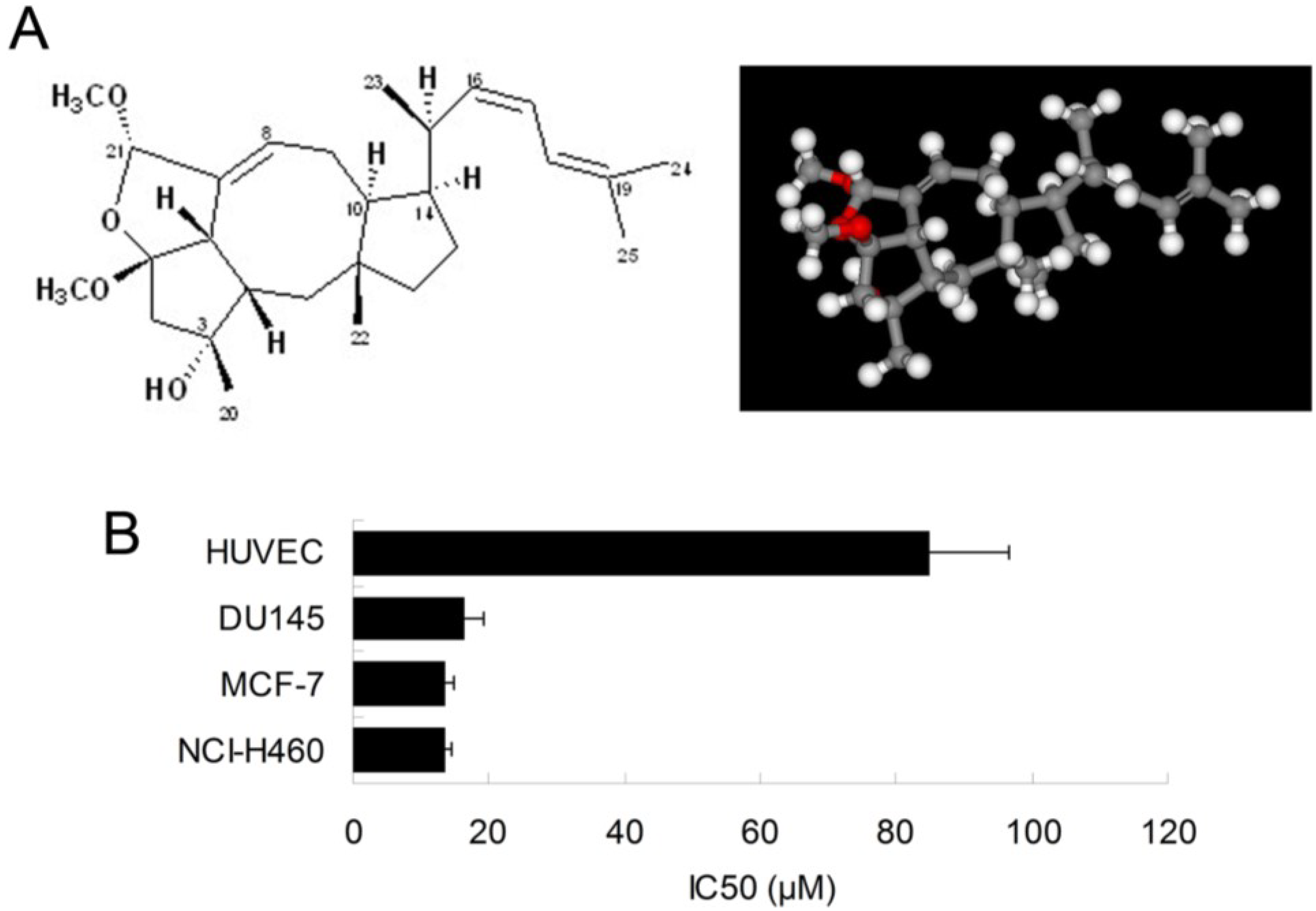
2.1. Ophiobolin O Inhibits the Proliferation of MCF-7 Cells
We have previously shown that the natural compound ophiobolin O isolated from
Aspergillus ustus 094102 inhibited the growth of human breast cancer cells [
4]. In this study, we further examined the inhibitory effect of ophiobolin O on proliferation of other cell lines, such as HUVEC (Human umbilical vein endothelial cell line), DU145 (Human prostate cancer cell line) and NCI-H460 (Human large cell lung carcinoma cell line). The results demonstrated that breast cancer cells MCF-7 was significantly sensitive to ophiobolin O, with IC
50 value of 13.45 ± 1.26 μM. The IC
50 values were 84.96 ± 11.73 μM, 16.48 ± 2.68 μM and 13.55 ± 1.01 μM when ophiobolin O treated HUVEC, DU145 and NCI-H460 cells, respectively. It is worth noting that normal human umbilical vein endothelial cells (HUVEC) are less sensitive to ophiobolin O compared with other cancer cell lines (
Figure 1B), suggesting that ophiobolin O has a potent anti-cancer activity that preferentially kills cancer cells, especially breast cancer cells, over normal cells. This selectivity led us to question the mechanism by which ophiobolin O exerted its effect on MCF-7 cells.
2.2. Ophiobolin O Induces G1 Arrest in MCF-7 Cells
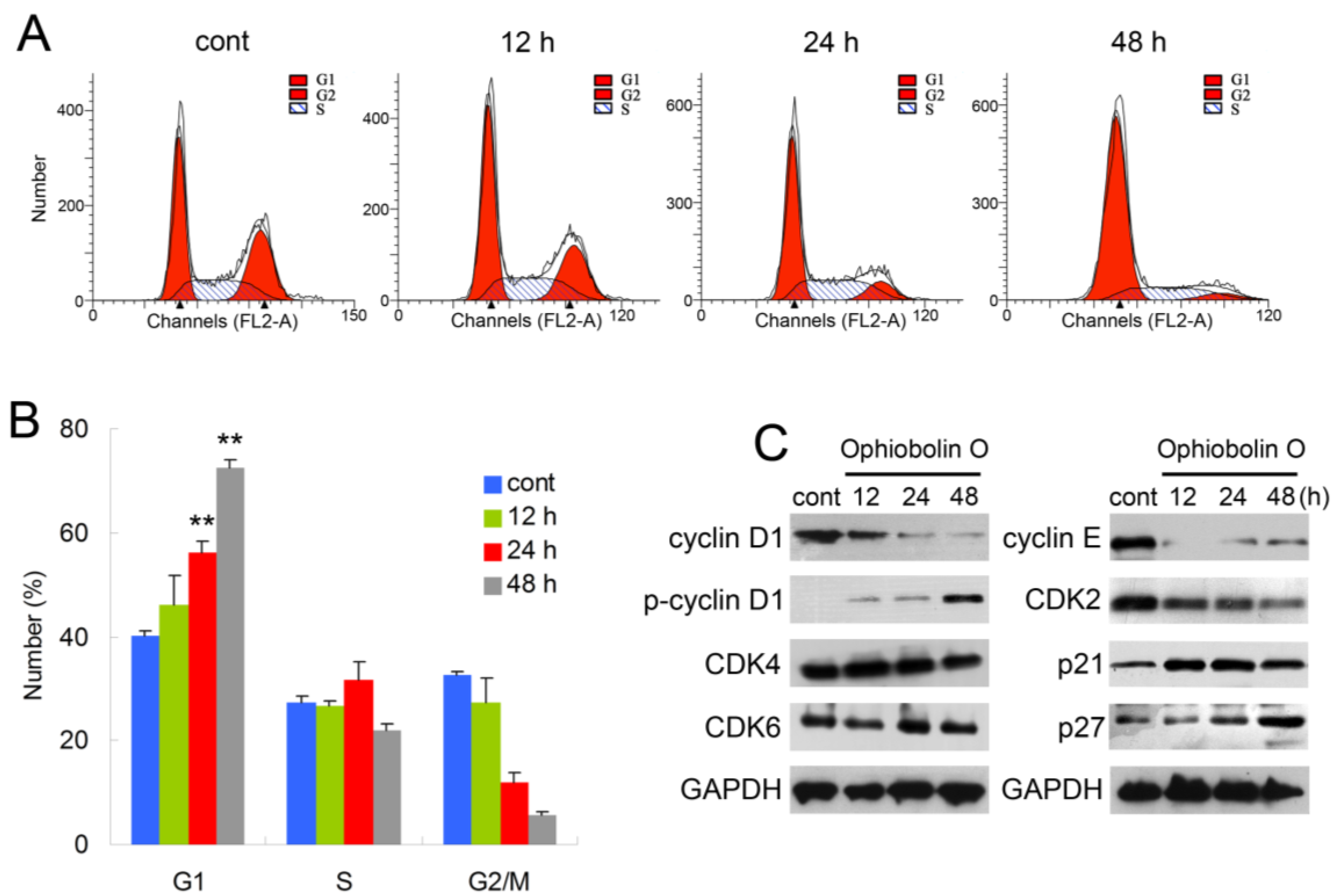
In order to test whether the inhibition effect of ophiobolin O was related to cell cycle progression, cell cycle distribution and related checkpoint factors were studied. MCF-7 cells were treated with 15 μM ophiobolin O for 12 h, 24 h and 48 h, respectively. The results showed that cells treated with ophiobolin O accumulated progressively in G1 phase (
Figure 2A,B). Compared with the negative control, treatment with ophiobolin O resulted in a significant increase in the proportion of G1 phase cells (control: 40.19% ± 1.03%; 12 h: 46.09% ± 5.79%; 24 h: 56.18% ± 2.19%; 48 h: 72.56% ± 1.35%) and about a 1.8-fold increase after 48 h. Meanwhile, G2/M reduced appreciably from 32.57% ± 0.69% to 5.53% ± 0.88% during 48 h incubation. These results suggest that G1 phase arrest accounts for the anti-proliferative effect of ophiobolin O observed in MCF-7 cells. Besides, ophiobolin O treatment resulted in a time-dependent decrease in the protein expression of cyclin D1, cyclin E, CDK2 and increase of p-cyclin D1 (Thr286), p21 and p27. However, the protein levels of CDK4 and CDK6 were not significantly changed during ophiobolin O treatment (
Figure 2b).
Figure 2.
Ophiobolin O induces cell cycle arrest in MCF-7 cells. (A) Ophiobolin O caused cell cycle arrest at the G1 phase. Cells were treated with vehicle and 15 μM Ophiobolin O for 12, 24, and 48 h, and then cell cycle distribution was assessed using flow cytometry; (B) The percentage of cells in different phases of the cell cycle was represented by a bar diagram. The percentage of cells in each population was shown as mean ± SD from three independent experiments. * p < 0.05, ** p < 0.01; (C) Western blot analysis of G1 transition-related proteins were analyzed by Western blotting assay. GAPDH was used as an equal loading control.
Figure 2.
Ophiobolin O induces cell cycle arrest in MCF-7 cells. (A) Ophiobolin O caused cell cycle arrest at the G1 phase. Cells were treated with vehicle and 15 μM Ophiobolin O for 12, 24, and 48 h, and then cell cycle distribution was assessed using flow cytometry; (B) The percentage of cells in different phases of the cell cycle was represented by a bar diagram. The percentage of cells in each population was shown as mean ± SD from three independent experiments. * p < 0.05, ** p < 0.01; (C) Western blot analysis of G1 transition-related proteins were analyzed by Western blotting assay. GAPDH was used as an equal loading control.
2.3. Putative Protein Targets for Ophiobolin O
Previously, we observed that ophiobolin O-induced cell apoptosis was regulated via activation of MAPK signaling pathways in MCF-7 cells; but for cell cycle arrest, we did not elucidate how the ophiobolin O induced G1 phase arrest. Furthermore, to establish the anti-cancer mechanism of ophiobolin O, an inverse docking (INVDOCK) analysis was applied to identify the possible targets of ophiobolin O. Using the INVDOCK program, 90 putative proteins were extracted from the Protein Data Bank. Of these, three proteins were closely related to apoptosis and G1 phase arrest (
Table 1). TNF (Tumor necrosis factor) is related to cell apoptosis [
7]; MAP2K1 (Dual specificity mitogen-activated protein kinase kinase 1; ERK), which is a member of MAPKs, has been proved to be activated during the ophiobolin O-induced apoptosis in MCF-7 cells [
4]; GSK3β (Glycogen synthase kinase-3 beta)regulates G1 phase transition through Phosphorylation of cyclin D1 which is one of the key regulatory proteins controlling the transition fromG1 to S phase [
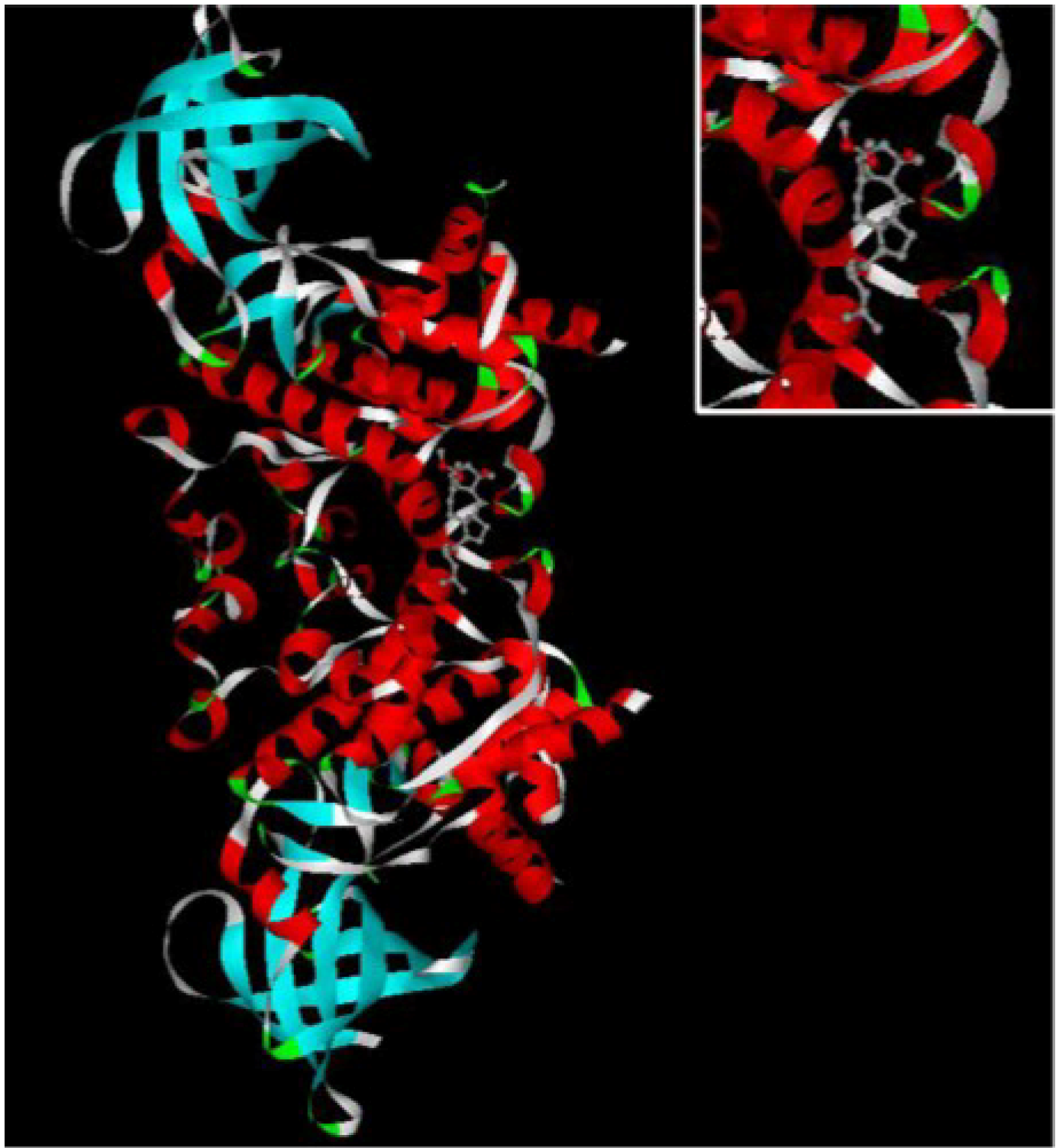
8], therefore, in order to study the mechanism of ophiobolin O-induced G1 phase arrest, 3-D structure of GSK3β was chosen to explore its binding interaction with ophiobolin O. The illustration of ophiobolin O docked to GSK3β using the INVDOCK program is shown in
Figure 3. Such an analysis indicated that ophiobolin O could form a reasonable drug-protein complex with GSK3β, which provided a hypothesis that ophiobolin O may induce G1 arrest of MCF-7 cells through interaction with GSK3β/cyclin D1 signaling.
Table 1.
Apoptotic or G1 phase-related proteins predicted by INVDOCK to bind to ophiobolin O.
Table 1.
Apoptotic or G1 phase-related proteins predicted by INVDOCK to bind to ophiobolin O.
| Compond | Protein | Gene | Gene ID | Species | Ligand-Protein Interaction Energy Value |
|---|
| Ophiobolin O | Glycogen synthase kinase-3beta | GSK-3β | 2932 | Human | −35.8 |
| Tumor necrosis factor | TNF | 7124 | Human | −37.5 |
| Dual specificity mitogen-activated protein kinase kinase 1 | MAP2K1 | 5604 | Human | −37.6 |
Figure 3.
The INVDOCK analysis of ophiobolin O binds to GSK3β. Illustration of the ophiobolin O molecule docked into the GSK3β protein, modeled using the INVDOCK program; upright: the Amplification of binding area.
Figure 3.
The INVDOCK analysis of ophiobolin O binds to GSK3β. Illustration of the ophiobolin O molecule docked into the GSK3β protein, modeled using the INVDOCK program; upright: the Amplification of binding area.
2.4. Ophiobolin O Induces G1 Arrest of MCF-7 Cells through Interaction with AKT/GSK3β/Cyclin D1 Signaling
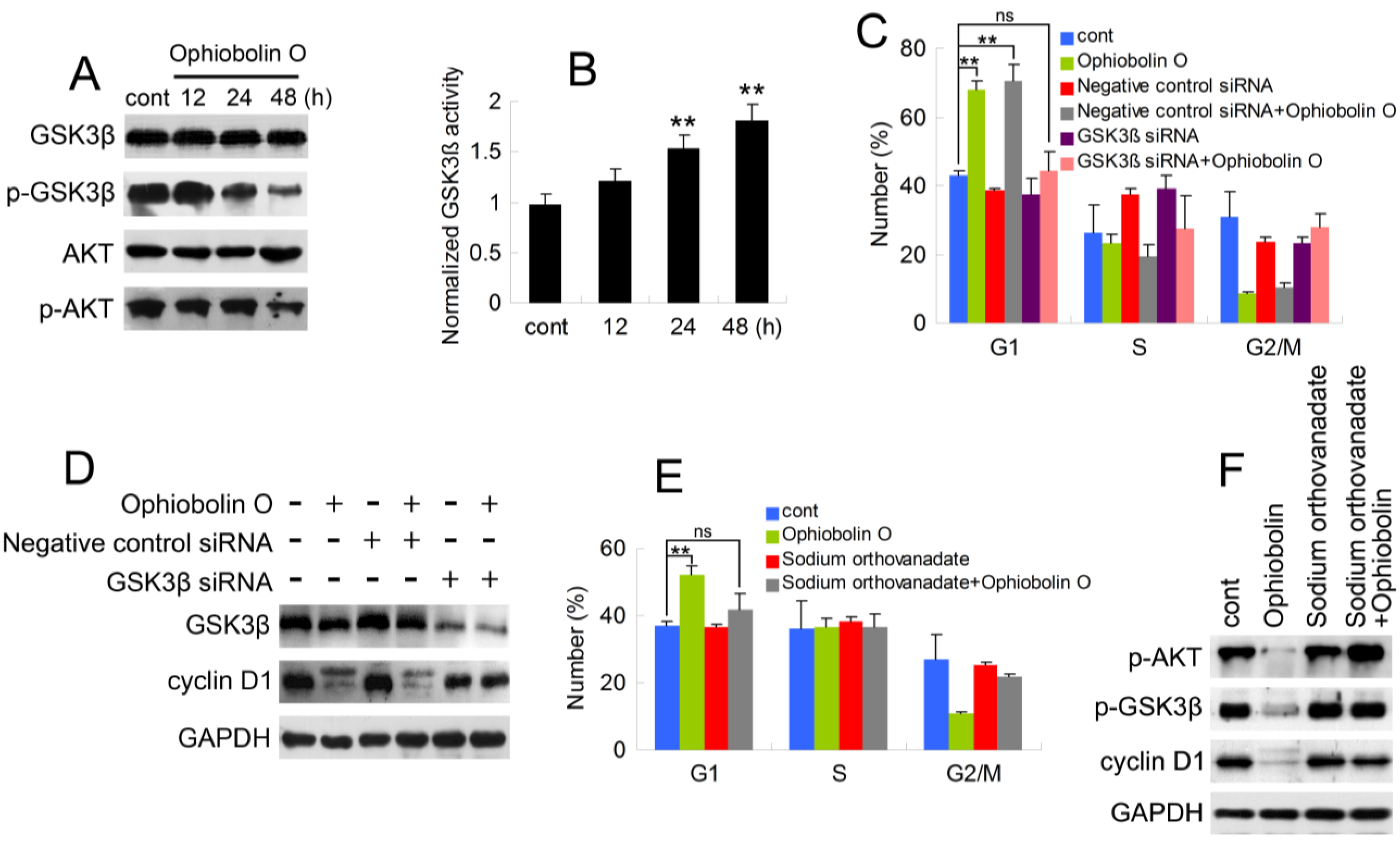
As described above, it is essential to check whether GSK3β is involved in ophiobolin O-induced G1 phase arrest. Therefore, protein level of GSK3β was analyzed by Western blotting. Furthermore, consider that Akt/GSK3β signaling plays a critical role in cyclin D1 expression and carcinogenesis, we also examined AKT levels. As shown in
Figure 4A, ophiobolin O treatment reduced the phosphorylation level of Akt (Ser 473) and GSK3β (Ser 9), indicating an increased activity of GSK3β. And GSK3β activity assay proved the increased enzyme activity of GSK3β
in vitro (
Figure 4B). Furthermore, we used siRNA to knock-down GSK3β, and observed that cells transfected with GSK3β siRNA were not sensitive to ophiobolin O anymore: ophiobolin O-induced G1 phase arrest in MCF-7 cells was significantly abolished; and the decrease of cyclin D1 expression was brought to normal levels (
Figure 4C,D). Finally, phosphatase inhibitor sodium orthovanadate was applied to halt dephosphorylation of AKT and GSK3β, and the pre-treatment also blocked ophiobolin O-induced G1 phase arrest and cyclin D1 reduction.
Figure 4.
AKT/GSK3β signaling is involved in ophiobolin O-induced G1 phase arrest in MCF-7 cells. (A) GSK3β, p-GSK3β (Ser 9), AKT and p-AKT (Ser 473) were detected after ophiobolin O-treatment for 48h by western blotting analysis; (B) Statistical results showing the normalized enzyme activities of GSK3β. Cells were treated with 15 μM ophiobolin O, harvested at 12 h, 24 h and 48 h. GSK3β activity was determined using GSK3β enzyme activity detection kit, and normalized versus control group; (C) Cells transfected with GSK3β siRNA or not were treated with or without 15 μM ophiobolin O for 48 h, then harvested for cell cycle analysis or (D) western blotting assay against GSK3β and cyclin D1. GAPDH was used to ensure equal protein loading; (E) Cells pre-treated with sodium orthovanadate or not were incubated with or without 15 μM ophiobolin O for 48 h, then harvested for cell cycle analysis or (F) western blotting assay against p-AKT, p-GSK3β and cyclin D1. Values were means ± SD of three independent experiments. * p < 0.05, ** p < 0.01 versus control group.
Figure 4.
AKT/GSK3β signaling is involved in ophiobolin O-induced G1 phase arrest in MCF-7 cells. (A) GSK3β, p-GSK3β (Ser 9), AKT and p-AKT (Ser 473) were detected after ophiobolin O-treatment for 48h by western blotting analysis; (B) Statistical results showing the normalized enzyme activities of GSK3β. Cells were treated with 15 μM ophiobolin O, harvested at 12 h, 24 h and 48 h. GSK3β activity was determined using GSK3β enzyme activity detection kit, and normalized versus control group; (C) Cells transfected with GSK3β siRNA or not were treated with or without 15 μM ophiobolin O for 48 h, then harvested for cell cycle analysis or (D) western blotting assay against GSK3β and cyclin D1. GAPDH was used to ensure equal protein loading; (E) Cells pre-treated with sodium orthovanadate or not were incubated with or without 15 μM ophiobolin O for 48 h, then harvested for cell cycle analysis or (F) western blotting assay against p-AKT, p-GSK3β and cyclin D1. Values were means ± SD of three independent experiments. * p < 0.05, ** p < 0.01 versus control group.
![Marinedrugs 13 00431 g004]()
Taken together, these results suggest that ophiobolin O treatment reduces the phosphorylation of Akt and subsequent phosphorylation of GSK3β, indicating Akt inactivation and GSK3β activation respectively. This might contribute to cyclinD1 degradation and G1-phase arrest, cause GSK3βis a critical regulator of cyclin D1 expression [
6,
9,
10].
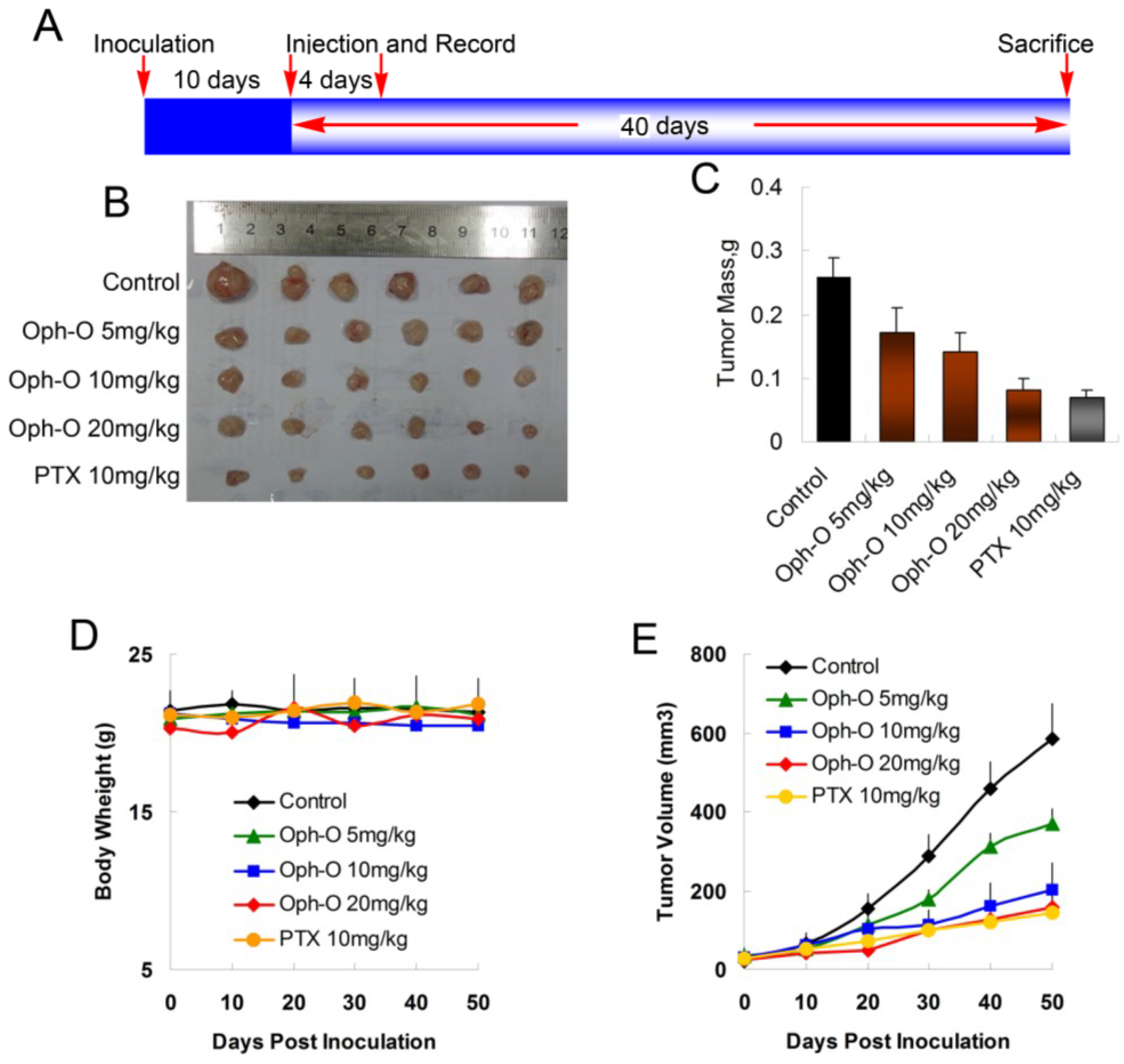
2.5. Ophiobolin O Inhibits Tumor Xenograft Growth
To evaluate whether ophiobolin O inhibits tumor growth
in vivo, MCF-7 cells were injected s.c. into the right armpit of six-week old BALB/c female athymic mice. The injected carcinoma cells grew into palpable tumors in the nude mice within 10 days (
Figure 5A). Paclitaxel (PTX)-treated mice (10 mg/kg) were used as positive control to assess the effect and toxicity of ophiobolin O. In this study, the tumor growth in mice treated with ophiobolin O (5, 10, or 20 mg/kg/day) was dose-dependently slowed (
Figure 5B,C). Tumor volume was significantly reduced during ophiobolin O treatment (
Figure 5E). The inhibitory rates at 50th day of 5, 10, and 20 mg/kg ophiobolin O treatment group were 34.62%, 46.15%, and 69.23%, respectively. The 20 mg/kg ophiobolin O treatment showed nearly equivalent effect compared to positive control (inhibition rate of 73.08%). Furthermore, no significant weight loss was observed during the course of ophiobolin O treatment, indicating there is feasibility for upward adjustment of dosage for improved treatment outcomes (
Figure 5D).
Figure 5.
Effect of ophiobolin O on tumor growth. (A) BALB/c male athymic mice were injected 5 × 106 MCF-7 cells s.c. for the development of subcutaneous tumors. The mice were randomized into five groups and treated with Vehicle (1% DMSO), 10 mg/kg PTX or various concentrations of ophiobolin O (5, 10, or 20 mg/kg) i.v. every four days until sacrifice according to the protocol in panel (A); (B) Tumor image from various treatment groups; (C) Average tumor mass at sacrifice; (D) Tumor volume measurements; (E) Body weight during the treatment.
Figure 5.
Effect of ophiobolin O on tumor growth. (A) BALB/c male athymic mice were injected 5 × 106 MCF-7 cells s.c. for the development of subcutaneous tumors. The mice were randomized into five groups and treated with Vehicle (1% DMSO), 10 mg/kg PTX or various concentrations of ophiobolin O (5, 10, or 20 mg/kg) i.v. every four days until sacrifice according to the protocol in panel (A); (B) Tumor image from various treatment groups; (C) Average tumor mass at sacrifice; (D) Tumor volume measurements; (E) Body weight during the treatment.
2.6. Discussion
So far, studies have shown that ophiobolin O significantly suppressed tumor growth in vivo and in vitro. It triggered cell cycle arrest at G1 phase through interaction with AKT/GSK3β/cyclin D1 signaling.
In our obvious studies, significant attention was focused on the apoptosis induced by ophiobolin O, and the important role of MAPK signaling pathway involved in apoptotic death in MCF-7 cells. MAPK signaling cascades have been proved to play essential roles in the regulation of a wide variety of cellular processes [
11], and several key signaling components and phosphorylation events participate in regulating the cell cycle, apoptosis and even tumorigenesis [
12,
13]. JNK, ERK and p38 are three members of the MAPK family [
14]. The JNK pathway is considered to be responsible for the apoptotic response induced by several anti-tumor agents [
15], and we have reported that the activation of JNK induced by ophiobolin O triggered apoptosis through the phosphorylation of Bcl-2 in MCF-7 cells. Otherwise, ERK and p38 activation were also observed during ophiobolin O treatment. The p38
SAPK2 and ERK2 have been shown to regulate G1 transition through interaction with cyclin D1 stability by phosphorylating T286 in some reports [
16,
17,
18]. However, here we declare that AKT/GSK3β/cyclin D1 signaling is responsible for ophiobolin O-induced cyclin D1 degradation and G1 arrest.
In tumor cells, the abnormal molecular activity directly induces the uncontrolled cell proliferation, which is one of the hallmarks of cancer. The majority of human cancers have been reported to have alterations in the function of cell cycle regulatory proteins [
19,
20,
21]. Cyclin D1 and cyclin E are the key regulatory proteins controlling the transition from G1 to S phase by binding to Cdk4/Cdk6 and CDK2 [
12]. Considering the crucial role of cyclin D1, it is not surprising that its expression is down-regulated in cells stimulated with anti-proliferative cytokines. This down-regulation generally manifests at the level of protein stabilization, which was accelerated via the phosphatidylinositol 3-kinase Akt/GSK3β pathway [
9]. In normal cells, the levels of cyclin D1 begin to rise early in G1 phase and continue to accumulate until the G1/S-phase boundary when levels rapidly decline [
13,
14]; glycogen synthase kinase 3β (GSK3β) induces rapid proteolysis of cyclin D1 by phosphorylating cyclin D1 on threonine residue 286 (Thr 286) [
8]. Phosphorylation facilitates cyclin D1 nuclear export by the exportin-chromosome maintenance region 1 (CRM1), and the phosphorylated form of the cyclin is subsequently degraded within the cytoplasm [
22,
23,
24]. In this manner, GSK3β regulates nuclear export and stability of cyclin D1 to ensure the normal mitosis. Usually, GSK3β has a higher level of basal activity in tumor cells than in their normal counterparts [
25]. Thus, the inactivation of GSK3β and subsequent up-regulation of cyclin D1 may have a critical role in human cancer cells. In these cells, phosphorylation at Ser 9 by AKT is a major means to inactivate GSK3β [
26,
27].
In our study, no obvious amount of changes of total GSK3β were observed during the treatment. In contrast, numerous phosphorylation form of GSK3β (Ser 9) was detected in control cells, and showed marked time-dependent decrease after ophiobolin O treatment (
Figure 4A), consistently with increase of GSK3β activity (
Figure 4B). Therefore, the expression of cyclin D1 reduced and phosphorylated cyclin D1 on threonine residue 286 was subsequently increased (
Figure 2C). These results indicated that the inactivation of GSK3β contributes to MCF-7 cell proliferation, and GSK3β/cyclin D1 cell signaling maybe involved in ophiobolin O-induced G1 phase arrest. The INVDOCK was designed to confirm the potential targets related with ophiobolin O-induced anti-neoplastic effect, and the results noted that ophiobolin O could directly bind to GSK3β (
Table 1 and
Figure 3). It is accepted that small-molecule drugs generally exert their therapeutic functions by binding to the cavities of proteins to influence their biological activities [
28]. This binding may influence phosphorylation of GSK3β, therefore activate GSK3β and down-regulate cyclin D1 to induce G1 phase arrest. To investigate weather the putative protein target-GSK3β is responsible for ophiobolin O-induced cyclin D1 degradation and G1 arrest, we used siRNA to knock-down GSK3β, and noticed ophiobolin O-induced reduction of cyclin D1 and G1 arrest were significantly abolished (
Figure 4C–E). Otherwise, considering GSK3β as a Akt-inactivating factor, we also detected the AKT effect during ophiobolin O treatment. It appeared that ophiobolin O inhibited the phosphorylation level of Akt at Ser 473. Furthermore, we used the sodium orthovanadate to up-regulate Akt activity. The sodium orthovanadate is an inhibitor of tyrosine phosphatases, alkaline phosphatases and a number of ATPases; commonly used to improve the activation of protein by inhibiting the dephosphorylation or promoting phosphorylation. It prevents decreased Akt-Ser-473 phosphorylation in the CA1 region, which up-regulates Akt activity [
29]. In our study, sodium orthovanadate was applied to halt dephosphorylation of AKT and GSK3β, and the pre-treatment also blocked ophiobolin O-induced G1 phase arrest and cyclin D1 reduction. Therefore, we conclude that Ophiobolin O induces G1 arrest of MCF-7 cells through interaction with AKT/GSK3β/cyclin D1 signaling.
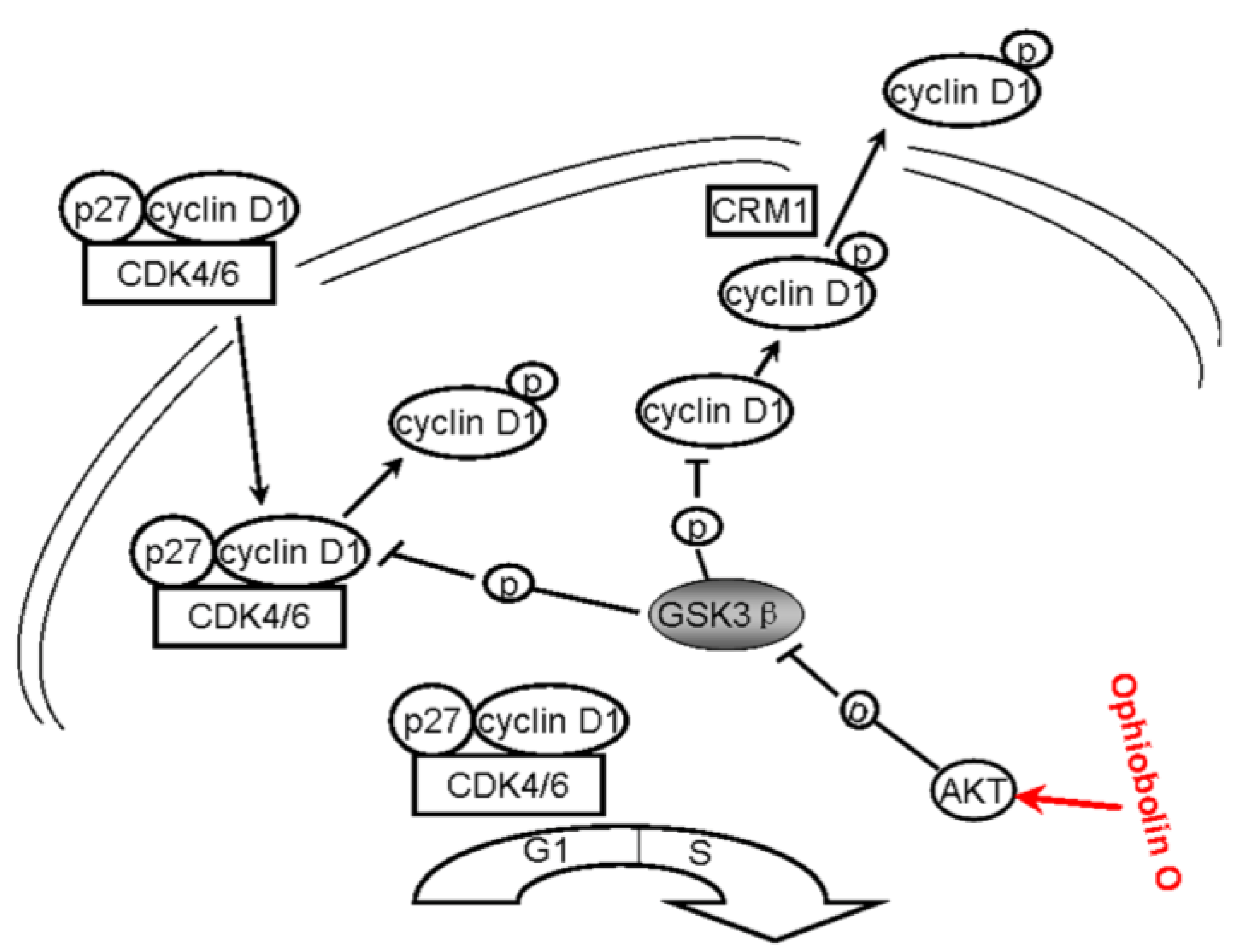
All above strongly suggest that ophiobolin O may target AKT/GSK3β/cyclin D1 signaling. The increase of active form of GSK3β accelerates cyclin D1 degradation, and inventually induces G1 phase arrest in MCF-7 cells. Cyclin D1 regulation and the probable mechanisms of ophiobolin O-induced G1 phase arrest are characterized in
Figure 6.
Figure 6.
A schematic demonstration for ophiobolin O inducing G1 arrest in MCF-7 cells through interaction with AKT/GSK3β/cyclin D1 signaling.
Figure 6.
A schematic demonstration for ophiobolin O inducing G1 arrest in MCF-7 cells through interaction with AKT/GSK3β/cyclin D1 signaling.