Synthesis and Characterization of N-Doped Porous TiO2 Hollow Spheres and Their Photocatalytic and Optical Properties
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Monodisperse MF Microspheres
2.3. Coating the MF Microspheres with Titania
2.4. Preparation of TiO2 Hollow Spheres
2.5. Photocatalytic Reaction
2.6. Characterization
3. Results and Discussion
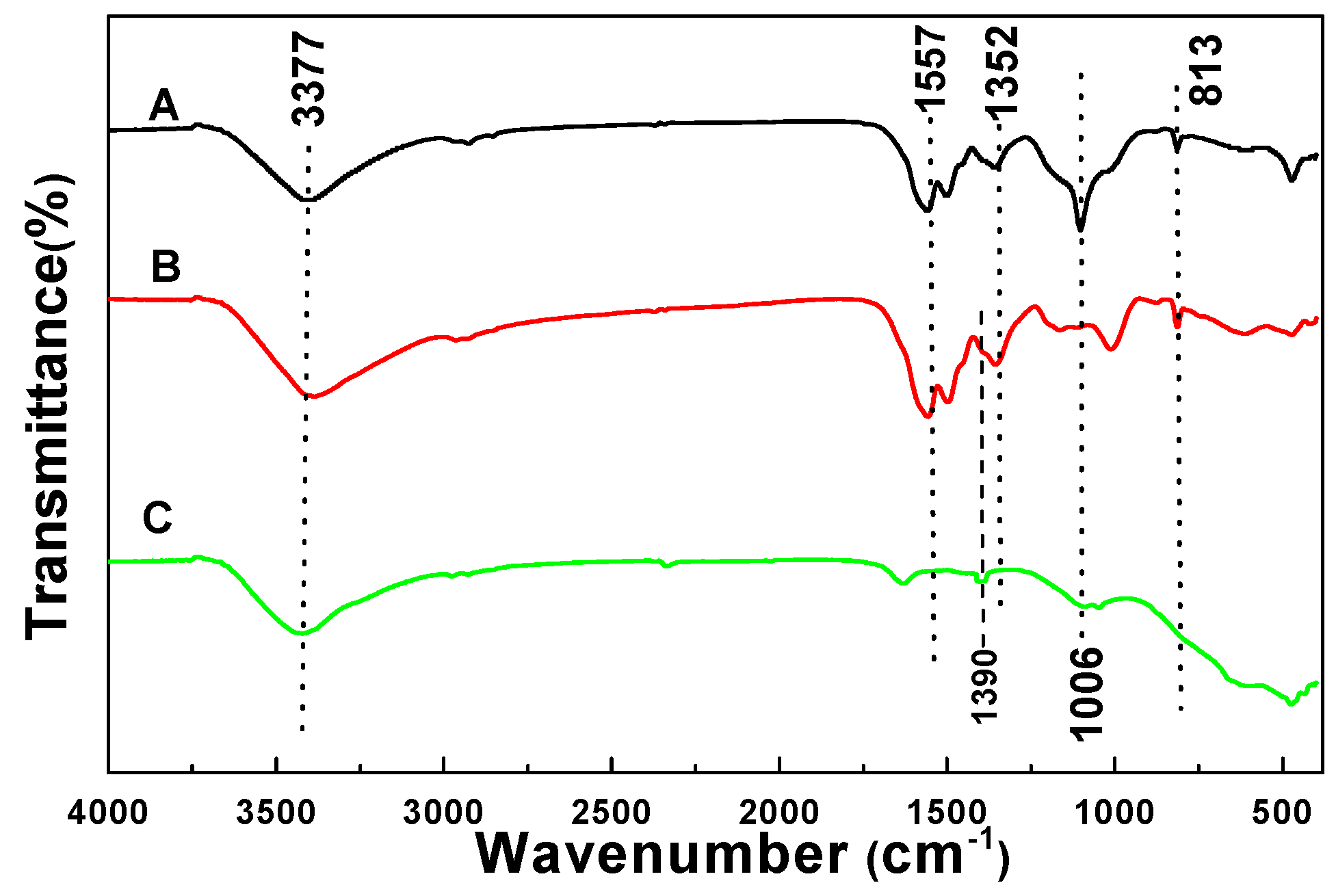
3.1. FTIR Analysis
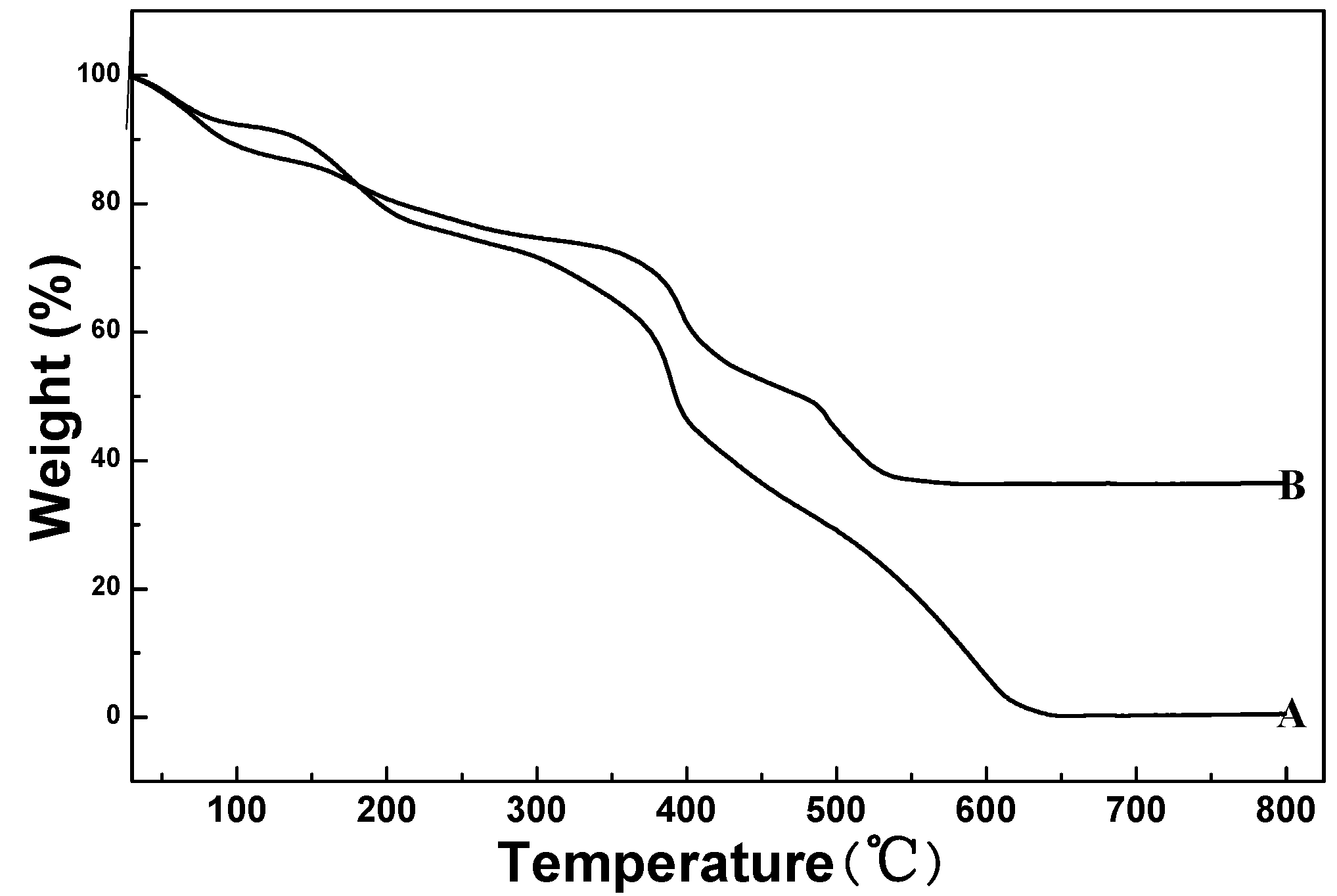
3.2. Thermogravimetric Analysis
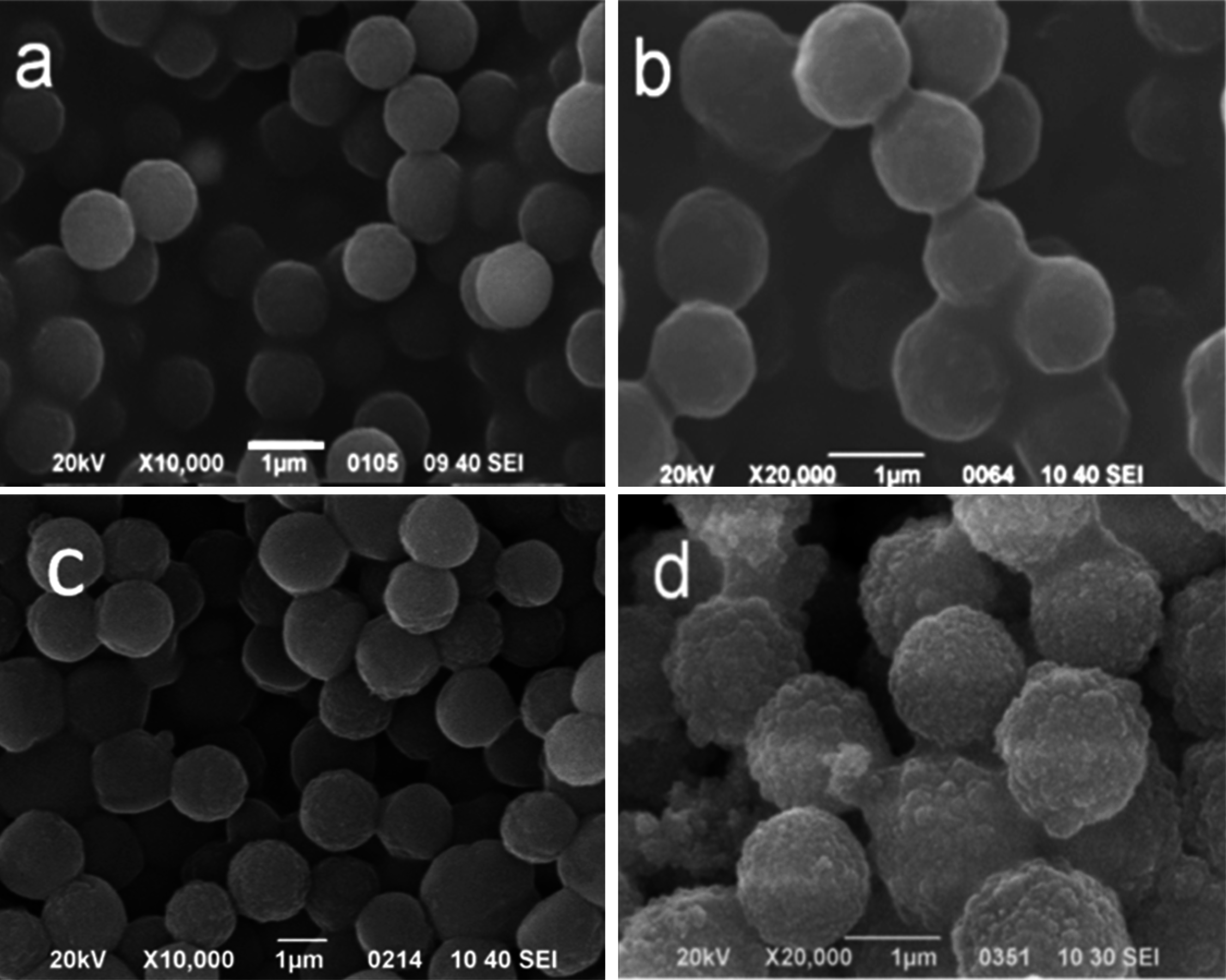
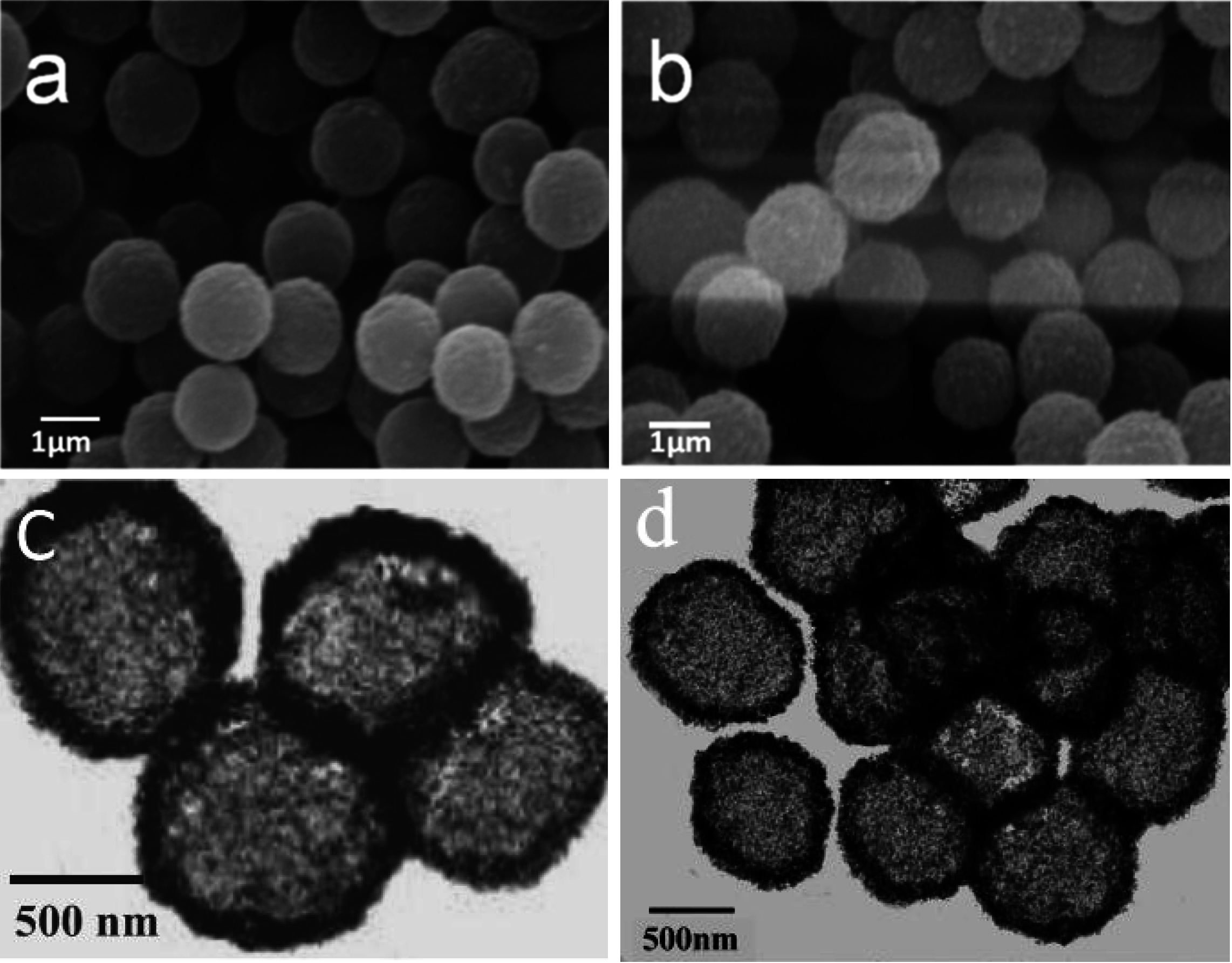
3.3. SEM and TEM Analysis
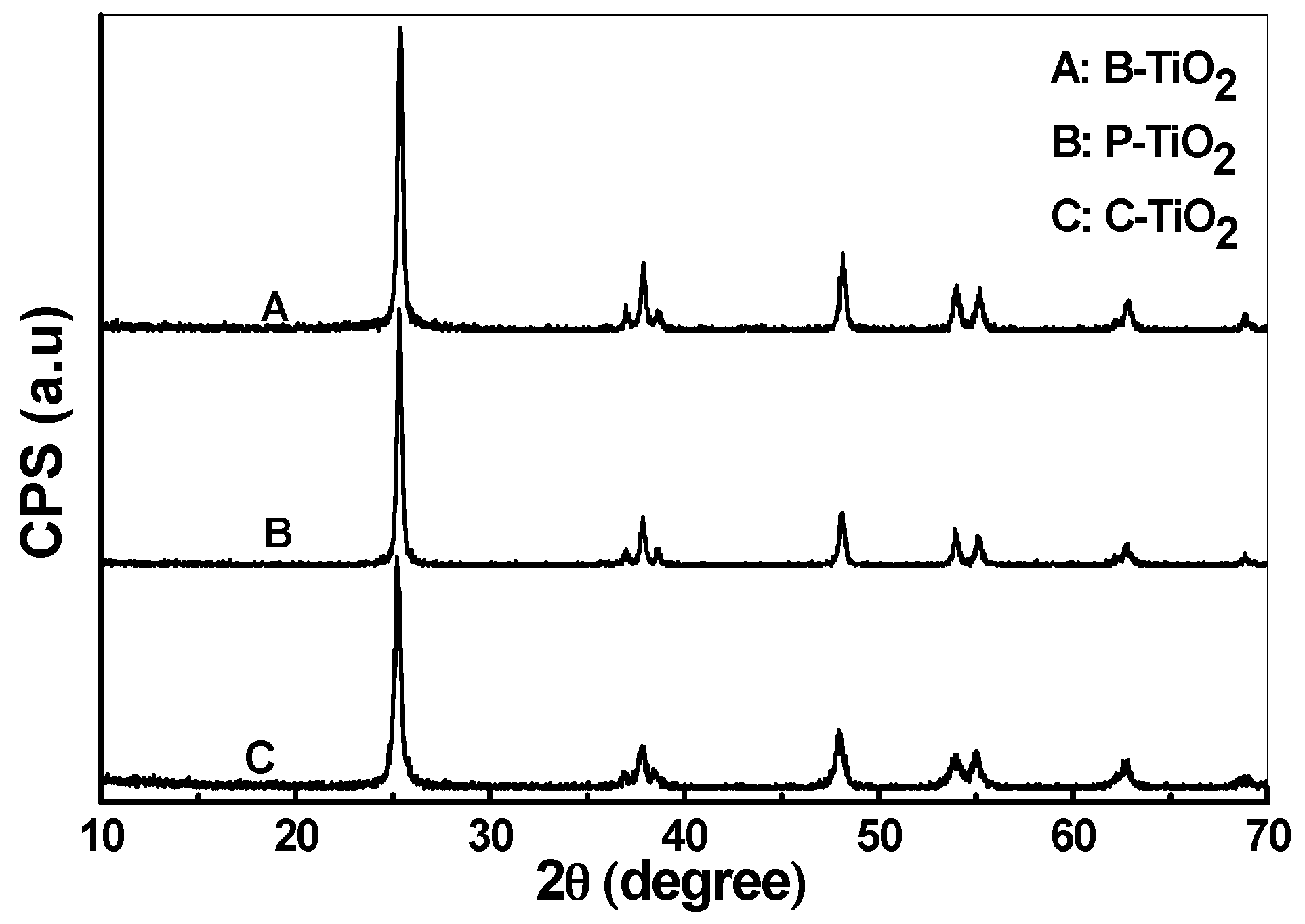
3.4. XRD Analysis
3.5. Nitrogen Adsorption–Desorption Analysis
3.6. XPS Analysis
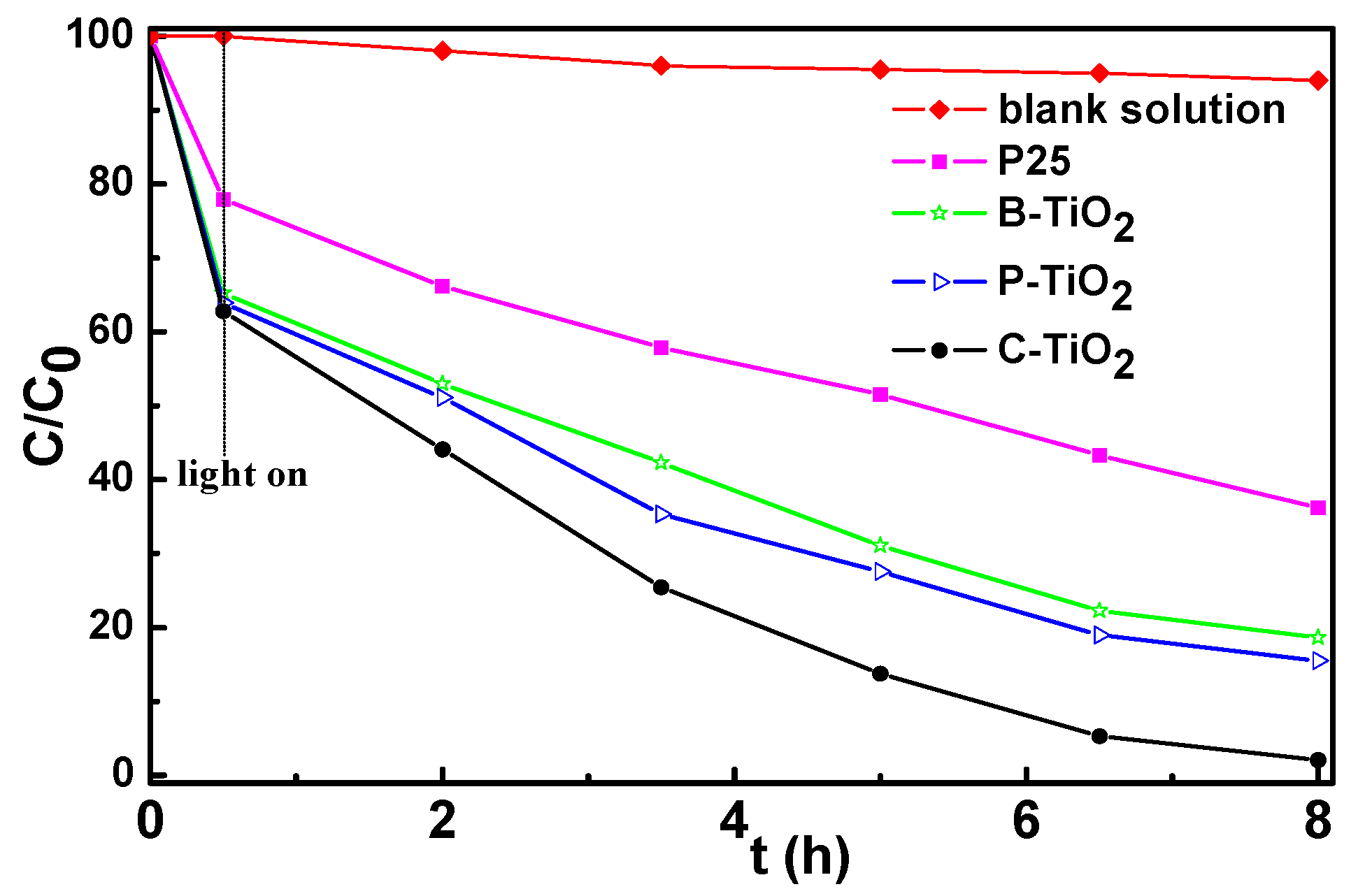
3.7. Photocatalytic Properties
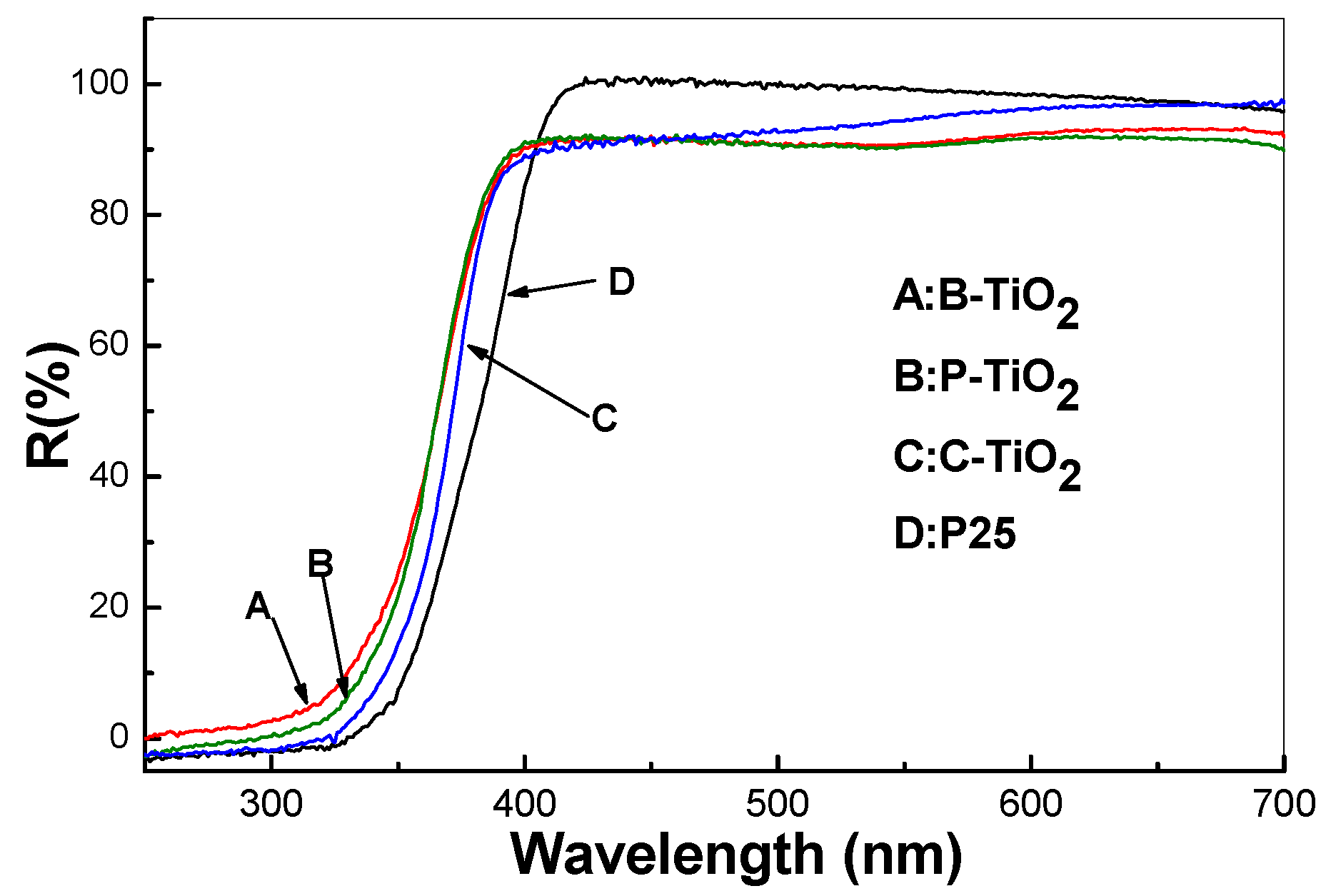
3.8. Optical Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mahoney, L.; Koodali, R.T. Versatility of Evaporation-Induced Self-Assembly (EISA) method for preparation of mesoporous TiO2 for energy and environmental Applications. Materials 2014, 7, 2697–2746. [Google Scholar] [CrossRef]
- Fagan, R.; McCormack, D.E.; Hinder, S.J.; Pillai, S.C. Photocatalytic properties of g-C3N4-TiO2 heterojunctions under UV and visible light conditions. Materials 2016, 9, 286. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, N.; Yang, L.J.; Lin, Q. Sorption behavior of nano-TiO2 for the removal of selenium ions from aqueous solution. J. Hazard. Mater. 2009, 170, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.A.; Bahnemann, D.W. Mesoporous titania photocatalysts: Preparation, characterization and reaction mechanisms. J. Mater. Chem. 2011, 21, 11686–11707. [Google Scholar] [CrossRef]
- Manthina, V.; Agrios, A.G. Band edge engineering of composite photoanodes for dye-sensitized solar cells. Electrochim. Acta 2015, 169, 416–423. [Google Scholar] [CrossRef]
- Pan, J.H.; Dou, H.Q.; Xiong, Z.G.; Xu, C.; Ma, J.Z.; Zhao, X.S. Porous photocatalysts for advanced waterpurifications. J. Mater. Chem. 2010, 20, 4512–4528. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Ge, X.; Wang, C. Synthesis of titania in ethanol/acetic acid mixture solvents: Phase and morphology variations. Cryst. Growth Des. 2009, 9, 4301–4307. [Google Scholar] [CrossRef]
- Liu, H.; Li, H.L.; Ding, Z.L.; Wang, H.Y.; Guo, P.Z.; Yu, J.Q.; Wang, C.G.; Zhao, X.S. Preparation of porous hollow SiO2 spheres by a modified stöber process using MF microspheres as templates. J. Cluster. Sci. 2012, 23, 273–285. [Google Scholar] [CrossRef]
- Deng, T.S.; Marlow, F. Synthesis of monodisperse polystyrene@vinyl-SiO2 core-shell particles and hollow SiO2 spheres. Chem. Mater. 2012, 24, 536–542. [Google Scholar] [CrossRef]
- Wu, G.L.; Wu, H.J.; Wang, K.K.; Zheng, C.H.; Wang, Y.Q.; Feng, A.L. Facile synthesis and application of multi-shelled SnO2 hollow spheres in lithium ion battery. RSC Adv. 2016, 6, 58069–58076. [Google Scholar] [CrossRef]
- Chen, J.F.; Hua, Z.J.; Yan, Y.S.; Zakhidov, A.A.; Baughman, R.H.; Xu, L.B. Template synthesis of ordered arrays of mesoporous titania spheres. Chem. Commun. 2010, 46, 1872–1874. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.D.; Tian, Q.F.; Zhou, H.; Liu, Q.; Liu, P.; Zhong, H.M. Hierarchical porous TiO2@C hollow microspheres: One-pot synthesis and enhanced visible-light photocatalysis. J. Mater. Chem. 2012, 22, 7036–7042. [Google Scholar] [CrossRef]
- Zhao, W.; Feng, L.L.; Yang, R.; Zheng, J.; Li, X.G. Synthesis, characterization, and photocatalytic properties of Ag modified hollow SiO2/TiO2 hybrid microspheres. Appl. Catal. B Environ. 2011, 103, 181–189. [Google Scholar] [CrossRef]
- Bryan, J.D.; Heald, S.M.; Chambers, S.A.; Gamelin, D.R. Strong room temperature ferromagnetism in Co2+-doped TiO2 made from colloidal nanocrystals. J. Am. Chem. Soc. 2004, 126, 11640–11647. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Wang, H.Q.; Sen, G.; Wu, Z.B.; Lee, S.C. Enhanced visible light photocatalytic activity of novel Pt/C-doped TiO2/PtCl4 three-component nanojunction system for degradation of toluene in air. J. Hazard. Mater. 2011, 187, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Subagio, D.P.; Srinivasan, M.; Lim, M.; Lim, T.T. Photocatalytic degradation of bisphenol A by nitrogen-doped TiO2 hollow sphere in a vis-LED photoreactor. Appl. Catal. B 2010, 95, 414–422. [Google Scholar] [CrossRef]
- Xiang, Q.J.; Yu, J.G.; Wang, W.G.; Jaroniec, M. Nitrogenself-doped nanosized TiO2 sheets with exposed {001} facets for enhanced visible-light photocatalytic activity. Chem. Commun. 2011, 47, 6906–6908. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.B.; Liu, X.W.; Nan, C.Y.; Liu, Y.X.; Wang, D.S.; Chen, X.Q. C/N-sensitized self-assemblyof mesostructured TiO2 nanospheres with significantly enhanced photocatalytic activity. New J. Chem. 2013, 37, 2582–2588. [Google Scholar] [CrossRef]
- Serpone, N. Is the band gap of pristine TiO2 narrowed by anion- and cation-doping of titanium dioxide in second-generation photocatalysts? J. Phys. Chem. B 2006, 110, 24287–24293. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Gole, J.L.; Stout, J.D.; Burda, C.; Lou, Y.B.; Chen, X.B. Highly efficient formation of visible light tunable TiO2−xNx photocatalysts and their transformation at the nanoscale. J. Phys. Chem. B 2004, 108, 1230–1240. [Google Scholar] [CrossRef]
- Sakthivel, S.; Janczarek, M.; Kisch, H. Visible light activity and photoelectrochemical properties of nitrogen-doped TiO2. J. Phys. Chem. B 2004, 108, 19384–19387. [Google Scholar] [CrossRef]
- Xiong, Z.G.; Zhao, X.S. Nitrogen-doped titanate-anatase core-shell nanobelts with exposed {101} anatase facets and enhanced visible light photocatalytic activity. J. Am. Chem. Soc. 2012, 134, 5754–5757. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Moya, S.; Lichtenfeld, H.; Casoli, A. The decomposition process of melamine formaldehyde cores: The key step in the fabrication of ultrathin polyelectrolyte multilayer capsules. Macromol. Mater. Eng. 2001, 286, 355–358. [Google Scholar] [CrossRef]
- Jagadale, T.C.; Takale, S.P.; Sonawane, R.S.; Joshi, H.M.; Patil, S.I.; Kale, B.B.; Ogale, S.B. N-doped TiO2 nanoparticle based visible light photocatalyst by modified peroxide sol-gel method. J. Phys. Chem. C 2008, 112, 14595–14602. [Google Scholar] [CrossRef]
- Wang, P.; Chen, D.; Tang, F.Q. Preparation of titania-coated polystyrene particles in mixed solvents by ammonia catalysis. Langmuir 2006, 22, 4832–4835. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.P.; Kalita, S.J. Synthesis, processing and characterization of nanocrystalline titanium dioxide. Mater. Sci. Eng. A 2006, 435–436, 327–332. [Google Scholar] [CrossRef]
- Anuradha, T.V.; Ranganathan, S. Nanocrystalline TiO2 by three different synthetic approaches: A comparison. Bull. Mater. Sci. 2007, 30, 263–269. [Google Scholar] [CrossRef]
- Gao, L.D.; Le, Y.; Wang, J.X.; Chen, J.F. Preparation and characterization of titania nanotubes with mesostructured walls. Mater. Lett. 2006, 60, 3882–3886. [Google Scholar] [CrossRef]
- Sathish, M.; Viswanathan, B.; Viswanath, R.; Gopinath, C.S. Synthesis, characterization, electronic structure, and photocatalytic activity of nitrogen-doped TiO2 nanocatalyst. Chem. Mater. 2005, 17, 6349–6353. [Google Scholar] [CrossRef]
- Wang, J.; Tafen, D.N.; Lewis, J.P.; Hong, Z.L.; Manivannan, A.; Zhi, M.J.; Li, M.; Wu, N.Q. Origin of photocatalytic activity of nitrogen-doped TiO2 nanobelts. J. Am. Chem. Soc. 2009, 131, 12290–12297. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, C.X.; Zhang, M.; Yang, J.Y.; Zhang, Z.J. Enhanced visible light photocatalytic activity of N-doped TiO2 in relation to single-electron-trapped oxygen vacancy and doped-nitrogen. Appl. Catal. B Environ. 2010, 100, 84–90. [Google Scholar] [CrossRef]
- Morikawa, T.; Asahi, R.; Ohwaki, T.; Aoki, A.; Taga, Y. Band-gap narrowing of titanium dioxide by nitrogen doping. Jpn. J. Appl. Phys. 2001, 40, L561–L563. [Google Scholar] [CrossRef]
- Wu, N.; Wang, J.; Tafen, D.; Wang, H.; Zheng, J.G.; Lewis, J.P.; Liu, X.; Leonard, S.S.; Manivannan, A. Shape-enhanced photocatalytic activity of single-crystalline anatase TiO2 {101} nanobelts. J. Am. Chem. Soc. 2010, 19, 6679–6685. [Google Scholar] [CrossRef] [PubMed]
- Serpone, N.; Lawless, D.; Khairutdinov, R. Size effects on thephotophysical properties of colloidal anatase TiO2 particles: Size quantization versus direct transitions in this indirect semiconductor? J. Phys. Chem. 1995, 99, 16646–16654. [Google Scholar] [CrossRef]
- Li, H.L.; Zhu, Y.C.; Chen, S.G.; Palchik, O.; Xiong, J.P.; Koltypin, Y.; Gedanken, A. A novel ultrasound-assisted approach to the synthesis of CdSe and CdS nanoparticles. J. Solid State Chem. 2003, 172, 102–110. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.X.; Ji, W.; Mao, Z.; Mao, H.J.; Wang, Y.; Wang, X.; Ruan, W.D.; Zhao, B.; Lombardi, J.R. Raman investigation of nanosized TiO2: Effect of crystallite size and quantum confinement. J. Phys. Chem. C 2012, 116, 8792–8797. [Google Scholar] [CrossRef]
- Kormann, C.; Bahnemann, D.; Hoffmann, M.R. Preparation and characterization of quantum-size titanium dioxide. J. Phys. Chem. 1988, 92, 5196–5201. [Google Scholar] [CrossRef]
- Brus, L. Electronic wave functions in semiconductor clusters: Experiment and theory. J. Phys. Chem. 1986, 90, 2555–2560. [Google Scholar] [CrossRef]
- Burda, C.; Lou, Y.B.; Chen, X.B.; Samia, A.C.S.; Stout, J.; Gole, J.L. Enhanced nitrogen doping in TiO2 nanoparticles. Nano Lett. 2003, 3, 1049–1051. [Google Scholar] [CrossRef]
- Konjhodzic, D.; Bretinger, H.; Marlow, F. Structure and properties of low-n mesoporous silica films for optical applications. Thin Solid Films 2006, 495, 333–337. [Google Scholar] [CrossRef]




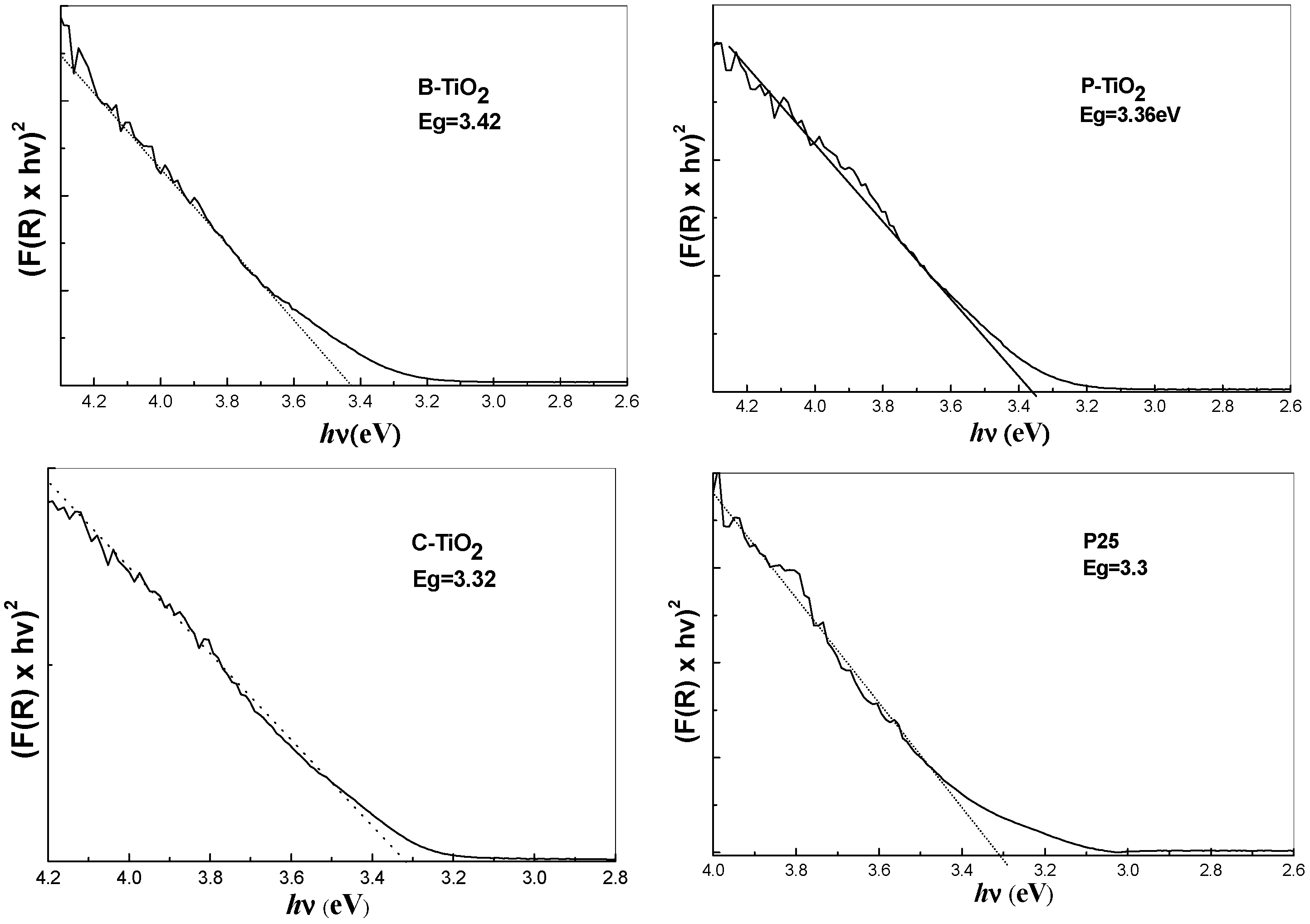
| Sample Name | Specific Surface Area | Pore Volume | Average Pore Width |
|---|---|---|---|
| P25 | 50 | - a | - b |
| B-TiO2 | 98 | 0.13 | 8.7 |
| P-TiO2 | 128 | 0.30 | 10.6 |
| C-TiO2 | 131 | 0.41 | 12.9 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Liu, H.; Fu, A.; Wu, G.; Xu, M.; Pang, G.; Guo, P.; Liu, J.; Zhao, X.S. Synthesis and Characterization of N-Doped Porous TiO2 Hollow Spheres and Their Photocatalytic and Optical Properties. Materials 2016, 9, 849. https://doi.org/10.3390/ma9100849
Li H, Liu H, Fu A, Wu G, Xu M, Pang G, Guo P, Liu J, Zhao XS. Synthesis and Characterization of N-Doped Porous TiO2 Hollow Spheres and Their Photocatalytic and Optical Properties. Materials. 2016; 9(10):849. https://doi.org/10.3390/ma9100849
Chicago/Turabian StyleLi, Hongliang, Hui Liu, Aiping Fu, Guanglei Wu, Man Xu, Guangsheng Pang, Peizhi Guo, Jingquan Liu, and Xiu Song Zhao. 2016. "Synthesis and Characterization of N-Doped Porous TiO2 Hollow Spheres and Their Photocatalytic and Optical Properties" Materials 9, no. 10: 849. https://doi.org/10.3390/ma9100849





