar] [CrossRef] [PubMed]
- Feigl, B.; Lovie-Kitchin, J.; Brown, B. Objective functional assessment of age-related maculopathy: A special application for the multifocal electroretinogram. Clin. Exp. Optom. J. Aust. Optom. Assoc. 2005, 88, 304–312. [Google Scholar] [CrossRef]
- Birch, D.G. Surrogate electroretinographic markers for assessing therapeutic efficacy in the retina. Expert Rev. Mol. Diagn. 2004, 4, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Osborne, N.N.; Casson, R.J.; Wood, J.P.M.; Chidlow, G.; Graham, M.; Melena, J. Retinal ischemia: Mechanisms of damage and potential therapeutic strategies. Prog. Retin. Eye Res. 2004, 23, 91–147. [Google Scholar] [CrossRef] [PubMed]
- Curcio, C.A. Photoreceptor topography in ageing and age-related maculopathy. Eye 2001, 15, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Kaidzu, S.; Anderson, R.E. Protective effects of soft acrylic yellow filter against blue light-induced retinal damage in rats. Exp. Eye Res. 2006, 83, 1493–1504. [Google Scholar] [CrossRef] [PubMed]
- Noell, W.K.; Walker, V.S.; Kang, B.S.; Berman, S. Retinal damage by light in rats. Investig. Ophthalmol. 1966, 5, 450–473. [Google Scholar]
- Sasaki, M.; Yuki, K.; Kurihara, T.; Miyake, S.; Noda, K.; Kobayashi, S.; Ishida, S.; Tsubota, K.; Ozawa, Y. Biological role of lutein in the light-induced retinal degeneration. J. Nutr. Biochem. 2012, 23, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, T.; Shimazawa, M.; Nakanishi, T.; Ohno, Y.; Inoue, Y.; Tsuruma, K.; Ishibashi, T.; Hara, H. The protective effects of a dietary carotenoid, astaxanthin, against light-induced retinal damage. J. Pharmacol. Sci. 2013, 123, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, F.; Waterhouse, J.; Nason, J.; Kalt, W. Prophylactic neuroprotection by blueberry-enriched diet in a rat model of light-induced retinopathy. J. Nutr. Biochem. 2013, 24, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Narimatsu, T.; Ozawa, Y.; Miyake, S.; Kubota, S.; Yuki, K.; Nagai, N.; Tsubota, K. Biological effects of blocking blue and other visible light on the mouse retina. Clin. Exp. Ophthalmol. 2014, 42, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Roehlecke, C.; Schumann, U.; Ader, M.; Brunssen, C.; Bramke, S.; Morawietz, H.; Funk, R.H.W. Stress reaction in outer segments of photoreceptors after blue light irradiation. PLoS ONE 2013, 8, e71570. [Google Scholar] [PubMed]
- Calzia, D.; Barabino, S.; Bianchini, P.; Garbarino, G.; Oneto, M.; Caicci, F.; Diaspro, A.; Tacchetti, C.; Manni, L.; Candiani, S.; et al. New findings in ATP supply in rod outer segments: Insights for retinopathies. Biol. Cell 2013, 105, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Calzia, D.; Oneto, M.; Caicci, F.; Bianchini, P.; Ravera, S.; Bartolucci, M.; Diaspro, A.; Degan, P.; Manni, L.; Traverso, C.E.; et al. Effect of polyphenolic phytochemicals on ectopic oxidative phosphorylation in rod outer segments of bovine retina. Br. J. Pharmacol. 2015, 172, 3890–3903. [Google Scholar] [CrossRef] [PubMed]
- Hafezi, F.; Marti, A.; Munz, K.; Reme, C.E. Light-induced apoptosis: Differential timing in the retina and pigment epithelium. Exp. Eye Res. 1997, 64, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, Y.; Shimizu, S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis 2007, 12, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Surh, Y.J. Potentiation of cellular antioxidant capacity by Bcl-2: Implications for its antiapoptotic function. Biochem. Pharmacol. 2003, 66, 1371–1379. [Google Scholar] [CrossRef]
- Perche, O.; Doly, M.; Ranchon-Cole, I. Caspase-dependent apoptosis in light-induced retinal degeneration. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2753–2759. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.; Maslim, J.; Valter-Kocsi, K.; Mervin, K.; Bowers, F.; Chu, Y.; Barnett, N.; Provis, J.; Lewis, G.; Fisher, S.K.; et al. Mechanisms of photoreceptor death and survival in mammalian retina. Prog. Retin. Eye Res. 1999, 18, 689–735. [Google Scholar] [CrossRef]
- Kunchithapautham, K.; Rohrer, B. Apoptosis and autophagy in photoreceptors exposed to oxidative stress. Autophagy 2007, 3, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Seregard, S.; Algvere, P.V. Photochemical damage of the retina. Surv. Ophthalmol. 2006, 51, 461–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, D.; Liu, Y.; Wang, D.; Liu, J.; Ji, B. The protective effects of berry-derived anthocyanins against visible light-induced damage in human retinal pigment epithelial cells. J. Sci. Food Agric. 2015, 95, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, J.R.; Gregory-Roberts, E.; Yamamoto, K.; Blonska, A.; Ghosh, S.K.; Ueda, K.; Zhou, J. The bisretinoids of retinal pigment epithelium. Prog. Retin. Eye Res. 2012, 31, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yanase, E.; Feng, X.; Siegel, M.M.; Sparrow, J.R. Structural characterization of bisretinoid A2E photocleavage products and implications for age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2010, 107, 7275–7280. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, J.R.; Zhou, J.; Ben-Shabat, S.; Vollmer, H.; Itagaki, Y.; Nakanishi, K. Involvement of oxidative mechanisms in blue-light-induced damage to A2E-laden RPE. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1222–1227. [Google Scholar]
- Romeo, G.; Liu, W.L.; Asnaghi, V.; Kern, T.S.; Lorenzi, M. Activation of nuclear factor-kappa B induced by diabetes and high glucose regulates a proapoptotic program in retinal pericytes. Diabetes 2002, 51, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, H.; Al-Shabrawey, M.; Caldwell, R.W.; Caldwell, R.B. Inflammation and diabetic retinal microvascular complications. J. Cardiovasc. Dis. Res. 2011, 2, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Costa, M.B.; Gerhardinger, C. IL-1 beta is upregulated in the diabetic retina and retinal vessels: Cell-specific effect of high glucose and iL-1 beta autostimulation. PLoS ONE 2012, 7, e36949. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Liao, S.; Mi, H.; Guo, C.; Qi, D.; Li, F.; Zhang, C.; Yang, Z. Hesperidin prevents retinal and plasma abnormalities in streptozotocin-induced diabetic rats. Molecules 2012, 17, 12868–12881. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed]
- Behl, Y.; Krothapalli, P.; Desta, T.; DiPiazza, A.; Roy, S.; Graves, D.T. Diabetes-enhanced tumor necrosis factor-alpha production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy. Am. J. Pathol. 2008, 172, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Grisanti, S.; Tatar, O. The role of vascular endothelial growth factor and other endogenous interplayers in age-related macular degeneration. Prog. Retin. Eye Res. 2008, 27, 372–390. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Jun, J.-H.; Jung, E.-H.; Koo, B.A.; Kim, Y.S. Epigalloccatechin-3-gallate inhibits ocular neovascularization and vascular permeability in human retinal pigment epithelial and human retinal microvascular endothelial cells via suppression of MMP-9 and VEGF activation. Molecules 2014, 19, 12150–12172. [Google Scholar] [CrossRef] [PubMed]
- Bush, R.A.; Lei, B.; Tao, W.; Raz, D.; Chan, C.C.; Cox, T.A.; Santos-Muffley, M.; Sieving, P.A. Encapsulated cell-based intraocular delivery of ciliary neurotrophic factor in normal rabbit: Dose-dependent effects on ERG and retinal histology. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2420–2430. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhao, L.; Lu, F.; Yang, X.; Deng, Q.; Ji, B.; Huang, F. Retinoprotective Effects of Bilberry Anthocyanins via Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Mechanisms in a Visible Light-Induced Retinal Degeneration Model in Pigmented Rabbits. Molecules 2015, 20, 22395-22410. https://doi.org/10.3390/molecules201219785
Wang Y, Zhao L, Lu F, Yang X, Deng Q, Ji B, Huang F. Retinoprotective Effects of Bilberry Anthocyanins via Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Mechanisms in a Visible Light-Induced Retinal Degeneration Model in Pigmented Rabbits. Molecules. 2015; 20(12):22395-22410. https://doi.org/10.3390/molecules201219785
Chicago/Turabian StyleWang, Yong, Liang Zhao, Feng Lu, Xue Yang, Qianchun Deng, Baoping Ji, and Fenghong Huang. 2015. "Retinoprotective Effects of Bilberry Anthocyanins via Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Mechanisms in a Visible Light-Induced Retinal Degeneration Model in Pigmented Rabbits" Molecules 20, no. 12: 22395-22410. https://doi.org/10.3390/molecules201219785