Acute Effects of Transdermal Administration of Jojoba Oil on Lipid Metabolism in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
2.3. Experimental Protocol
2.4. Biochemical Analysis of Serum
2.5. RNA Isolation and Gene Expression Analysis Using Real-Time Polymerase Chain Reaction (Real-Time PCR)
2.6. Statistical Analysis
3. Results
3.1. Effects on Serum Biochemical Data 30 Minutes after Transdermal Administration of Jojoba Oil
3.2. Changes in Expression Levels of Lipid Metabolism-Related Genes in Various Tissues/Organs after Transdermal Administration of Jojoba Oil
3.2.1. Changes in the Expression of Lipid Degradation-Related Genes
3.2.2. Changes in the Expression of Fatty Acid Trafficking-Related Genes
3.2.3. Changes in the Expression of Lipogenesis-Related Genes
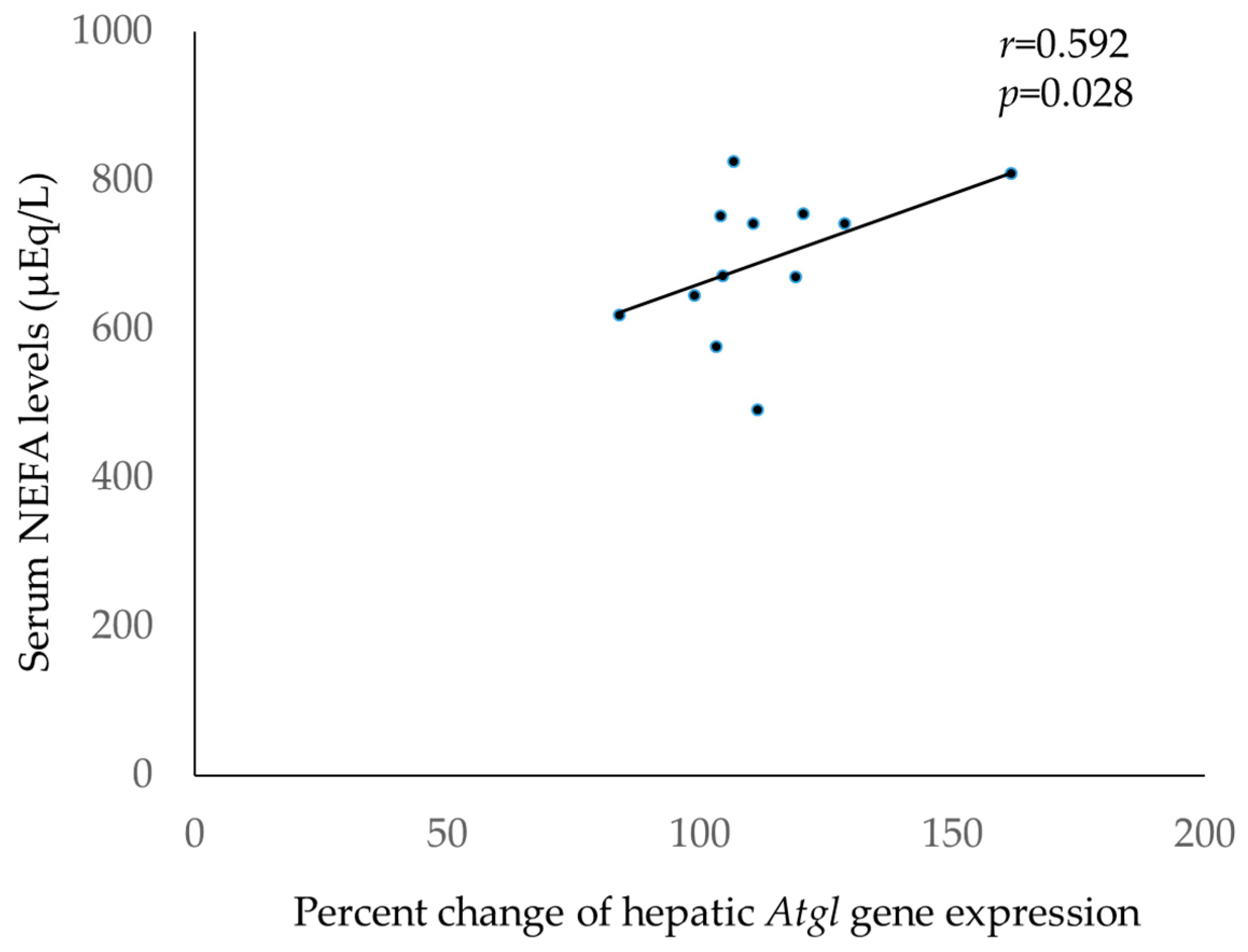
3.3. Correlation between Lipid Metabolism-Related Genes Expressed in Various Tissues/Organs and Serum NEFA Levels after Transdermal Administration of Jojoba Oil
3.3.1. Correlations between Lipid Degradation-Related Genes Expressed in Various Tissues/Organs and Serum NEFA Levels after Transdermal Administration of Jojoba Oil
3.3.2. Correlations between Fatty Acid Trafficking-Related Genes Expressed in Various Tissues/Organs and Serum NEFA Levels after Transdermal Administration of Jojoba Oil
3.3.3. Correlations between Lipogenesis-Related Genes Expressed in Various Tissues/Organs and Serum NEFA Levels after Transdermal Administration of Jojoba Oil
3.3.4. Correlations between Nuclear Transcription Factors Expressed in Various Tissues/Organs and Serum NEFA Levels after Transdermal Administration of Jojoba Oil
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kokoska, L.; Kloucek, P.; Leuner, O.; Novy, P. Plant-derived products as antibacterial and antifungal agents in human health care. Curr. Med. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraj, P.; Muthukumar, K.; Sabarirajan, J.; Nachiappan, V. Antihyperlipidemic activity of Cassia auriculata flowers in triton WR 1339 induced hyperlipidemic rats. Exp. Toxicol. Pathol. 2013, 65, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Ha, K.B.; Park, D.H.; Fang, Y.; Kim, J.H.; Park, M.G.; Woo, R.M.; Kim, W.J.; Park, I.K.; Choi, J.Y.; et al. Plant-derived compounds regulate formation of the insect juvenile hormone receptor complex. Pestic. Biochem. Physiol. 2018, 50, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Field, T. Massage therapy research review. Complement. Ther. Clin. Pract. 2016, 24, 19–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boehm, K.; Büssing, A.; Ostermann, T. Aromatherapy as an adjuvant treatment in cancer care—A descriptive systematic review. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 503–518. [Google Scholar] [CrossRef] [PubMed]
- Best, T.M.; Hunter, R.; Wilcox, A.; Haq, F. Effectiveness of sports massage for recovery of skeletal muscle from strenuous exercise. Clin. J. Sport Med. 2008, 18, 446–460. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.K.; Zhong, L.; Santiago, J.L. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Gassenmeier, T.; Busch, P.; Hensen, H.; Seipel, W. Some aspects of refatting the skin. Cosmet. Toilet. 1998, 113, 89–92. [Google Scholar]
- Loden, M. Effect of moisturizers on epidermal barrier function. Clin. Dermatol. 2012, 30, 286–296. [Google Scholar] [CrossRef]
- Stamatas, G.N.; de Sterke, J.; Hauser, M.; von Stetten, O.; van der Pol, A. Lipid uptake and skin occlusion following topical application of oils on adult and infant skin. J. Dermatol. Sci. 2008, 50, 135–142. [Google Scholar] [CrossRef]
- Patzelt, A.; Lademann, J.; Richter, H.; Darvin, M.E.; Schanzer, S.; Thiede, G.; Sterry, W.; Vergou, T.; Hauser, M. In vivo investigations on the penetration of various oils and their influence on the skin barrier. Skin Res. Technol. 2012, 18, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Tiidus, P.M. Manual massage and recovery of muscle function following exercise: A literature review. J. Orthop. Sports Phys. Ther. 1997, 25, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E. Does post-exercise massage treatment reduce delayed onset muscle soreness? A systematic review. Br. J. Sports Med. 1998, 32, 212–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brian, J.H. Physiological, psychological and performance effects of massage therapy in sport: A review of the literature. Phys. Ther. Sport 2001, 2, 165–170. [Google Scholar]
- Brummitt, J. The role of massage in sports performance and rehabilitation: Current evidence and future direction. N. Am. J. Sports Phys. Ther. 2008, 3, 7–21. [Google Scholar] [PubMed]
- Guo, J.; Li, L.; Gong, Y.; Zhu, R.; Xu, J.; Zou, J.; Chen, X. Massage alleviates delayed onset muscle soreness after strenuous exercise: A systematic review and meta-analysis. Front. Physiol. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Callaghan, M.J. The role of massage in the management of the athlete: A review. Br. J. Sports Med. 1993, 27, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Stefaniak, A.B.; Harvey, C.J. Dissolution of materials in artificial skin surface film liquids. Toxicol. In Vitro 2006, 20, 1265–1283. [Google Scholar] [CrossRef] [PubMed]
- Yermanos, D.M.; Duncan, C.C. Quantitative and qualitative characteristics of jojoba seed. J. Am. Oil Chem. Soc. 1976, 53, 80–82. [Google Scholar] [CrossRef]
- Miwa, T.K. Jojoba oil wax esters and derived fatty acids and alcohols: Gas chromatographic analyses. J. Am. Oil Chem. Soc. 1971, 48, 259–264. [Google Scholar] [CrossRef]
- Fujisaki, R.; Kamei, K.; Yamamura, M.; Nishiya, H.; Inouye, S.; Takahashi, M.; Abe, S. In vitro and in vivo anti-plasmodial activity of essential oils, including hinokitiol. Southeast Asian J. Trop. Med. Public Health 2012, 43, 270–279. [Google Scholar] [PubMed]
- Jäger, W.; Buchbauer, G.; Jirovetz, L.; Fritzer, M. Percutaneous absorption of lavender oil from a massage oil. J. Soc. Cosmet. Chem. 1992, 43, 49–54. [Google Scholar]
- Ranzato, E.; Martinotti, S.; Burlando, B. Wound healing properties of jojoba liquid wax: An in vitro study. J. Ethnopharmacol. 2011, 134, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Pazyar, N.; Yaghoobi, R.; Ghassemi, M.R.; Kazerouni, A.; Rafeie, E.; Jamshydian, N. Jojoba in dermatology: A succinct review. G. Ital. Dermatol. Venereol. 2013, 148, 687–691. [Google Scholar] [PubMed]
- Al-Obaidi, J.R.; Halabi, M.F.; Al-Khalifah, N.S.; Asanar, S.; Al-Soqeer, A.A.; Attia, M.F. A review on plant importance, biotechnological aspects, and cultivation challenges of jojoba plant. Biol. Res. 2017, 50, 25. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, R.; Strauss, J.G.; Haemmerle, G.; Schoiswohl, G.; Birner-Gruenberger, R.; Riederer, M.; Lass, A.; Neuberger, G.; Eisenhaber, F.; Hermetter, A.; et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 2004, 306, 1383–1386. [Google Scholar] [CrossRef]
- Finck, B.N.; Gropler, M.C.; Chen, Z.; Leone, T.C.; Croce, M.A.; Harris, T.E.; Lawrence, J.C., Jr.; Kelly, D.P. Lipin 1 is an inducible amplifier of the hepatic PGC-1α/PPARα regulatory pathway. Cell Metab. 2006, 4, 199–210. [Google Scholar] [CrossRef]
- Motojima, K.; Passilly, P.; Peters, J.M.; Gonzalez, F.J.; Latruffe, N. Expression of putative fatty acid transporter genes are regulated by peroxisome proliferator-activated receptor alpha and gamma activators in a tissue- and inducer-specific manner. J. Biol. Chem. 1998, 273, 16710–16714. [Google Scholar] [CrossRef]
- Schmuth, M.; Ortegon, A.M.; Mao-Qiang, M.; Elias, P.M.; Feingold, K.R.; Stahl, A. Differential expression of fatty acid transport proteins in epidermis and skin appendages. J. Investig. Dermatol. 2005, 125, 1174–1181. [Google Scholar] [CrossRef]
- Schaffer, J.E.; Lodish, H.F. Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell 1994, 79, 427–436. [Google Scholar] [CrossRef]
- Kusunoki, J.; Kanatani, A.; Moller, D.E. Modulation of fatty acid metabolism as a potential approach to the treatment of obesity and the metabolic syndrome. Endocrine 2006, 29, 91–100. [Google Scholar] [CrossRef]
- Sethi, G.; Shanmugam, M.K.; Kumar, A.P. SREBP-1c as a molecular bridge between lipogenesis and cell cycle progression of clear cell renal carcinoma. Biosci. Rep. 2017, 37, BSR20171270. [Google Scholar] [CrossRef] [PubMed]
- Ferré, P.; Foufelle, F. Hepatic steatosis: A role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes. Metab. 2010, 12, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Jabraeile, M.; Rasooly, A.S.; Farshi, M.R.; Malakouti, J. Effect of olive oil massage on weight gain in preterm infants: A randomized controlled clinical trial. Niger. Med. J. 2016, 57, 160–163. [Google Scholar] [PubMed]
- Sankaranarayanan, K.; Mondkar, J.A.; Chauhan, M.M.; Mascarenhas, B.M.; Mainkar, A.R.; Salvi, R.Y. Oil massage in neonates: An open randomized controlled study of coconut versus mineral oil. Indian Pediatr. 2005, 42, 877–884. [Google Scholar]
- Soriano, C.R.; Martinez, F.E.; Jorge, S.M. Cutaneous application of vegetable oil as a coadjutant in the nutritional management of preterm infants. J. Pediatr. Gastroenterol. Nutr. 2000, 31, 387–390. [Google Scholar] [CrossRef]
- Li, X.; Zhong, Q.; Tang, L. A meta-analysis of the efficacy and safety of using oil massage to promote infant growth. J. Pediatr. Nurs. 2016, 31, e313–e322. [Google Scholar] [CrossRef]
- Solanki, K.; Matnani, M.; Kale, M.; Joshi, K.; Bavdekar, A.; Bhave, S.; Pandit, A. Transcutaneous absorption of topically massaged oil in neonates. Indian Pediatr. 2005, 42, 998–1005. [Google Scholar]
- Li, B.S.; Cary, J.H.; Maibach, H.I. Should we instruct patients to rub topical agents into skin? The evidence. J. Dermatol. Treat. 2019, 30, 328–332. [Google Scholar] [CrossRef]
- Bos, J.D.; Meinardi, M.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef]
- Choe, C.; Schleusener, J.; Lademann, J.; Darvin, M.E. In vivo confocal Raman microscopic determination of depth profiles of the stratum corneum lipid organization influenced by application of various oils. J. Dermatol. Sci. 2017, 87, 183–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Name | MW [g/mol] | Content [%] |
|---|---|---|
| Eicosenoic acid | 310.51 | 73.4 |
| Erucic acid | 338.57 | 14.7 |
| Oleic acid | 282.47 | 8.3 |
| Name | Accession no. | Forward | Reverse |
|---|---|---|---|
| Atgl | NM_025802.3 | TGTGGCCTCATTCCTCCTAC | TCGTGGATGTTGGTGGAGCT |
| Hsl | NM_010719.5 | GCTGGGCTGTCAAGCACTGT | GTAACTGGGTAGGCTGCCAT |
| Lpl | NM_008509.2 | CCAATGGAGGCACTTTCCA | TGGTCCACGTCTCCGAGTC |
| Cpt-1a | XM_006531658.3 | CCAGGCTACAGTGGGACATT | AAGGAATGCAGGTCCACATC |
| Cd36 | NM_001159558.1 | CCGGGCCAACGTAGAAAACA | CCTCCAAACACAGCCAGGAC |
| FABPpm | NM_010325.2 | AGCGGCTGACCAAGGAGTT | GACCCCTGCCACGGAGAT |
| FATP-1 | NM_011977.4 | GGCTCCTGGAGCAGGAACA | ACGGAAGTCCCAGAAACCAA |
| FATP-2 | NM_011978.2 | TTCGGGAACCACAGGTCTTC | GCAAGGCTTGTCCCATACCTT |
| FATP-3 | NM_001316688.1 | CAGCTCTACAGCCATGTTTCTGA | CAAAGATTCCTGGAGCCTGAGA |
| FATP-4 | NM_011989.5 | GGCTTCCCTGGTGTACTATGGAT | ACGATGTTTCCTGCTGAGTGGTA |
| FATP-5 | NM_009512.2 | TTTCTGGGGTTGGCCAAGTT | TGGCCAAGGTAGAAGCAGTG |
| FATP-6 | NM_001081072.1 | GGCTTGAGGATGCCGCTTA | GTACTCTGGGCTCATGCTATGAAGT |
| Acc-1 | XM_011248667.1 | ATTGGGCACCCCAGAGCTA | CCCGCTCCTTCAACTTGCT |
| Acc-2 | XM_006530113.3 | GGGCTCCCTGGATGACAAC | TTCCGGGAGGAGTTCTGGA |
| Fas | NM_007988.3 | CCTGGATAGCATTCCGAACCT | AGCACATCTCGAAGGCTACACA |
| Scd-1 | NM_009127.4 | TTCTTGCGATACACTCTGGTGC | CGGGATTGAATGTTCTTGTCGT |
| Srebp-1c | XM_006532716.2 | GGAGCCATGGATTGCACATT | GGCCCGGGAAGTCACTGT |
| Lpin-1 | NM_001355598.1 | CCATTCACAGCGAGTCTTCA | TGGAAGGGGAATCTGTCTTG |
| Ppar-a | XM_006520624.3 | TCTGTGGGCTCACTGTTCT | AGGGCTCATCCTGTCTTTG |
| Sirt-1 | NM_001159289.2 | GCAACAGCATCTTGCCTGAT | GTGCTACTGGTCTCACTT |
| Actb | NM_007393.5 | CCTCCCTGGAGAAGAGCTATG | TTACGGATGTCAACGTCACAC |
| Control | Jojoba Oil | ||
|---|---|---|---|
| ALB | (g/dL) | 2.8 ± 0.1 | 2.9 ± 0.0 |
| BUN | (mg/dL) | 25.9 ± 1.1 | 23.8 ± 1.0 |
| CRE | (mg/dL) | 0.1 ± 0.0 | 0.1 ± 0.0 |
| UA | (mg/dL) | 2.0 ± 0.2 | 2.2 ± 0.1 |
| AST | (IU/L) | 99.5 ± 15.4 | 87.0 ± 6.8 |
| ALP | (IU/L) | 401.7 ± 21.9 | 414.7 ± 17.0 |
| CK | (IU/L) | 388.0 ± 102.0 | 216.7 ± 45.1 |
| T-CHO | (mg/dL) | 73.8 ± 2.3 | 70.0 ± 1.9 |
| TG | (mg/dL) | 67.5 ± 7.7 | 62.0 ± 6.7 |
| PL | (mg/dL) | 154.3 ± 4.0 | 148.7 ± 5.5 |
| NEFA | (μEq/L) | 626.2 ± 36.0 | 757.7 ± 22.8 * |
| LDL-C | (mg/dL) | 4.8 ± 0.4 | 4.3 ± 0.6 |
| HDL-C | (mg/dL) | 47.0 ± 1.5 | 44.3 ± 1.4 |
| TBA | (μmol/L) | 1.2 ± 0.2 | 1.0 ± 0.0 |
| GLU | (mg/dL) | 239.5 ± 17.5 | 223.7 ± 12.2 |
| LA | (mg/dL) | 81.0 ± 6.9 | 66.8 ± 5.3 |
| T-KB | (μmol/L) | 317.0 ± 25.5 | 338.7 ± 25.4 |
| Liver | WAT | Skin | BAT | Plantaris Muscle | Heart | |
|---|---|---|---|---|---|---|
| Lipid degradation | ||||||
| Atgl | 123.2 ± 8.0 * | 138.4 ± 22.8 | 68.0 ± 3.6 | 102.5 ± 3.6 | 74.5 ± 8.2 | 92.4 ± 3.1 |
| Hsl | 95.0 ± 4.0 | 134.7 ± 21.3 | 55.4 ± 4.9 † | 114.8 ± 2.9 | 84.3 ± 5.2 | 80.4 ± 9.1 |
| Lpl | 94.9 ± 3.8 | 135.7 ± 20.8 | 90.7 ± 15.4 | 99.0 ± 8.2 | 81.2 ± 6.1 | 90.0 ± 5.4 |
| Cpt-1a | 112.1 ± 6.8 | 112.9 ± 36.0 | 103.7 ± 8.2 | 89.6 ± 3.5 | 80.9 ± 10.6 | 99.3 ± 6.6 |
| Fatty acid trafficking | ||||||
| Cd36 | 140.4 ± 23.6 | 113.1 ± 16.4 | 108.6 ± 10.0 | 110.9 ± 3.5 | 94.6 ± 9.8 | 102.1 ± 6.4 |
| FABPpm | 101.6 ± 9.4 | 47.6 ± 34.4 | 64.7 ± 7.4 † | 128.3 ± 34.2 | 88.5 ± 6.4 | 94.6 ± 6.2 |
| FATP-1 | 83.0 ± 4.9 | 89.1 ± 16.3 | 30.0 ± 3.8 † | 103.6 ± 6.6 | 61.0 ± 22.1 | 96.6 ± 3.4 |
| FATP-2 | 112.6 ± 13.8 | 77.2 ± 52.1 | N.D. | 123.8 ± 11.6 | 87.2 ± 79.1 | 36.7 ± 20.9 |
| FATP-3 | 198.4 ± 21.5 ** | 100.8 ± 15.9 | 43.6 ± 4.8 † | 84.2 ± 4.1 | 76.5 ± 7.0 | 140.7 ± 30.1 |
| FATP-4 | 80.8 ± 3.9 * | 84.2 ± 26.3 | 69.0 ± 5.0 † | 97.4 ± 4.9 | 76.3 ± 8.7 | 107.5 ± 8.4 |
| FATP-5 | 112.6 ± 11.0 | 53.3 ± 29.1 | N.D. | 39.7 ± 6.9 | N.D. | 106.6 ± 36.7 |
| FATP-6 | N.D. | 35.2 ± 15.5 | N.D. | 16.3 ± 4.8 | 65.5 ± 20.1 | 40.9 ± 14.5 |
| Lipogenesis | ||||||
| Acc-1 | 91.4 ± 6.6 | 119.7 ± 19.0 | 53.3 ± 3.6 † | 87.6 ± 3.7 * | 103.2 ± 5.8 | 58.7 ± 15.0 |
| Acc-2 | 81.5 ± 6.6 | 138.0 ± 22.6 | 40.4 ± 3.4 * | 100.2 ± 5.2 | 74.4 ± 5.4 * | 91.5 ± 5.6 |
| Fas | 89.7 ± 9.9 | 99.3 ± 16.9 | 85.3 ± 4.0 | 87.5 ± 2.6 * | 97.2 ± 12.5 | 51.1 ± 20.7 |
| Scd-1 | 81.0 ± 7.1 | 133.4 ± 28.0 | 81.9 ± 16.9 | 96.0 ± 3.8 | 158.6 ± 28.5 † | 81.7 ± 29.3 |
| Nuclear transcrption factors | ||||||
| Srebp-1c | 94.6 ± 7.7 | 95.5 ± 5.5 | 52.6 ± 9.4 ** | 87.2 ± 2.1 ** | 58.1 ± 2.6 * | 78.0 ± 7.0 |
| Lpin-1 | 284.8 ± 68.6 * | 130.5 ± 14.7 | 43.1 ± 5.0 * | 95.5 ± 3.2 | 75.9 ± 7.6 | 76.4 ± 4.2 |
| Ppar-a | 92.1 ± 8.3 | 125.6 ± 16.5 | 14.3 ± 4.2 * | 129.8 ± 12.2 | 119.1 ± 44.0 | 85.9 ± 6.3 |
| Sirt-1 | 157.6 ± 16.7 * | 90.7 ± 9.7 | 127.1 ± 6.1 † | 89.4 ± 2.6 | 116.2 ± 13.2 | 112.8 ± 7.2 |
| Liver | WAT | Skin | BAT | Plantaris Muscle | Heart | |
|---|---|---|---|---|---|---|
| Lipid degradation | ||||||
| Atgl | 0.592 * | 0.392 | −0.385 | 0.095 | −0.294 | −0.179 |
| Hsl | −0.178 | 0.025 | −0.427 † | 0.235 | −0.179 | 0.196 |
| Lpl | −0.046 | 0.231 | 0.123 | 0.277 | −0.018 | −0.469 |
| Cpt-1a | 0.133 | −0.063 | 0.168 | −0.196 | −0.098 | −0.200 |
| Fatty acid trafficking | ||||||
| Cd36 | 0.528 † | −0.035 | −0.032 | 0.179 | −0.287 | −0.263 |
| FABPpm | 0.049 | −0.081 | −0.133 | 0.182 | −0.266 | −0.375 |
| FATP-1 | 0.305 | 0.028 | −0.284 | 0.238 | −0.203 | −0.298 |
| FATP-2 | 0.098 | 0.091 | N.D. | 0.091 | −0.071 | 0.395 |
| FATP-3 | 0.452 | 0.312 | −0.501 † | −0.361 | −0.168 | 0.462 |
| FATP-4 | −0.126 | −0.319 | −0.357 | −0.004 | −0.413 | −0.231 |
| FATP-5 | 0.203 | −0.238 | N.D. | −0.056 | N.D. | 0.035 |
| FATP-6 | N.D. | −0.207 | N.D. | −0.132 | −0.237 | −0.074 |
| Lipogenesis | ||||||
| Acc-1 | 0.025 | 0.277 | −0.147 | −0.137 | 0.025 | 0.347 |
| Acc-2 | 0.770 | 0.326 | −0.280 | 0.109 | −0.333 | −0.392 |
| Fas | −0.084 | −0.004 | 0.070 | −0.228 | 0.294 | 0.487 |
| Scd-1 | 0.105 | 0.259 | −0.123 | 0.155 | 0.490 | 0.581 * |
| Nuclear transcrption factors | ||||||
| Srebp-1c | −0.389 | −0.119 | −0.438 | −0.497 | −0.567 † | −0.291 |
| Lpin-1 | 0.583 † | 0.228 | −0.543 | 0.130 | −0.158 | −0.609 * |
| Ppar-a | −0.123 | 0.028 | −0.452 | 0.109 | 0.053 | −0.424 |
| Sirt-1 | 0.592 † | 0.238 | 0.308 | −0.333 | −0.238 | 0.147 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, Y.; Ma, S.; Tominaga, T.; Yokoyama, K.; Kitatani, K.; Horikawa, K.; Suzuki, K. Acute Effects of Transdermal Administration of Jojoba Oil on Lipid Metabolism in Mice. Medicina 2019, 55, 594. https://doi.org/10.3390/medicina55090594
Matsumoto Y, Ma S, Tominaga T, Yokoyama K, Kitatani K, Horikawa K, Suzuki K. Acute Effects of Transdermal Administration of Jojoba Oil on Lipid Metabolism in Mice. Medicina. 2019; 55(9):594. https://doi.org/10.3390/medicina55090594
Chicago/Turabian StyleMatsumoto, Yutaka, Sihui Ma, Takaki Tominaga, Keiko Yokoyama, Kanae Kitatani, Kazumasa Horikawa, and Katsuhiko Suzuki. 2019. "Acute Effects of Transdermal Administration of Jojoba Oil on Lipid Metabolism in Mice" Medicina 55, no. 9: 594. https://doi.org/10.3390/medicina55090594







