Nanostructure-Enabled and Macromolecule-Grafted Surfaces for Biomedical Applications
Abstract
:1. Introduction
2. Surface Functionalization of Biomaterials
2.1. Grafting by Chemical Means
2.2. Plasma-Induced Grafting
2.3. Ultraviolet-Induced Photo-Initiated Grafting
2.4. Radiation Grafting
2.5. Enzymatic Grafting
2.6. Nanoparticle Grafting
2.7. Laser Treatment
2.8. Ion Implantation
3. Antifouling Biomaterials
3.1. Poly(ethylene glycol)
3.2. Hyperbranched Fluoropolymers
3.3. Zwitterions
4. Nanostructured Film Surfaces
4.1. Canonical Thrombogenic Cascade
4.2. Films for Thrombosis Applications
4.2.1. Nano-Structured and Roughened Surfaces
4.2.2. Macromolecule-Grafted Surfaces
4.2.3. Composites and Inorganic Coatings
4.3. Films for Drug Delivery Applications
4.3.1. Oral Drug Delivery
4.3.2. Grafted Surfaces for Topical Applications
4.4. Nanotextured Films in Other Biomedical Applications
5. Nanostructured Fiber Surfaces
5.1. Surface Nanostructure for Wound Healing
5.2. Drug Eluting Stents
5.3. Nanofiber Scaffolds for Tissue Engineering
6. Future Directions
7. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Asri, R.I.M.; Harun, W.S.W.; Samykano, M.; Lah, N.A.C.; Ghani, S.A.C.; Tarlochan, F.; Raza, M.R. Corrosion and surface modification on biocompatible metals: A review. Mater. Sci. Eng. C 2017, 77, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Ayyavoo, J.; Nguyen, T.P.N.; Jun, B.-M.; Kim, I.-C.; Kwon, Y.-N. Protection of polymeric membranes with antifouling surfacing via surface modifications. Colloids Surf. Physicochem. Eng. Asp. 2016, 506, 190–201. [Google Scholar] [CrossRef]
- Duque Sánchez, L.; Brack, N.; Postma, A.; Pigram, P.J.; Meagher, L. Surface modification of electrospun fibres for biomedical applications: A focus on radical polymerization methods. Biomaterials 2016, 106, 24–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef]
- Nikolic, V.; Velickovic, S.; Popovic, A. Biodegradation of polystyrene-graft-starch copolymers in three different types of soil. Environ. Sci. Pollut. Res. 2014, 21, 9877–9886. [Google Scholar] [CrossRef] [PubMed]
- Pergal, M.; Antic, V.; Tovilovic, G.; Nestorov, J.; Vasiljevic-Radovic, D.; Djonlagic, J. In vitro biocompatibility evaluation of novel urethane–siloxane co-polymers based on poly(ε-caprolactone)-block-poly(dimethylsiloxane)-block-poly(ε-caprolactone). J. Biomater. Sci. Polym. Ed. 2012, 23, 1629–1657. [Google Scholar] [PubMed]
- Kim, P.-H.; Kim, S.W. Polymer-based delivery of glucagon-like peptide-1 for the treatment of diabetes. ISRN Endocrinol. 2012, 2012, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi Sejoubsari, R.; Martinez, A.P.; Kutes, Y.; Wang, Z.; Dobrynin, A.V.; Adamson, D.H. “Grafting-through”: Growing polymer brushes by supplying monomers through the surface. Macromolecules 2016, 49, 2477–2483. [Google Scholar] [CrossRef]
- Kang, S.M.; Hwang, N.S.; Yeom, J.; Park, S.Y.; Messersmith, P.B.; Choi, I.S.; Langer, R.; Anderson, D.G.; Lee, H. One-step multipurpose surface functionalization by adhesive catecholamine. Adv. Funct. Mater. 2012, 22, 2949–2955. [Google Scholar] [CrossRef] [PubMed]
- McGinty, K.M.; Brittain, W.J. Hydrophilic surface modification of poly(vinyl chloride) film and tubing using physisorbed free radical grafting technique. Polymer 2008, 49, 4350–4357. [Google Scholar] [CrossRef]
- Zhao, J.; Li, J.; Feng, Y.; Yin, J. A novel approach to synthesis of functional CPVC and CPE or graft copolymers- in situ chlorinating graft. Polym. Adv. Technol. 2007, 18, 822–828. [Google Scholar] [CrossRef]
- Alves, N.M.; Mano, J.F. Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int. J. Biol. Macromol. 2008, 43, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-P.; Zhou, X.-P.; Cui, W.; Xie, X.-L.; Tong, S.-Y. Maleic anhydride-grafted linear low-density polyethylene with low gel content. Polym. Eng. Sci. 2009, 49, 673–679. [Google Scholar] [CrossRef]
- Vicente, G.; Aguado, J.; Serrano, D.P.; Sánchez, N. HDPE chemical recycling promoted by phenol solvent. J. Anal. Appl. Pyrolysis 2009, 85, 366–371. [Google Scholar] [CrossRef]
- Kato, K.; Uchida, E.; Kang, E.-T.; Uyama, Y.; Ikada, Y. Polymer surface with graft chains. Prog. Polym. Sci. 2003, 28, 209–259. [Google Scholar] [CrossRef]
- Jenkins, D.W.; Hudson, S.M. Review of vinyl graft copolymerization featuring recent advances toward controlled radical-based reactions and illustrated with chitin/chitosan trunk polymers. Chem. Rev. 2001, 101, 3245–3274. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Mountrichas, G.; Zhang, G.; Pispas, S. Thermoresponsive core−shell brush copolymers with poly(propylene oxide)-block-poly(ethylene oxide) side chains via a “grafting from” technique. Macromolecules 2010, 43, 1771–1777. [Google Scholar] [CrossRef]
- Scherf, U.; Gutacker, A.; Koenen, N. All-conjugated block copolymers. Acc. Chem. Res. 2008, 41, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Roach, P.; Eglin, D.; Rohde, K.; Perry, C.C. Modern biomaterials: A review—Bulk properties and implications of surface modifications. J. Mater. Sci. Mater. Med. 2007, 18, 1263–1277. [Google Scholar] [CrossRef] [PubMed]
- Price, M.; Reiners, J.J.; Santiago, A.M.; Kessel, D. Monitoring singlet oxygen and hydroxyl radical formation with fluorescent probes during photodynamic therapy. Photochem. Photobiol. 2009, 85, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Mountrichas, G.; Zhang, G.; Pispas, S. Amphiphilic polystyrene-b-poly(p-hydroxystyrene-g-ethylene oxide) block−graft copolymers via a combination of conventional and metal-free anionic polymerization. Macromolecules 2009, 42, 8661–8668. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Radical addition–fragmentation chemistry in polymer synthesis. Polymer 2008, 49, 1079–1131. [Google Scholar] [CrossRef]
- Saito, T.; Mather, B.D.; Costanzo, P.J.; Beyer, F.L.; Long, T.E. Influence of site-specific sulfonation on acrylic graft copolymer morphology. Macromolecules 2008, 41, 3503–3512. [Google Scholar] [CrossRef]
- Durmaz, Y.Y.; Kumbaraci, V.; Demirel, A.L.; Talinli, N.; Yagci, Y. Graft copolymers by the combination of ATRP and photochemical acylation process by using benzodioxinones. Macromolecules 2009, 42, 3743–3749. [Google Scholar] [CrossRef]
- Liston, E.M.; Martinu, L.; Wertheimer, M.R. Plasma surface modification of polymers for improved adhesion: A critical review. J. Adhes. Sci. Technol. 1993, 7, 1091–1127. [Google Scholar] [CrossRef]
- Wenzel, A.; Yanagishita, H.; Kitamoto, D.; Endo, A.; Haraya, K.; Nakane, T.; Hanai, N.; Matsuda, H.; Koura, N.; Kamusewitz, H.; et al. Effects of preparation condition of photoinduced graft filling-polymerized membranes on pervaporation performance. J. Membr. Sci. 2000, 179, 69–77. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Yamahara, S.; Nakao, S.; Kimura, S. Preparation of pervaporation membranes for removal of dissolved organics from water by plasma-graft filling polymerization. J. Membr. Sci. 1994, 95, 39–49. [Google Scholar] [CrossRef]
- Park, H.; Lee, K.Y.; Lee, S.J.; Park, K.E.; Park, W.H. Plasma-treated poly(lactic-co-glycolic acid) nanofibers for tissue engineering. Macromol. Res. 2007, 15, 238–243. [Google Scholar] [CrossRef]
- Zhang, P.; Henthorn, D.B. Synthesis of PEGylated single wall carbon nanotubes by a photoinitiated graft from polymerization. AIChE J. 2010, 56, 1610–1615. [Google Scholar] [CrossRef]
- Wu, G.; Xie, Y.; Ou, E.; Zhang, L.; Xiong, Y.; Xu, W. Preparation, characterization, and properties of sodium montmorillonite clay/poly(styrene-butadiene-styrene) containing quaternary ammonium cations and photoinitiator nanocomposites via ultraviolet exposure. J. Appl. Polym. Sci. 2010, 118, 1675–1682. [Google Scholar] [CrossRef]
- Wang, X.; Colavita, P.E.; Streifer, J.A.; Butler, J.E.; Hamers, R.J. Photochemical grafting of alkenes onto carbon surfaces: Identifying the roles of electrons and holes. J. Phys. Chem. C 2010, 114, 4067–4074. [Google Scholar] [CrossRef]
- Deng, J.; Wang, L.; Liu, L.; Yang, W. Developments and new applications of UV-induced surface graft polymerizations. Prog. Polym. Sci. 2009, 34, 156–193. [Google Scholar] [CrossRef]
- Krenkova, J.; Lacher, N.A.; Svec, F. Highly efficient enzyme reactors containing trypsin and endoproteinase LysC immobilized on porous polymer monolith coupled to MS suitable for analysis of antibodies. Anal. Chem. 2009, 81, 2004–2012. [Google Scholar] [CrossRef] [PubMed]
- Na, C.-K.; Park, H.-J. Preparation of acrylic acid grafted polypropylene nonwoven fabric by photoinduced graft polymerization with preabsorption of monomer solution. J. Appl. Polym. Sci. 2009, 114, 387–397. [Google Scholar] [CrossRef]
- Lv, X.; Song, W.; Ti, Y.; Qu, L.; Zhao, Z.; Zheng, H. Gamma radiation-induced grafting of acrylamide and dimethyl diallyl ammonium chloride onto starch. Carbohydr. Polym. 2013, 92, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Wang, Z.; Li, H.; Xu, L.; Peng, J.; Zhai, M.; Yang, C.; Li, J.; Wei, G. Adsorption of Cr(VI) using silica-based adsorbent prepared by radiation-induced grafting. J. Hazard. Mater. 2009, 166, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Alkan Gürsel, S.; Gubler, L.; Gupta, B.; Scherer, G.G. Radiation Grafted Membranes. In Fuel Cells I; Scherer, G.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 157–217. ISBN 978-3-540-69755-8. [Google Scholar]
- Goel, N.K.; Rao, M.S.; Kumar, V.; Bhardwaj, Y.K.; Chaudhari, C.V.; Dubey, K.A.; Sabharwal, S. Synthesis of antibacterial cotton fabric by radiation-induced grafting of [2-(Methacryloyloxy)ethyl]trimethylammonium chloride (MAETC) onto cotton. Radiat. Phys. Chem. 2009, 78, 399–406. [Google Scholar] [CrossRef]
- Liu, R.; Saunders, B.R. Thermoresponsive surfaces prepared using adsorption of a cationic graft copolymer: A versatile method for triggered particle capture. J. Colloid Interface Sci. 2009, 338, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gao, K. The study on methyl methacrylate graft-copolymerized composite separator prepared by pre-irradiation method for Li-ion batteries. Surf. Coat. Technol. 2010, 204, 2822–2828. [Google Scholar] [CrossRef]
- Chen, J.; Iwata, H.; Maekawa, Y.; Yoshida, M.; Tsubokawa, N. Grafting of polyethylene by γ-radiation grafting onto conductive carbon black and application as novel gas and solute sensors. Radiat. Phys. Chem. 2003, 67, 397–401. [Google Scholar] [CrossRef]
- Sampaio, S.; Taddei, P.; Monti, P.; Buchert, J.; Freddi, G. Enzymatic grafting of chitosan onto Bombyx mori silk fibroin: Kinetic and IR vibrational studies. J. Biotechnol. 2005, 116, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Hanefeld, U.; Gardossi, L.; Magner, E. Understanding enzyme immobilisation. Chem. Soc. Rev. 2009, 38, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Hossain, K.M.G.; González, M.D.; Lozano, G.R.; Tzanov, T. Multifunctional modification of wool using an enzymatic process in aqueous–organic media. J. Biotechnol. 2009, 141, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Aracri, E.; Fillat, A.; Colom, J.F.; Gutiérrez, A.; del Río, J.C.; Martínez, Á.T.; Vidal, T. Enzymatic grafting of simple phenols on flax and sisal pulp fibres using laccases. Bioresour. Technol. 2010, 101, 8211–8216. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.H.; Waldmann, H. An enzyme-initiated domino hydroxylation-oxidation-carbo-diels-alder reaction cascade. Tetrahedron Lett. 1996, 37, 3833–3836. [Google Scholar] [CrossRef]
- Sheldon, R.A. Enzyme immobilization: The quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Schober, J.M.; Komarova, Y.A.; Chaga, O.Y.; Akhmanova, A.; Borisy, G.G. Microtubule-targeting-dependent reorganization of filopodia. J. Cell Sci. 2007, 120, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.; Kwon, S.; Jeong, S. Preparation of biodegradable polymer/silver nanoparticles composite and its antibacterial efficacy. J. Nanosci. Nanotechnol. 2009, 9, 1098–1102. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Bansal, V.; Chaudhary, M.; Basu, A.; Bhonde, R.R.; Sastry, M. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: A microscopic overview. Langmuir 2005, 21, 10644–10654. [Google Scholar] [CrossRef] [PubMed]
- Parizek, M.; Kasalkova, N.; Bacakova, L.; Slepicka, P.; Lisa, V.; Blazkova, M.; Svorcik, V. Improved adhesion, growth and maturation of vascular smooth muscle cells on polyethylene grafted with bioactive molecules and carbon particles. Int. J. Mol. Sci. 2009, 10, 4352–4374. [Google Scholar] [CrossRef] [PubMed]
- Guerrouache, M.; Mahouche-Chergui, S.; Chehimi, M.M.; Carbonnier, B. Site-specific immobilisation of gold nanoparticles on a porous monolith surface by using a thiol–yne click photopatterning approach. Chem. Commun. 2012, 48, 7486–7488. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.R.; Osberg, K.D.; Macfarlane, R.J.; Langille, M.R.; Mirkin, C.A. Templated techniques for the synthesis and assembly of plasmonic nanostructures. Chem. Rev. 2011, 111, 3736–3827. [Google Scholar] [CrossRef] [PubMed]
- Bolle, M.; Lazare, S. Large scale excimer laser production of submicron periodic structures on polymer surfaces. Appl. Surf. Sci. 1993, 69, 31–37. [Google Scholar] [CrossRef]
- Borowiec, A.; Haugen, H.K. Subwavelength ripple formation on the surfaces of compound semiconductors irradiated with femtosecond laser pulses. Appl. Phys. Lett. 2003, 82, 4462–4464. [Google Scholar] [CrossRef]
- Granados, E.; Martinez-Calderon, M.; Gomez, M.; Rodriguez, A.; Olaizola, S.M. Photonic structures in diamond based on femtosecond UV laser induced periodic surface structuring (LIPSS). Opt. Express 2017, 25, 15330–15335. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-C.; Reinhardt, H.; Hillebrecht, P.; Hampp, N.A. Photochemical oreparation of sub-wavelength heterogeneous laser-induced periodic surface structures. Adv. Mater. 2012, 24, 1994–1998. [Google Scholar] [CrossRef] [PubMed]
- Slepicka, P.; Nedela, O.; Siegel, J.; Krajcar, R.; Kolska, Z.; Svorcik, V. Ripple polystyrene nano-pattern induced by KrF laser. Express Polym. Lett. 2014, 8, 459–466. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, A.I.; Nealey, P.F.; Murphy, C.J. Responses of human keratocytes to micro- and nanostructured substrates. J. Biomed. Mater. Res. 2004, 71A, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Michaljaničová, I.; Slepička, P.; Rimpelová, S.; Slepičková Kasálková, N.; Švorčík, V. Regular pattern formation on surface of aromatic polymers and its cytocompatibility. Appl. Surf. Sci. 2016, 370, 131–141. [Google Scholar] [CrossRef]
- Walachova, K.; Bacakova, L.; Dvorankova, B.; Svorcik, V. Biocompatibility of polymers modified by high-energy ions. Chem. Listy 2002, 96, 19–24. [Google Scholar]
- Carella, E.; Leon, M.; Sauvage, T.; Gonzalez, M. On ion implantation and damage effect in Li2TiO3 as a fusion breeder blanket: A technological approach for degradation testing. Fusion Eng. Des. 2014, 89, 1529–1533. [Google Scholar] [CrossRef]
- Chang, Z.; Laverne, J.A. Hydrogen production in γ-ray and helium-ion radiolysis of polyethylene, polypropylene, poly(methyl-methacrylate), and polystyrene. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 1656–1661. [Google Scholar] [CrossRef]
- Bacakova, L.; Svorcik, V.; Rybka, V.; Mićek, I.; Hnatowicz, V.; Lisā, V.; Kocourek, F. Adhesion and proliferation of cultured human aortic smooth muscle cells on polystyrene implanted with N+, F+ and Art+ ions: Correlation with polymer surface polarity and carbonization. Biomaferiok 1996, 17, 1121–1126. [Google Scholar]
- Shekhawat, N.; Aggarwal, S.; Sharma, A.; Nair, K.G.M. Surface hardening in N+ implanted polycarbonate. J. Mater. Sci. 2015, 50, 3005–3013. [Google Scholar] [CrossRef]
- Kasper, C.; Bartsch, I. Tissue Engineering III: Cell-Surface Interactions for Tissue Culture; Springer: Heidelberg, Germany, 2012; ISBN 978-3-642-28281-2. [Google Scholar]
- Damodaran, V.B.; Murthy, N.S. Bio-inspired strategies for designing antifouling biomaterials. Biomater. Res. 2016, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Magin, C.M.; Cooper, S.P.; Brennan, A.B. Non-toxic antifouling strategies. Mater. Today 2010, 13, 36–44. [Google Scholar] [CrossRef]
- Dalsin, J.L.; Messersmith, P.B. Bioinspired antifouling polymers. Mater. Today 2005, 8, 38–46. [Google Scholar] [CrossRef]
- Pape, A.C.H.; Ippel, B.D.; Dankers, P.Y.W. Cell and protein fouling properties of polymeric mixtures containing supramolecular poly(ethylene glycol) additives. Langmuir 2017, 33, 4076–4082. [Google Scholar] [CrossRef] [PubMed]
- Perrino, C.; Lee, S.; Choi, S.W.; Maruyama, A.; Spencer, N.D. A biomimetic alternative to poly(ethylene glycol) as an antifouling coating: Resistance to nonspecific protein adsorption of poly(l-lysine)-graft-dextran. Langmuir 2008, 24, 8850–8856. [Google Scholar] [CrossRef] [PubMed]
- Yune, P.S.; Kilduff, J.E.; Belfort, G. Fouling-resistant properties of a surface-modified poly(ether sulfone) ultrafiltration membrane grafted with poly(ethylene glycol)-amide binary monomers. J. Membr. Sci. 2011, 377, 159–166. [Google Scholar] [CrossRef]
- Zhou, Z.; Rajabzadeh, S.; Rajjak Shaikh, A.; Kakihana, Y.; Ishigami, T.; Sano, R.; Matsuyama, H. Preparation and characterization of antifouling poly(vinyl chloride-co-poly(ethylene glycol)methyl ether methacrylate) membranes. J. Membr. Sci. 2016, 498, 414–422. [Google Scholar] [CrossRef]
- Zhou, Z.; Rajabzadeh, S.; Shaikh, A.R.; Kakihana, Y.; Ma, W.; Matsuyama, H. Effect of surface properties on antifouling performance of poly(vinyl chloride-co-poly(ethylene glycol)methyl ether methacrylate)/PVC blend membrane. J. Membr. Sci. 2016, 514, 537–546. [Google Scholar] [CrossRef]
- Wang, Y.; Betts, D.E.; Finlay, J.A.; Brewer, L.; Callow, M.E.; Callow, J.A.; Wendt, D.E.; DeSimone, J.M. Photocurable amphiphilic perfluoropolyether/poly(ethylene glycol) networks for fouling-release coatings. Macromolecules 2011, 44, 878–885. [Google Scholar] [CrossRef]
- Yasani, B.R.; Martinelli, E.; Galli, G.; Glisenti, A.; Mieszkin, S.; Callow, M.E.; Callow, J.A. A comparison between different fouling-release elastomer coatings containing surface-active polymers. Biofouling 2014, 30, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Hirao, A.; Sugiyama, K.; Yokoyama, H. Precise synthesis and surface structures of architectural per- and semifluorinated polymers with well-defined structures. Prog. Polym. Sci. 2007, 32, 1393–1438. [Google Scholar] [CrossRef]
- Pollack, K.A.; Imbesi, P.M.; Raymond, J.E.; Wooley, K.L. Hyperbranched fluoropolymer-polydimethylsiloxane-poly(ethylene glycol) cross-linked terpolymer networks designed for marine and biomedical applications: Heterogeneous nontoxic antibiofouling surfaces. ACS Appl. Mater. Interfaces 2014, 6, 19265–19274. [Google Scholar] [CrossRef] [PubMed]
- Gudipati, C.S.; Finlay, J.A.; Callow, J.A.; Callow, M.E.; Wooley, K.L. The antifouling and fouling-release perfomance of hyperbranched fluoropolymer (HBFP)−poly(ethylene glycol) (PEG) composite coatings evaluated by adsorption of biomacromolecules and the green fouling alga Ulva. Langmuir 2005, 21, 3044–3053. [Google Scholar] [CrossRef] [PubMed]
- Alswieleh, A.M.; Cheng, N.; Canton, I.; Ustbas, B.; Xue, X.; Ladmiral, V.; Xia, S.; Ducker, R.E.; El Zubir, O.; Cartron, M.L.; et al. Zwitterionic poly(amino acid methacrylate) brushes. J. Am. Chem. Soc. 2014, 136, 9404–9413. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Li, Q.; Zhang, J.; Xu, T.; Zhao, W.; Yang, J.; Zhang, L. Antifouling zwitterionic hydrogel coating improves hemocompatibility of activated carbon hemoadsorbent. J. Colloid Interface Sci. 2017, 503, 168–177. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Gao, K.; Zhou, L.; Jiao, Z.; Wu, M.; Cao, J.; You, X.; Cai, Z.; Su, Y.; Jiang, Z. Zwitterionic materials for antifouling membrane surface construction. Acta Biomater. 2016, 40, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Lee, Y.; Phuong, L.T.; Seon, G.M.; Kim, E.; Park, J.C.; Yoon, H.; Park, K.D. Zwitterionic sulfobetaine polymer-immobilized surface by simple tyrosinase-mediated grafting for enhanced antifouling property. Acta Biomater. 2017, 61, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Schlenoff, J.B. Zwitteration: Coating surfaces with zwitterionic functionality to reduce nonspecific adsorption. Langmuir 2014, 30, 9625–9636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Xiong, S.; Shi, Y.S.; Zhu, J.; Hu, Q.L.; Liu, J.; Wang, Y. Antifouling enhancement of polyimide membrane by grafting DEDA-PS zwitterions. Chemosphere 2018, 198, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xie, Y.; Xiao, T.; Zhao, W.; Li, J.; Zhao, C. Tannic acid-inspiration and post-crosslinking of zwitterionic polymer as a universal approach towards antifouling surface. Chem. Eng. J. 2018, 337, 122–132. [Google Scholar] [CrossRef]
- Huang, C.-J.; Wang, L.-C.; Liu, C.-Y.; Chiang, A.S.T.; Chang, Y.-C. Natural zwitterionic organosulfurs as surface ligands for antifouling and responsive properties. Biointerphases 2014, 9, 029010. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Sundaram, H.S.; Wei, Z.; Li, C.; Yuan, Z. Applications of zwitterionic polymers. React. Funct. Polym. 2017, 118, 51–61. [Google Scholar] [CrossRef]
- Neuenschwander, P.F.; Jesty, J. Blood Coagulation. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Chichester, UK, 2011; ISBN 978-0-470-01617-6. [Google Scholar]
- Neuenschwander, P.F.; Fiore, M.M.; Morrissey, J.H. Factor VII autoactivation proceeds via interaction of distinct protease-cofactor and zymogen-cofactor complexes. Implications of a two-dimensional enzyme kinetic mechanism. J. Biol. Chem. 1993, 268, 21489–21492. [Google Scholar] [PubMed]
- Doshi, R.; Shah, J.; Jauhar, V.; Decter, D.; Jauhar, R.; Meraj, P. Comparison of drug eluting stents (DESs) and bare metal stents (BMSs) with STEMI: Who received BMS in the era of 2nd generation DES? Heart Lung 2018, 47, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, H.; Tekinay, A.B.; Guler, M.O. Selective adhesion and growth of vascular endothelial cells on bioactive peptide nanofiber functionalized stainless steel surface. Biomaterials 2011, 32, 8797–8805. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-K.; Park, C.; Jang, T.-S.; Kim, H.-E.; Jeong, S.-H. Enhanced mechanical stability of PTFE coating on nano-roughened NiTi for biomedical applications. Mater. Lett. 2018, 216, 12–15. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Gao, L.; Wang, S.; Mao, A.; Liu, B. Preparation of the micro/nano structures of the biomimetic coating stent for loading MiRNA126 by four-beam laser interference. Opt. Int. J. Light Electron Opt. 2017, 128, 247–252. [Google Scholar] [CrossRef]
- Saleem, S.; Ahmad, R.; Ayub, R.; Ikhlaq, U.; Jin, W.; Chu, P.K. Investigation of nano-structured Zirconium oxide film on Ti6Al4V substrate to improve tribological properties prepared by PIII&D. Appl. Surf. Sci. 2017, 394, 586–597. [Google Scholar]
- Huang, J.; Zhang, X.; Yan, W.; Chen, Z.; Shuai, X.; Wang, A.; Wang, Y. Nanotubular topography enhances the bioactivity of titanium implants. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, B.; Havakeshian, E.; Ensafi, A.A. Decoration of nanoporous stainless steel with nanostructured gold via galvanic replacement reaction and its application for electrochemical determination of dopamine. Sens. Actuators B Chem. 2015, 213, 484–492. [Google Scholar] [CrossRef]
- Alves, P.; Pinto, S.; Kaiser, J.-P.; Bruinink, A.; de Sousa, H.C.; Gil, M.H. Surface grafting of a thermoplastic polyurethane with methacrylic acid by previous plasma surface activation and by ultraviolet irradiation to reduce cell adhesion. Colloids Surf. B Biointerfaces 2011, 82, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Alves, P.; Cardoso, R.; Correia, T.R.; Antunes, B.P.; Correia, I.J.; Ferreira, P. Surface modification of polyurethane films by plasma and ultraviolet light to improve haemocompatibility for artificial heart valves. Colloids Surf. B Biointerfaces 2014, 113, 25–32. [Google Scholar] [CrossRef] [PubMed]
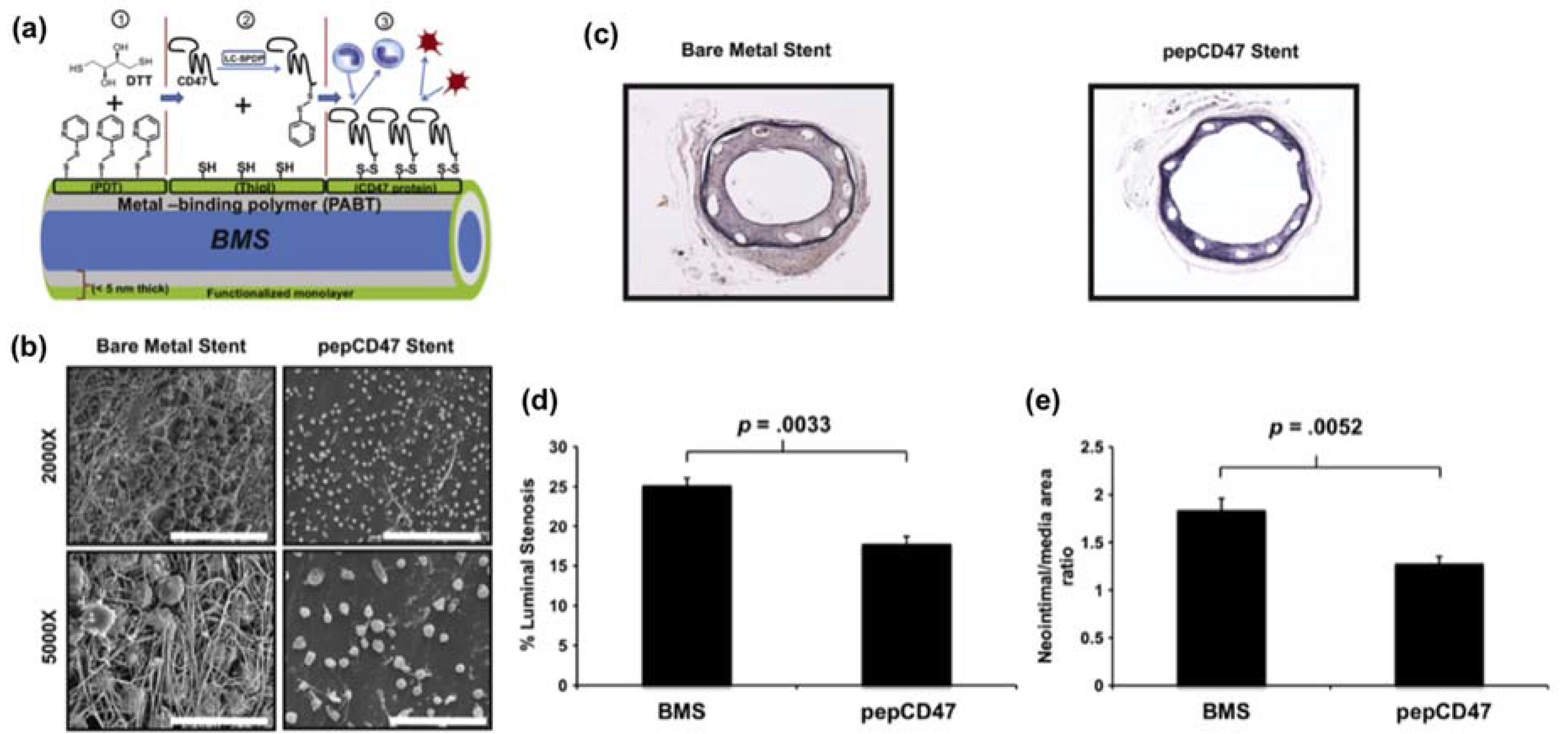
- Slee, J.B.; Alferiev, I.S.; Nagaswami, C.; Weisel, J.W.; Levy, R.J.; Fishbein, I.; Stachelek, S.J. Enhanced biocompatibility of CD47-functionalized vascular stents. Biomaterials 2016, 87, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Le Thi, P.; Seon, G.M.; Ryu, S.B.; Brophy, C.M.; Kim, Y.; Park, J.-C.; Park, K.D.; Cheung-Flynn, J.; Sung, H.-J. Heparin-functionalized polymer graft surface eluting MK2 inhibitory peptide to improve hemocompatibility and anti-neointimal activity. J. Control. Release 2017, 266, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Agarwal, R.; Tara, S.; Yi, T.; Lee, Y.-U.; Breuer, C.K.; Weiss, A.S.; Shinoka, T. Tropoelastin inhibits intimal hyperplasia of mouse bioresorbable arterial vascular grafts. Acta Biomater. 2017, 52, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Karagkiozaki, V.; Vavoulidis, E.; Karagiannidis, P.G.; Gioti, M.; Fatouros, D.G.; Vizirianakis, I.S.; Logothetidis, S. Development of a nanoporous and multilayer drug-delivery platform for medical implants. Int. J. Nanomed. 2012, 7, 5321–5338. [Google Scholar]
- Nakano, K.; Egashira, K.; Masuda, S.; Funakoshi, K.; Zhao, G.; Kimura, S.; Matoba, T.; Sueishi, K.; Endo, Y.; Kawashima, Y.; et al. Formulation of nanoparticle-eluting stents by a cationic electrodeposition coating technology. JACC Cardiovasc. Interv. 2009, 2, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Tan, H.; Han, D.; Fu, Q.; Jiang, L. No Platelet Can Adhere—Largely Improved Blood Compatibility on Nanostructured Superhydrophobic Surfaces. Small 2005, 1, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, W.; Zhang, J.; Sun, W.; Zhang, R.; Gu, H. Fabrication of a Novel Polymer-Free Nanostructured Drug-Eluting Coating for Cardiovascular Stents. ACS Appl. Mater. Interfaces 2013, 5, 10337–10345. [Google Scholar] [CrossRef] [PubMed]
- Loya, M.C.; Brammer, K.S.; Choi, C.; Chen, L.-H.; Jin, S. Plasma-induced nanopillars on bare metal coronary stent surface for enhanced endothelialization. Acta Biomater. 2010, 6, 4589–4595. [Google Scholar] [CrossRef] [PubMed]
- Maroni, A.; Del Curto, M.D.; Zema, L.; Foppoli, A.; Gazzaniga, A. Film coatings for oral colon delivery. Int. J. Pharm. 2013, 457, 372–394. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.M.; Palmeira-De-Oliveira, A.; Martinez-De-Oliveira, J.; Palmeira-De-Oliveira, R. Vaginal films for drug delivery. J. Pharm. Sci. 2013, 102, 2069–2081. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.; Kim, H.; Na, S.-J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef]
- Shukla, R.K.; Tiwari, A. Carbohydrate polymers: Applications and recent advances in delivering drugs to the colon. Carbohydr. Polym. 2012, 88, 399–416. [Google Scholar] [CrossRef]
- Karrout, Y.; Neut, C.; Wils, D.; Siepmann, F.; Deremaux, L.; Flament, M.-P.; Dubreuil, L.; Desreumaux, P.; Siepmann, J. Novel polymeric film coatings for colon targeting: Drug release from coated pellets. Eur. J. Pharm. Sci. 2009, 37, 427–433. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Du, Q.; Cao, D.; Xiang, B.; Fan, L. Study on colon-specific pectin/ethylcellulose film-coated 5-fluorouracil pellets in rats. Int. J. Pharm. 2008, 348, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Kyzioł, A.; Kyzioł, K. Surface Functionalization with Biopolymers via Plasma-Assisted Surface Grafting and Plasma-Induced Graft Polymerization—Materials for Biomedical Applications. In Biopolymer Grafting: Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 115–151. ISBN 978-0-12-810462-0. [Google Scholar]
- Pattanashetti, N.A.; Heggannavar, G.B.; Kariduraganavar, M.Y. Smart biopolymers and their biomedical applications. Procedia Manuf. 2017, 12, 263–279. [Google Scholar] [CrossRef]
- Hoffman, A.S. Stimuli-responsive polymers: Biomedical applications and challenges for clinical translation. Adv. Drug Deliv. Rev. 2013, 65, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Nishiyama, N.; Fukushima, S.; Koyama, H.; Yasuhiro, M.; Kataoka, K. Preparation and biological characterization of polymeric micelle drug carriers with intracellular pH-triggered drug release property: Tumor permeability, controlled subcellular drug distribution, and enhanced in vivo antitumor efficacy. Bioconjug. Chem. 2005, 16, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Torres-Lugo, M.; García, M.; Record, R.; Peppas, N.A. Physicochemical behavior and cytotoxic effects of p(methacrylic acid–g-ethylene glycol) nanospheres for oral delivery of proteins. J. Control. Release 2002, 80, 197–205. [Google Scholar] [CrossRef]
- Voulgari, E.; Bakandritsos, A.; Galtsidis, S.; Zoumpourlis, V.; Burke, B.P.; Clemente, G.S.; Cawthorne, C.; Archibald, S.J.; Tuček, J.; Zbořil, R.; et al. Synthesis, characterization and in vivo evaluation of a magnetic cisplatin delivery nanosystem based on PMAA-graft-PEG copolymers. J. Control. Release 2016, 243, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Jalababu, R.; Veni, S.S.; Reddy, K.V.N.S. Synthesis and characterization of dual responsive sodium alginate-g-acryloyl phenylalanine-poly N-isopropyl acrylamide smart hydrogels for the controlled release of anticancer drug. J. Drug Deliv. Sci. Technol. 2018, 44, 190–204. [Google Scholar] [CrossRef]
- Wang, K.; Xu, X.; Wang, Y.; Yan, X.; Guo, G.; Huang, M.; Luo, F.; Zhao, X.; Wei, Y.; Qian, Z. Synthesis and characterization of poly(methoxyl ethylene glycol-caprolactone-co-methacrylic acid-co-poly(ethylene glycol) methyl ether methacrylate) pH-sensitive hydrogel for delivery of dexamethasone. Int. J. Pharm. 2010, 389, 130–138. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, I.M. Enhanced pH-responsive carrier system based on alginate and chemically modified carboxymethyl chitosan for oral delivery of protein drugs: Preparation and in vitro assessment. Carbohydr. Polym. 2010, 80, 1125–1136. [Google Scholar] [CrossRef]
- Bae, W.K.; Park, M.S.; Lee, J.H.; Hwang, J.E.; Shim, H.J.; Cho, S.H.; Kim, D.-E.; Ko, H.M.; Cho, C.-S.; Park, I.-K.; et al. Docetaxel-loaded thermoresponsive conjugated linoleic acid-incorporated poloxamer hydrogel for the suppression of peritoneal metastasis of gastric cancer. Biomaterials 2013, 34, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Haji Mansor, M.; Najberg, M.; Contini, A.; Alvarez-Lorenzo, C.; Garcion, E.; Jérôme, C.; Boury, F. Development of a non-toxic and non-denaturing formulation process for encapsulation of SDF-1α into PLGA/PEG-PLGA nanoparticles to achieve sustained release. Eur. J. Pharm. Biopharm. 2018, 125, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Dang, Q.; Liu, K.; Zhang, Z.; Liu, C.; Liu, X.; Xin, Y.; Cheng, X.; Xu, T.; Cha, D.; Fan, B. Fabrication and evaluation of thermosensitive chitosan/collagen/α,β-glycerophosphate hydrogels for tissue regeneration. Carbohydr. Polym. 2017, 167, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Licciardi, M.; Amato, G.; Cappelli, A.; Paolino, M.; Giuliani, G.; Belmonte, B.; Guarnotta, C.; Pitarresi, G.; Giammona, G. Evaluation of thermoresponsive properties and biocompatibility of polybenzofulvene aggregates for leuprolide delivery. Int. J. Pharm. 2012, 438, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Tullii, G.; Desii, A.; Bossio, C.; Bellani, S.; Colombo, M.; Martino, N.; Antognazza, M.R.; Lanzani, G. Bimodal functioning of a mesoporous, light sensitive polymer/electrolyte interface. Org. Electron. 2017, 46, 88–98. [Google Scholar] [CrossRef]
- Indermun, S.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Modi, G.; Luttge, R.; Pillay, V. An interfacially plasticized electro-responsive hydrogel for transdermal electro-activated and modulated (TEAM) drug delivery. Int. J. Pharm. 2014, 462, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Mousavikhamene, Z.; Abdekhodaie, M.J.; Ahmadieh, H. Facilitation of transscleral drug delivery by drug loaded magnetic polymeric particles. Mater. Sci. Eng. C 2017, 79, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Sorribes-Soriano, A.; Esteve-Turrillas, F.A.; Armenta, S.; Montoya, A.; Herrero-Martínez, J.M.; de la Guardia, M. Magnetic molecularly imprinted polymers for the selective determination of cocaine by ion mobility spectrometry. J. Chromatogr. A 2018, 1545, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Samah, N.H.A.; Heard, C.M. Enhanced in vitro transdermal delivery of caffeine using a temperature- and pH-sensitive nanogel, poly(NIPAM-co-AAc). Int. J. Pharm. 2013, 453, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Hoffman, A.S. A novel approach for preparation of pH-sensitive hydrogels for enteric drug delivery. J. Control. Release 1991, 15, 141–152. [Google Scholar] [CrossRef]
- Sun, C.-C.; Chou, S.-F.; Lai, J.-Y.; Cho, C.-H.; Lee, C.-H. Dependence of corneal keratocyte adhesion, spreading, and integrin β1 expression on deacetylated chitosan coating. Mater. Sci. Eng. C 2016, 63, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.-F.; Lai, J.-Y.; Cho, C.-H.; Lee, C.-H. Relationships between surface roughness/stiffness of chitosan coatings and fabrication of corneal keratocyte spheroids: Effect of degree of deacetylation. Colloids Surf. B Biointerfaces 2016, 142, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Linez-Bataillon, P.; Monchau, F.; Bigerelle, M.; Hildebrand, H. In vitro MC3T3 osteoblast adhesion with respect to surface roughness of Ti6Al4V substrates. Biomol. Eng. 2002, 19, 133–141. [Google Scholar] [CrossRef]
- Cai, L.; Wang, K.; Wang, S. Poly(ethylene glycol)-grafted poly(propylene fumarate) networks and parabolic dependence of MC3T3 cell behavior on the network composition. Biomaterials 2010, 31, 4457–4466. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Cai, L.; Hao, F.; Xu, X.; Cui, M.; Wang, S. Distinct cell responses to substrates consisting of poly(ε-caprolactone) and poly(propylene fumarate) in the presence or absence of cross-links. Biomacromolecules 2010, 11, 2748–2759. [Google Scholar] [CrossRef] [PubMed]
- Amaral, I.F.; Cordeiro, A.L.; Sampaio, P.; Barbosa, M.A. Attachment, spreading and short-term proliferation of human osteoblastic cells cultured on chitosan films with different degrees of acetylation. J. Biomater. Sci. Polym. Ed. 2007, 18, 469–485. [Google Scholar] [CrossRef] [PubMed]
- Velzenberger, E.; Kirat, K.E.; Legeay, G.; Nagel, M.-D.; Pezron, I. Characterization of biomaterials polar interactions in physiological conditions using liquid–liquid contact angle measurements. Colloids Surf. B Biointerfaces 2009, 68, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Y.; Fan, Y.; Li, X. The use of bioactive peptides to modify materials for bone tissue repair. Regen. Biomater. 2017, 4, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Zamuner, A.; Brun, P.; Scorzeto, M.; Sica, G.; Castagliuolo, I.; Dettin, M. Smart biomaterials: Surfaces functionalized with proteolytically stable osteoblast-adhesive peptides. Bioact. Mater. 2017, 2, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Chollet, C.; Chanseau, C.; Remy, M.; Guignandon, A.; Bareille, R.; Labrugère, C.; Bordenave, L.; Durrieu, M.-C. The effect of RGD density on osteoblast and endothelial cell behavior on RGD-grafted polyethylene terephthalate surfaces. Biomaterials 2009, 30, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.-F.; Gunaseelan, S.; Kiellani, M.H.H.; Thottempudi, V.V.K.; Neuenschwander, P.; Nie, H. A review of injectable and implantable biomaterials for treatment and repair of soft tissues in wound healing. J. Nanotechnol. 2017, 2017, 1–15. [Google Scholar] [CrossRef]
- Gizaw, M.; Thompson, J.; Faglie, A.; Lee, S.-Y.; Neuenschwander, P.; Chou, S.-F. Electrospun fibers as a dressing material for drug and biological agent delivery in wound healing applications. Bioengineering 2018, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
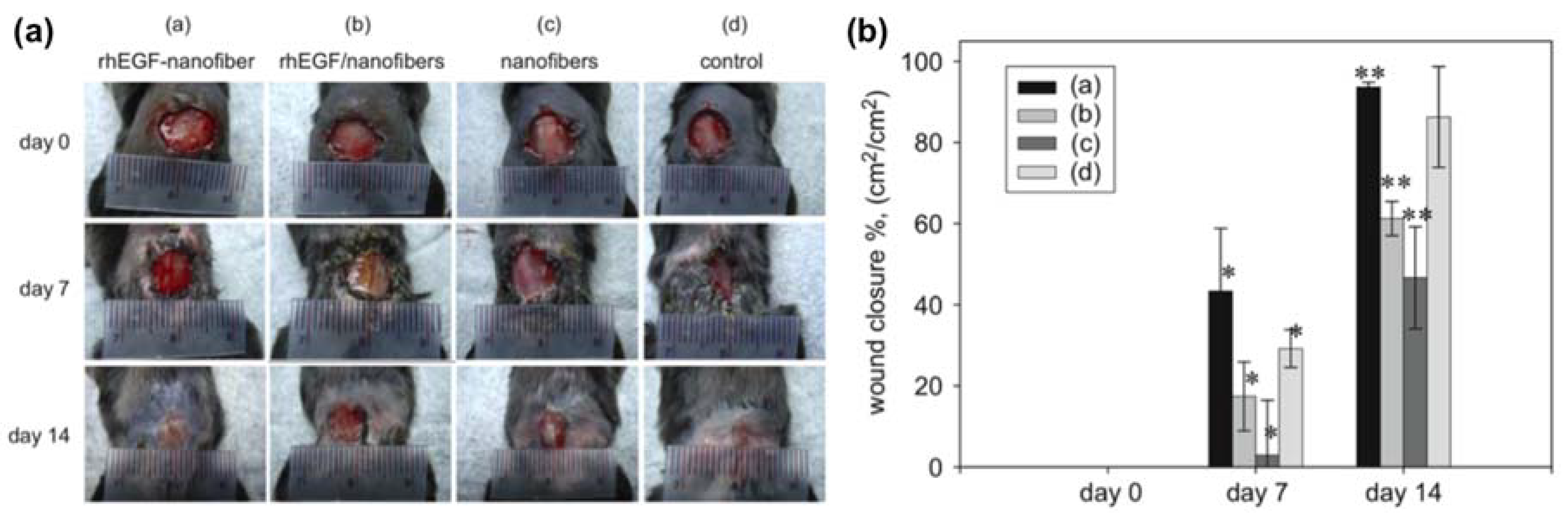
- Choi, J.S.; Leong, K.W.; Yoo, H.S. In vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF). Biomaterials 2008, 29, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Gümüşderelioğlu, M.; Dalkıranoğlu, S.; Aydın, R.S.T.; Çakmak, S. A novel dermal substitute based on biofunctionalized electrospun PCL nanofibrous matrix. J. Biomed. Mater. Res. A 2011, 98A, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Choi, S.H.; Yoo, H.S. Coaxial electrospun nanofibers for treatment of diabetic ulcers with binary release of multiple growth factors. J. Mater. Chem. 2011, 21, 5258–5267. [Google Scholar] [CrossRef]
- Song, D.W.; Kim, S.H.; Kim, H.H.; Lee, K.H.; Ki, C.S.; Park, Y.H. Multi-biofunction of antimicrobial peptide-immobilized silk fibroin nanofiber membrane: Implications for wound healing. Acta Biomater. 2016, 39, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Zhang, X.; Pi, Y.; Wang, X.; Jia, Z.; Zhu, J.; Dai, L.; Chen, W.; Yin, L.; Chen, H.; et al. Polycaprolactone electrospun mesh conjugated with an MSC affinity peptide for MSC homing in vivo. Biomaterials 2012, 33, 3375–3387. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Duan, Y.-Y.; Yu, J.; Lu, J.-W. Preparation and immobilization of soluble eggshell membrane protein on the electrospun nanofibers to enhance cell adhesion and growth. J. Biomed. Mater. Res. Part A 2008, 86, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Li, X.; Neoh, K.G.; Shi, Z.; Kang, E.T. Surface modification and antibacterial activity of electrospun polyurethane fibrous membranes with quaternary ammonium moieties. J. Membr. Sci. 2008, 320, 259–267. [Google Scholar] [CrossRef]
- Dumont, M.; Villet, R.; Guirand, M.; Montembault, A.; Delair, T.; Lack, S.; Barikosky, M.; Crepet, A.; Alcouffe, P.; Laurent, F.; et al. Processing and antibacterial properties of chitosan-coated alginate fibers. Carbohydr. Polym. 2018, 190, 31–42. [Google Scholar] [CrossRef] [PubMed]
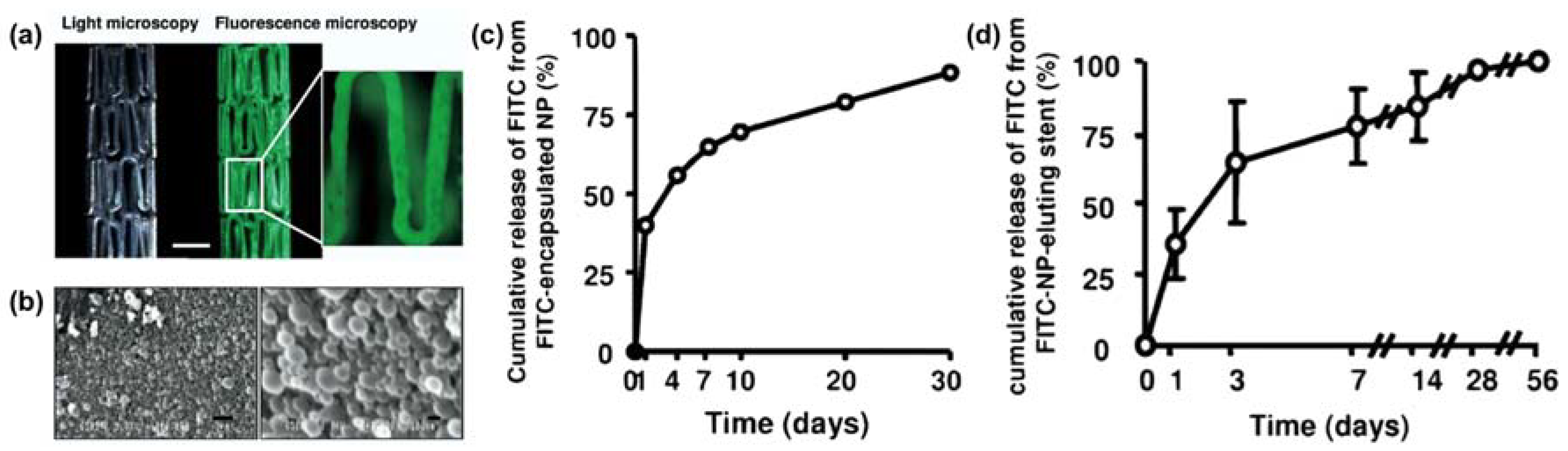
- Tang, J.; Liu, Y.; Zhu, B.; Su, Y.; Zhu, X. Preparation of paclitaxel/chitosan co-assembled core-shell nanofibers for drug-eluting stent. Appl. Surf. Sci. 2017, 393, 299–308. [Google Scholar] [CrossRef]
- Liu, K.-S.; Lee, C.-H.; Lee, D.; Liu, M.; Tsai, F.-C.; Tseng, Y.-Y. Sustained local delivery of high-concentration vancomycin from a hybrid biodegradable, antibiotic-eluting, nanofiber-loaded endovascular prosthesis for treatment of mycotic aortic aneurysms. J. Vasc. Surg. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Hsieh, M.-J.; Liu, S.-C.; Chen, J.-K.; Liu, S.-J.; Hsieh, I.-C.; Wen, M.-S.; Hung, K.-C. Novel bifurcation stents coated with bioabsorbable nanofibers with extended and controlled release of rosuvastatin and paclitaxel. Mater. Sci. Eng. C 2018, 88, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, J.; Zheng, Q.; Wu, Y.; Wu, B.; Huang, S.; Fang, W.; Guo, X. FGL-functionalized self-assembling nanofiber hydrogel as a scaffold for spinal cord-derived neural stem cells. Mater. Sci. Eng. C 2015, 46, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Nune, M.; Krishnan, U.M.; Sethuraman, S. PLGA nanofibers blended with designer self-assembling peptides for peripheral neural regeneration. Mater. Sci. Eng. C 2016, 62, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.-H.; Ramakrishna, S. Electrospun poly(ɛ-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 2008, 29, 4532–4539. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; He, W.; Yong, T.; Ramakrishna, S. Grafting of gelatin on electrospun poly(caprolactone) nanofibers to improve endothelial cell spreading and proliferation and to control cell orientation. Tissue Eng. 2005, 11, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
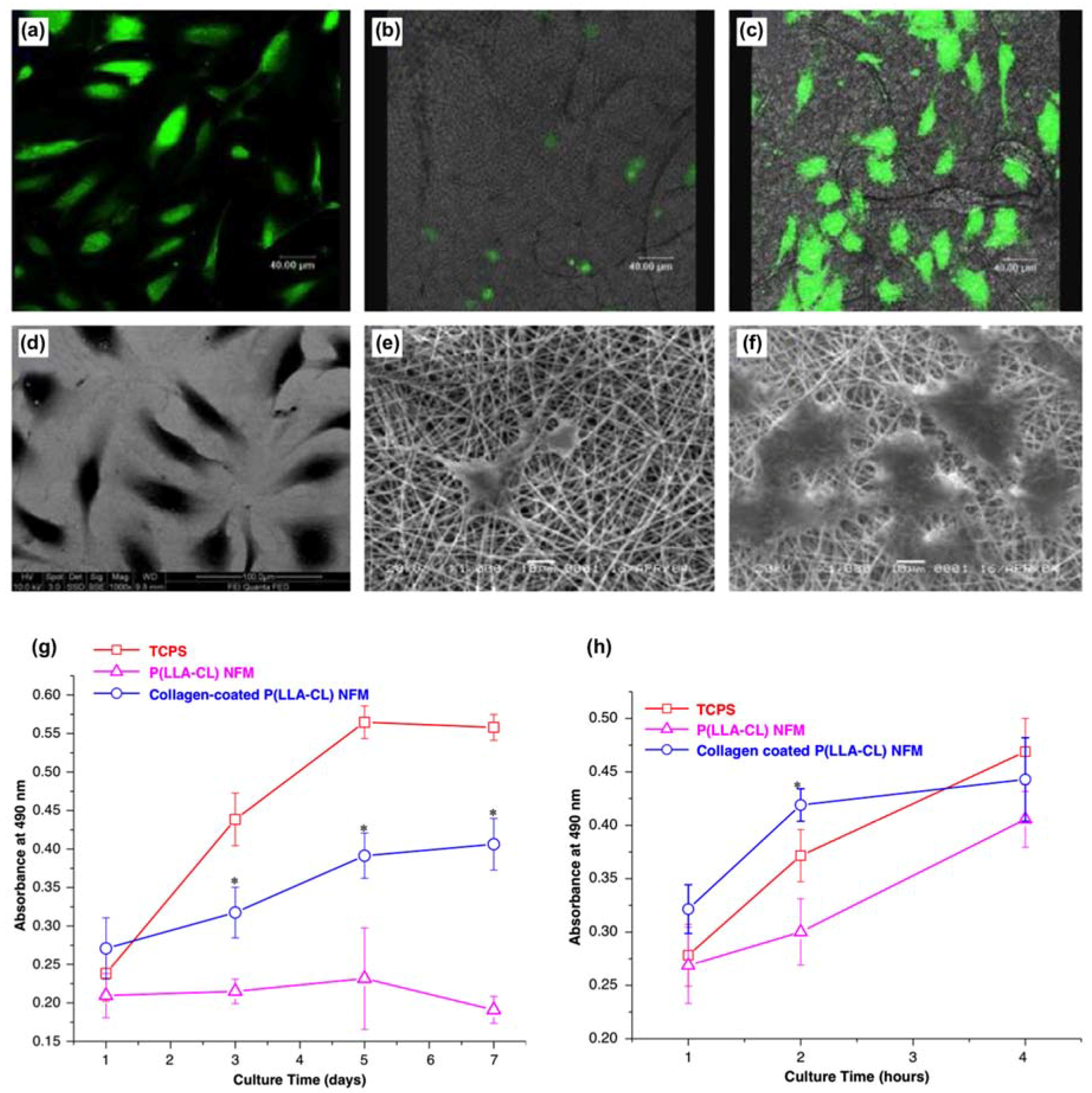
- He, W.; Ma, Z.; Yong, T.; Teo, W.E.; Ramakrishna, S. Fabrication of collagen-coated biodegradable polymer nanofiber mesh and its potential for endothelial cells growth. Biomaterials 2005, 26, 7606–7615. [Google Scholar] [CrossRef] [PubMed]
- Andalib, M.N.; Lee, J.S.; Ha, L.; Dzenis, Y.; Lim, J.Y. Focal adhesion kinase regulation in stem cell alignment and spreading on nanofibers. Biochem. Biophys. Res. Commun. 2016, 473, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Wyndaele, M.; Wyndaele, J.-J. Incidence, prevalence and epidemiology of spinal cord injury: What learns a worldwide literature survey? Spinal Cord 2006, 44, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Su, H.; Zeng, Y.; Liang, Y.-X.; Wong, W.M.; Ellis-Behnke, R.G.; So, K.-F.; Wu, W. Reknitting the injured spinal cord by self-assembling peptide nanofiber scaffold. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Hussain, A.N.; Sidra, L.; Sarfraz, Z.; Khalid, H.; Khan, M.; Manzoor, F.; Shahzadi, L.; Yar, M.; Rehman, I.U. Fabrication and in vivo evaluation of hydroxyapatite/carbon nanotube electrospun fibers for biomedical/dental application. Mater. Sci. Eng. C 2017, 80, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wolke, J.G.C.; Jansen, J.A. Biomimetic calcium phosphate coating on electrospun poly(ɛ-caprolactone) scaffolds for bone tissue engineering. Chem. Eng. J. 2008, 137, 154–161. [Google Scholar] [CrossRef]
- Hasan, A.; Memic, A.; Annabi, N.; Hossain, M.; Paul, A.; Dokmeci, M.R.; Dehghani, F.; Khademhosseini, A. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 2014, 10, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Çelik, E.E.; Rubio, J.M.A.; Gökmen, V. Behaviour of Trolox with macromolecule-bound antioxidants in aqueous medium: Inhibition of auto-regeneration mechanism. Food Chem. 2018, 243, 428–434. [Google Scholar] [CrossRef] [PubMed]



| Metal Stents | Films/Coatings | Methods | Functions | Ref. |
|---|---|---|---|---|
| Nickel-titanium (NiTi) | Polytetrafluoroethylene (PTFE) | The NiTi substrate was nano-roughened with target-ion induced plasma sputtering (TIPS) and coated with a 60% PTFE layer. | A better bonding of the PTFE film/coating to the NiTi stent for improvement in blood compatibility | [94] |
| Metal-stent | Phosphorylcholine | A four-beam laser interference is used to alter the metal-stent and next coated with a layered phosphorylcholine and miRNA126 coating. | Altering the surface profile of a metal stent to achieve drug delivery for coronary heart disease. | [95] |
| Ti6A14V alloy | Zirconium oxide | The surface of the Ti6A14V alloy is modified by implanting zirconium oxide using a plasma ion implanter. | The zirconium oxide film/coating on the Ti6A14V alloy aids wear resistance for medical implantations. | [96] |
| Titanium | TiO2 topography (TNT), (SLA), and (SLA/TNT) | Titanium implants. | Improving osseointegration and promotion of biological response to mesenchymal stem cells (MSC). | [97] |
| Stainless steel | (AU/NPSS) | The stainless-steel stent is encrusted with self-organized gold nanostructures. | Stainless steel/AU/NPSS is an electrochemical biosensor for measurements of dopamine levels. | [98] |
| Methacrylic acid (MAA) | Thermoplastic polyurethane (TPU) | MAA is grafted by argon plasma or UV light to TPU. | Enhancing blood compatibility and human bone marrow cell adhesion. | [99] |
| 2-hydroxyethyl methacrylate (HEMA) | Thermoplastic polyurethane (TPU) | HEMA is grafted by argon plasma or UV light to TPU. | Improving biocompatibility with lower levels of thrombogenicity for the use of coatings in heart valves. | [100] |
| Polyallylamine | CD47 | Polyallylamine bisphosphonate-modified. | Inhibition of cellular inflammatory response. | [101] |
| Polymers Under a Stimuli-Response | Applications | Drug Delivery | Ref. |
|---|---|---|---|
| 1. pH-sensitive polymers: | |||
| Poly(ethylene glycol)-poly-(aspartate hydrazine adriamycin) | Anticancer drug delivery | Adriamycin | [118] |
| Poly(methacrylic acid-grafted-poly(ethylene glycol) | Cytotoxic effects | Proteins and peptides | [119] |
| Drug delivery | Cisplatin | [120] | |
| N-Acryloyl-l-phenylalanine grafted sodium alginate copolymer, N-isopropylacrylamide, acrylamide | Anticancer drug delivery | Imatinib mesylate | [121] |
| Methoxyl poly(ethylene glycol)-poly(caprolactone)-acryloyl chloride, poly(ethylene glycol) methyl ether methacrylate, and methacrylic acid | Oral drug delivery | Dexamethasone | [122] |
| Alginate and chemical modified carboxymethyl chitosan | Oral drug delivery | Bovine serum albumin | [123] |
| 2. Temperature-sensitive polymers: | |||
| N-Acryloyl-l-phenylalanine grafted sodium alginate copolymer, N-isopropylacrylamide, acrylamide | Anticancer drug delivery | Imatinib mesylate | [121] |
| Conjugated linoleic acid coupled with pluronic F-127 | Peritoneal dissemination of gastric cancer | Docetaxel | [124] |
| Poly-(lactic-co-glycolic acid) and polyethylene glycol copolymer | Anticancer drug delivery | Chemokine stromal cell-derived factor-1α | [125] |
| Chitosan, collagen, α, β-glycerophosphate | Mimic extracellular microenvironment for tissue regeneration | Aid tissue regeneration | [126] |
| Polybenzofulvene derivative | Anticancer drug delivery | Leuprolide | [127] |
| 3. Light-sensitive polymers: | |||
| Region-regular poly(3-hexylthiophene) polymer | Use for triggered drug release or depolarization and hyperpolarization of the cell membrane | N/A | [128] |
| 4. Electro-responsive polymers: | |||
| Poly(ethyleneimine) and 1-vinylimidazole | Transdermal drug delivery | Indomethacin | [129] |
| 5. Magnetic-sensitive polymers: | |||
| Sodium alginate and iron oxide nanoparticles | Ocular drug delivery | Diclofenac sodium | [130] |
| Polyethylene glycol and 3-(trimethoxysilyl)propyl methacrylate coated magnetic nanoparticles | Cocaine recognition. | N/A | [131] |
| 6. Multi stimuli-responsive polymers: (e.g., pH and temperature-responsive) | |||
| N-isopropylacrylamide, acrylic acid | Use for drug delivery | Caffeine | [132] |
| N-isopropylacrylamide, acrylic acid, and vinyl terminated polydimethylsiloxane | Coatings on drug tablets | Indomethacin | [133] |
| Nanostructured Fibers | Growth Factors | Performance | Ref. |
|---|---|---|---|
| Polycaprolactone (PCL) and polyethylene glycol (PEG) coaxial fibers | Fibroblast growth factor (bFGF) and epidermal growth factor (EGF) | A study inferred that 2% of EGF was released in a week from PCL-PEG shell and 30% of bFGF encapsulated in the core was released in 12 h. | [149] |
| Immobilized fibers | Human cathelicidin peptide LL37 (Cysk-KR12) | Antimicrobial peptide motif (Cysk-KR12) was able to maintain antibacterial properties for 3 weeks. This study concluded that Cysk-KR12 activated keratinocytes, fibroblasts, and monocytes. | [150] |
| Polycaprolactone (PCL) | Peptide E7Arg-Gly-Asp peptide (RGD) | This study showed that PCL/E7 attained high percentage on MSCs growth than PCL/RGD and lowered inflammatory cells. | [151] |
| Polycaprolactone (PCL) | Soluble eggshell membrane protein (SEP) | A study stated that SEP-grafted PCL fibers were more hydrophilic than the blank PCL fibers, which promoted human dermal fibroblasts (HDFs) growth. | [152] |
| Hydrogel scaffold from FGL | FGL—A peptide motif from neural cell adhesion molecule | The nanofibrous morphology supported the migration and growth of spinal cord neural stem cells into a 3-dimensional scaffold. This study attained a promising treatment for spinal cord injuries using scaffold in tissue engineering. | [158] |
| PLGA or poly (lactic-co-glycolic acid) nanofiber scaffold | Peptide (RADA16-I-BMHP1) | A study integrated PLGA to a self-assembling peptide (RADA16-I-BMHP1) to assist adhesion and growth of rat Schwann cells. The peptide/PLGA blend upregulated genes expressions in the cultures: (SEMA3F), (NRP2), and (PLX1). | [159] |
| Polycaprolactone (PCL) | Gelatin molecules | A study manufactured PCL/Gelatin scaffold for nerve regeneration and 70/30 PCL/Gelatin to promote nerve stem cells in culture. | [160] |
| Polycaprolactone (PCL) | Gelatin molecules | A study suggested that PCL/gelatin assist spreading and growth of endothelial cells compared to a blank PCL surface. | [161] |
| Poly (l-lactic acid)-co-polycaprolactone fiber scaffold | Collagen | A study derived that collagen coated poly (l-lactic acid)-co-polycaprolactone fiber scaffold benefit attachment of endothelial cells in tissue engineering vascular grafts. | [162] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Small, M.; Faglie, A.; Craig, A.J.; Pieper, M.; Fernand Narcisse, V.E.; Neuenschwander, P.F.; Chou, S.-F. Nanostructure-Enabled and Macromolecule-Grafted Surfaces for Biomedical Applications. Micromachines 2018, 9, 243. https://doi.org/10.3390/mi9050243
Small M, Faglie A, Craig AJ, Pieper M, Fernand Narcisse VE, Neuenschwander PF, Chou S-F. Nanostructure-Enabled and Macromolecule-Grafted Surfaces for Biomedical Applications. Micromachines. 2018; 9(5):243. https://doi.org/10.3390/mi9050243
Chicago/Turabian StyleSmall, Madeline, Addison Faglie, Alexandra J. Craig, Martha Pieper, Vivian E. Fernand Narcisse, Pierre F. Neuenschwander, and Shih-Feng Chou. 2018. "Nanostructure-Enabled and Macromolecule-Grafted Surfaces for Biomedical Applications" Micromachines 9, no. 5: 243. https://doi.org/10.3390/mi9050243





