Phenolic Compounds from Merremia umbellata subsp. orientalis and Their Allelopathic Effects on Arabidopsis Seed Germination
Abstract
:1. Introduction
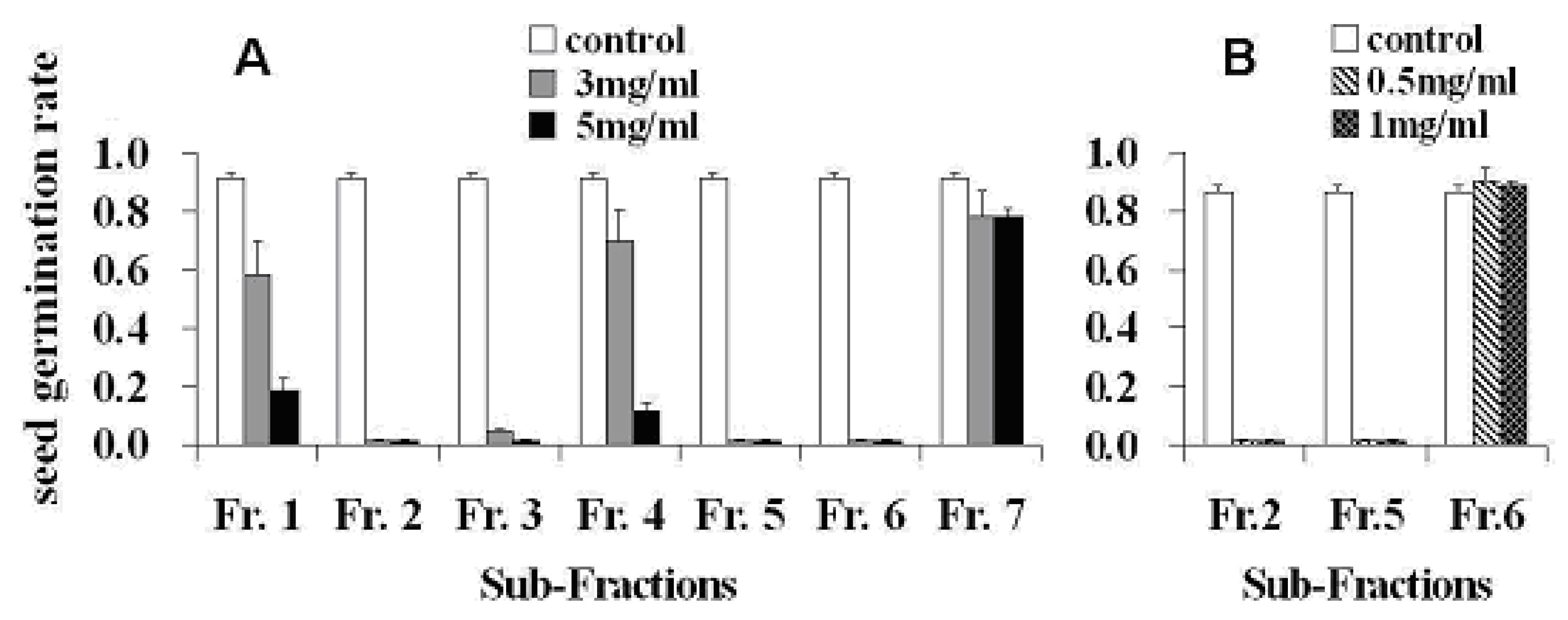
2. Results and Discussion
3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Hydrolysis, Sugar Permethylation and GC-MS Analysis
3.5. Seed Germination Bioassay
4. Conclusions
Acknowledgements
References and Notes
- Wu, D.L. Flora of Guangdong; Guangdong Science and Technology Press: Guangzhou, China, 2000; Volume 4, p. 345. [Google Scholar]
- Chen, B.H.; Wang, R.J.; Huang, X.L.; Zhou, L.X. Merremia boisiana - a newly recorded species from Guangdong, China. J. Trop. Subtrop. Bot. 2005, 13, 76–77. [Google Scholar]
- Fitter, A. Making Allelopathy Respectable. Science 2003, 301, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zheng, G.W.; Niu, X.M.; Li, W.Q.; Wang, F.S.; Li, S.H. Terpenes from Eupatorium adenophorum and their allelopathic effects on Arabidopsis seeds germination. J. Agr. Food Chem. 2009, 57, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, I.; Shibuya, H.; Yokokawa, Y.; Baek, N.I.; Ohashi, K.; Yoshikawa, M.; Nitta, A.; Wariadinata, H. Structures of merremosides B and D, new antiserotonic resin-glycosides from the tube of Merremia mammosa, an Indonesian folk medicine. Chem. Pharm. Bull. 1988, 36, 1618–1622. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, I.; Baek, N.I.; Ohashi, K.; Sakagami, M.; Yokokawa, Y.; Shibuya, H. Mammosides B and H1, new ionophoric resin-glycosides from the tube of Merremia mammosa, an Indonesian folk medicine. Chem. Pharm. Bull. 1989, 37, 1131–1133. [Google Scholar] [CrossRef]
- Kitagawa, I.; Baek, N.I.; Kawashima, K.; Yokokawa, Y.; Yoshikawa, M.; Ohashi, K.; Shibuya, H. Indonesian medicinal plants. XV. chemical structures of five new resin-glycosides, merremosides a, b, c, d, and e, from the tuber of Merremia mammosa (Convolvulaceae). Chem. Pharm. Bull. 1996, 44, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, I.; Baek, N.I.; Yokokawa, Y.; Yoshikawa, M.; Ohashi, K.; Shibuya, H. Indonesian medicinal plants. XVI. chemical structures of four new resin-glycosides, merremosides f, g, h1, and h2, from the tuber of Merremia mammosa (Convolvulaceae). Chem. Pharm. Bull. 1996, 44, 1693–1699. [Google Scholar] [CrossRef] [PubMed]
- Noda, N.; Tsuji, K.; Miyahara, K.; Yang, C.R. Resin glycosides. XXI. tuguajalapins I-X, the resin glycosides having long-chain fatty acid groups from the root of Merremia hungaiensis. Chem. Pharm. Bull. 1994, 42, 2011–2016. [Google Scholar] [CrossRef] [PubMed]
- Noda, N.; Tsuji, K.; Kawasaki, T.; Miyahara, K.; Hanazono, H.; Yang, C.R. A novel resin glycoside, merremin (tuguajalapin X dimer), from Merremia hungaiensis. Chem. Pharm. Bull. 1995, 43, 1061–1062. [Google Scholar] [CrossRef] [PubMed]
- Krishnappan, A.L.; Seetharaman, T.R. Flavonoids of Merremia tridentate. Fitoterapia 1992, 63, 190. [Google Scholar]
- Garcia-Argaez, A.N.; Perez-Amador, M.C.; Aguirre-Hernandez, E.; Martinez-Vazquez, M. Two new caffeate esters from roots of Merremia tuberosa and M. dissecta. Planta Med. 1999, 65, 678–679. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.M.; Rathor, O.S. Phenolic acids of Merremia emarginata Hallier F. Himalayan Chem. Pharm. Bull. 1985, 2, 12. [Google Scholar]
- Gao, G.C.; Wu, P.; Cao, H.L.; Lin, L.D.; Wei, X.Y. Phenolic compounds from Merremia boisiana (Convolvulaceae). J. Trop. Subtrop. Bot. 2006, 14, 233–237. [Google Scholar]
- Jenett-Siems, K.; Siems, K.; Witte, L.; Eich, E. Merrekentrones A−D, ipomeamarone-like furanosesquiterpenes from Merremia kentrocaulos. J. Nat. Prod. 2001, 64, 1471–1473. [Google Scholar] [CrossRef] [PubMed]
- Jenett-Siems, K.; Weigl, R.; Böhm, A.; Mann, P.; Tofern-Reblin, B.; Ott, S.C.; Ghomian, A.; Kaloga, M.; Siems, K.; Witte, L.; Hilker, M.; Müller, F.; Eich, E. Chemotaxonomy of the pantropical genus Merremia (Convolvulaceae) based on the distribution of tropane alkaloids. Phytochemistry 2005, 66, 1448–1464. [Google Scholar] [CrossRef] [PubMed]
- Kelley, C.J.; Harruff, R.C.; Carmack, M. Polyphenolic acids of Lithospermum ruderale. II. Carbon-13 nuclear magnetic resonance of lithospermic and rosmarinic acids. J. Org. Chem. 1976, 41, 449–454. [Google Scholar] [CrossRef]
- Kelley, C.J.; Mahajan, J.R.; Brooks, L.C.; Neubert, L.A.; Breneman, W.R.; Carmack, M. Polyphenolic acids of Lithospermum ruderale (Boraginaceae). I. Isolation and structure determination of lithospermic acid. J. Org. Chem. 1975, 40, 1804–1805. [Google Scholar] [CrossRef]
- Rahman, A.U.; Bhatti, M.K.; Akhtar, F.; Choudhary, M.I. Alkaloids of Fumaria indica. Phytochemistry 1992, 31, 2869–2872. [Google Scholar] [CrossRef]
- Wu, T.S.; Ou, L.F.; Teng, C.M. Aristolochic acids, aristolactam alkaloids and amides from Aristolochia kankauensis. Phytochemistry 1994, 36, 1063–1068. [Google Scholar] [PubMed]
- Schnarr, G.W.; Vyas, D.M.; Szarek, W.A. Carbon-13 nuclear magnetic resonance spectra of acyclic carbohydrate derivatives: alditols, 1,2-bis(phenylhydrazones), and dithioacetals. J. Chem. Soc. Perkin Trans. 1979, 1, 496–503. [Google Scholar] [CrossRef]
- Zhang, W.X.; Bao, W.F. Studies on the chemical constituents of Xanthoceras sorbifolia Bunge. Acta Pharm. Sinica 2000, 35, 124–127. [Google Scholar]
- Shen, C.C.; Changa, Y.S.; Hott, L.K. Nuclear magnetic resonance studies of 5,7-dihydroxyflavonoids. Phytochemistry 1993, 34, 843–845. [Google Scholar] [CrossRef]
- Youssef, D.; Frahm, A.W. Constituents of the Egyptian Centaurea scoparia; III. Phenolic constituents of the aerial parts. Planta Med. 1995, 61, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Kalinova, J.; Vrchotova, N.; Triska, J. Exudation of allelopathic substances in buckwheat (Fagopyrum esculentum Moench). J. Agr. Food Chem. 2007, 55, 6453–6459. [Google Scholar] [CrossRef] [PubMed]
- Reigosa, M.J.; Malvido-Pazos, E. Phytotoxic effects of 21 plant secondary metabolites on Arabidopsis thaliana germination and root growth. J. Chem. Ecol. 2007, 33, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Cipollini, D.; Stevenson, R.; Enright, S.; Eyles, A.; Bonello, P. Phenolic metabolites in leaves of the invasive shrub, Lonicera maackii, and their potential phytotoxicand anti-herbivore effects. J. Chem. Ecol. 2008, 34, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Durrant, W.E.; Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Loake, G.; Grant, M. Salicylic acid in plant defence-the players and protagonists. Curr. Opin. Plant Biol. 2007, 10, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.I.; Raskin, I. Glucosylation of salicylic acid in Nicotiana tabacum Cv. Xanthi-nc. Phytopathol. 1998, 88, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Bertin, C.; Weston, L.A.; Huang, T.; Jander, G.; Owens, T.; Meinwald, J.; Schroeder, F.C. Grass roots chemistry: metatyrosine, an herbicidal nonprotein amino acid. Proc. Natl. Acad. Sci. USA 2007, 104, 16964–16969. [Google Scholar] [CrossRef] [PubMed]
- Diana, H.; Dudy, B.Z. An improved, simple, hydroponic method for growing Arabidopsis thaliana. Plant Mol. Biol. Rep. 2003, 21, 59–63. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |

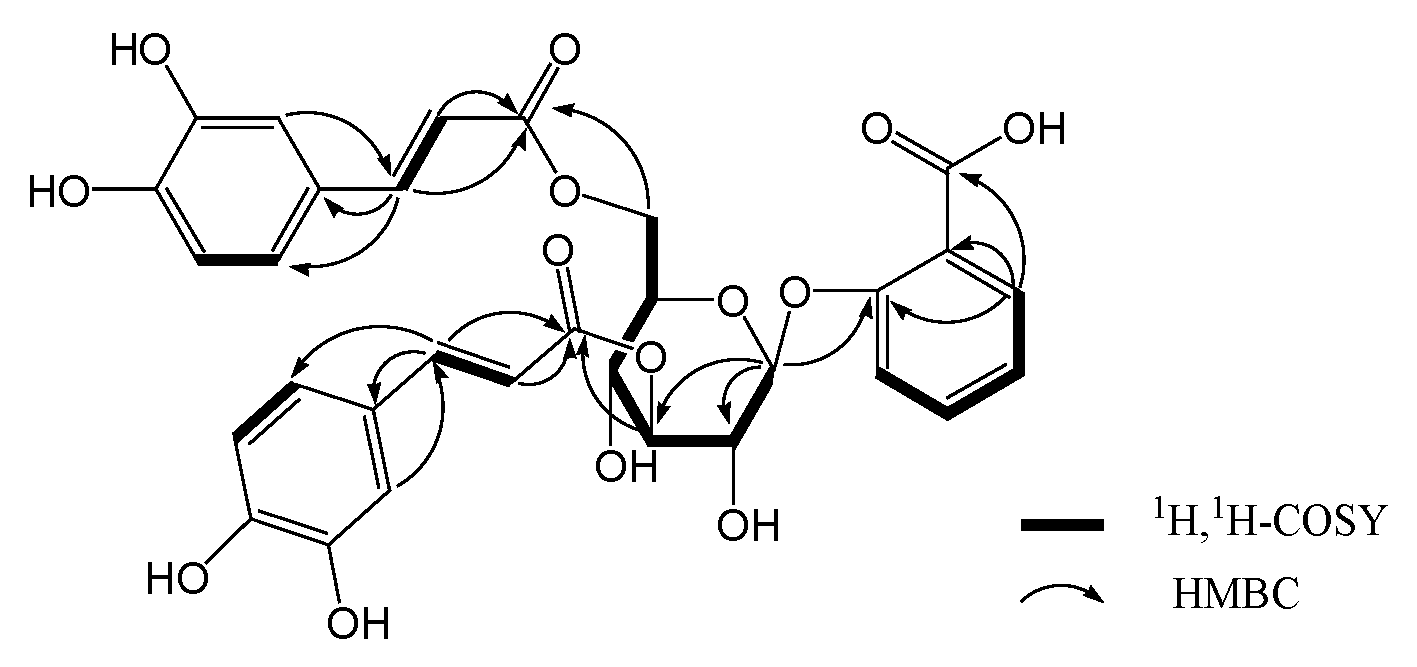

| No. | δ (C) | δ (H) | No. | δ (C) | δ (H) |
|---|---|---|---|---|---|
| 1 | 123.0 | 1″ | 127.9 | ||
| 2 | 158.4 | 2″ | 115.3 | 7.07 (1H, d, 2.4) | |
| 3 | 119.2 | 7.81 (1H, dd, 7.8, 1.2) | 3″ | 146.9 | |
| 4 | 134.9 | 7.42 (1H, dt, 7.8, 1.2) | 4″ | 149.7 | |
| 5 | 124.0 | 7.10 (1H, dt, 7.8, 1.2) | 5″ | 116.6 | 6.80 (1H, d, 8.4) |
| 6 | 132.5 | 7.34 (1H, dd, 7.8, 1.2) | 6″ | 123.0 | 6.79 (1H, dd, 8.4, 2.4) |
| 7 | 169.7 | 7″ | 147.3 | 7.62 (1H, d, 16.2) | |
| 1′ | 103.9 | 5.04 (1H, d, 7.8) | 8″ | 115.2 | 6.36 (1H, d, 16.2) |
| 2′ | 73.3 | 3.78 (1H, dd, 9.6, 7.8) | 9″ | 168.9 | |
| 3′ | 78.1 | 5.19 (1H, dd, 9.6, 9.0) | 1‴ | 127.7 | |
| 4′ | 70.0 | 3.68 (1H, dd, 9.6, 9.0) | 2‴ | 115.2 | 7.06 (1H, d, 1.8) |
| 5′ | 75.8 | 3.87 (1H, m) | 3‴ | 146.8 | |
| 6′a | 64.3 | 4.56 (1H, dd, 12.0, 1.8) | 4‴ | 149.6 | |
| 6′b | 4.44 (1H, dd, 12.0, 6.6) | 5‴ | 116.5 | 6.78 (1H, d, 8.4) | |
| 6‴ | 122.9 | 6.69 (1H, dd, 8.4, 1.8) | |||
| 7‴ | 147.2 | 7.59 (1H, d, 15.6) | |||
| 8‴ | 114.9 | 6.31 (1H, d, 15.6) | |||
| 9‴ | 168.9 |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yan, J.; Bi, H.-H.; Liu, Y.-Z.; Zhang, M.; Zhou, Z.-Y.; Tan, J.-W. Phenolic Compounds from Merremia umbellata subsp. orientalis and Their Allelopathic Effects on Arabidopsis Seed Germination. Molecules 2010, 15, 8241-8250. https://doi.org/10.3390/molecules15118241
Yan J, Bi H-H, Liu Y-Z, Zhang M, Zhou Z-Y, Tan J-W. Phenolic Compounds from Merremia umbellata subsp. orientalis and Their Allelopathic Effects on Arabidopsis Seed Germination. Molecules. 2010; 15(11):8241-8250. https://doi.org/10.3390/molecules15118241
Chicago/Turabian StyleYan, Jian, Hai-Hong Bi, Yong-Zhu Liu, Mei Zhang, Zhong-Yu Zhou, and Jian-Wen Tan. 2010. "Phenolic Compounds from Merremia umbellata subsp. orientalis and Their Allelopathic Effects on Arabidopsis Seed Germination" Molecules 15, no. 11: 8241-8250. https://doi.org/10.3390/molecules15118241