Phase Morphology and Mechanical Properties of Cyclic Butylene Terephthalate Oligomer-Containing Rubbers: Effect of Mixing Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Processing
2.2. Testing Methods
3. Results
3.1. Melting of CBT
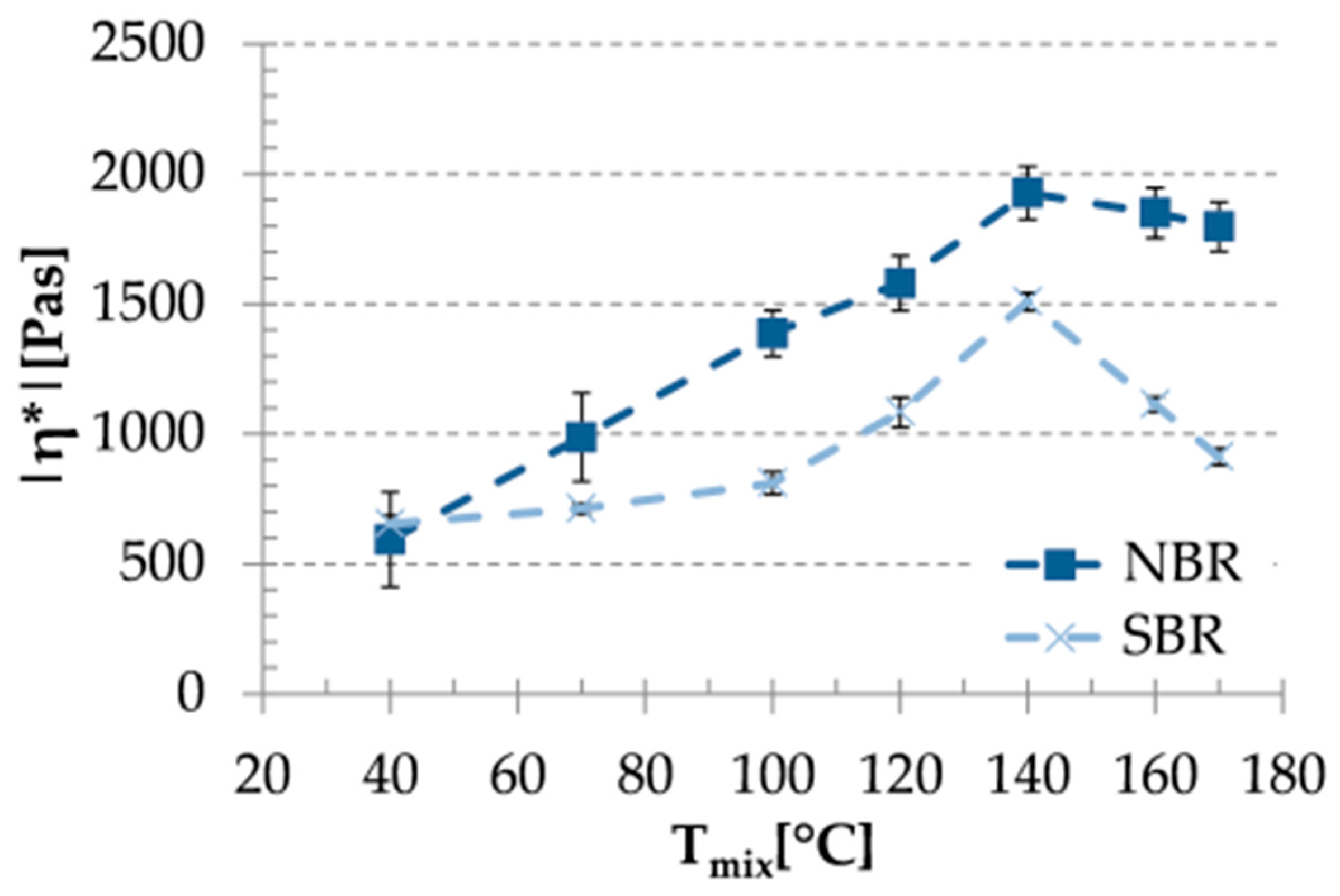
3.2. Rheological Properties
3.3. Curing Properties
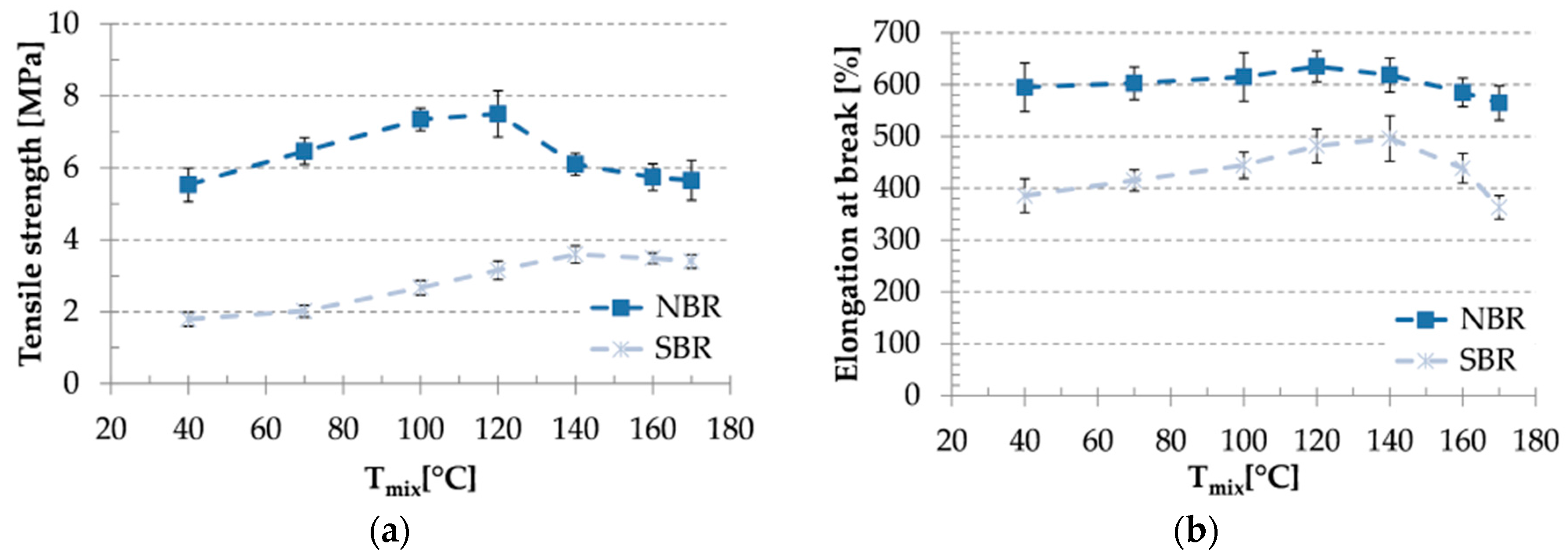
3.4. Mechanical Properties
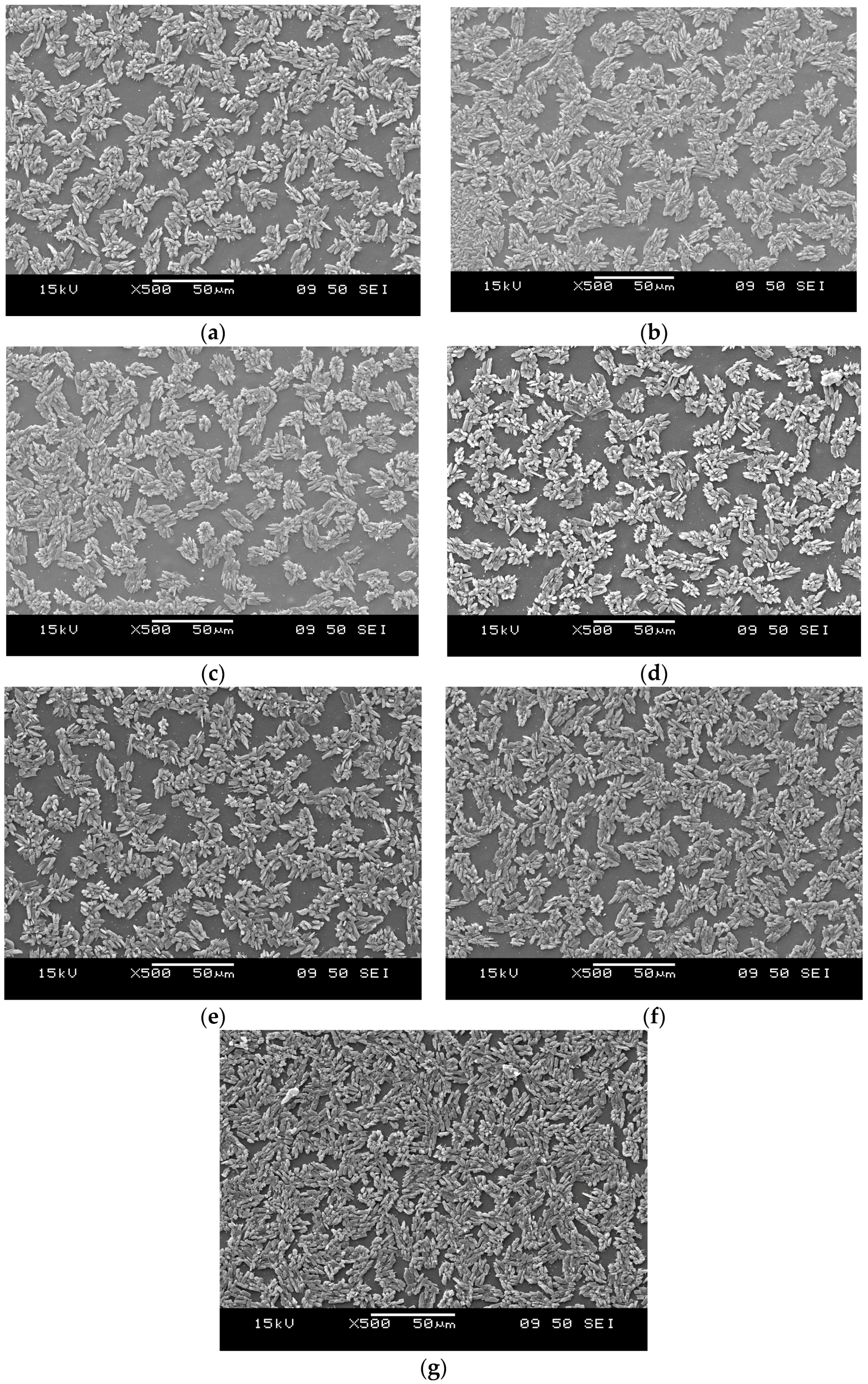
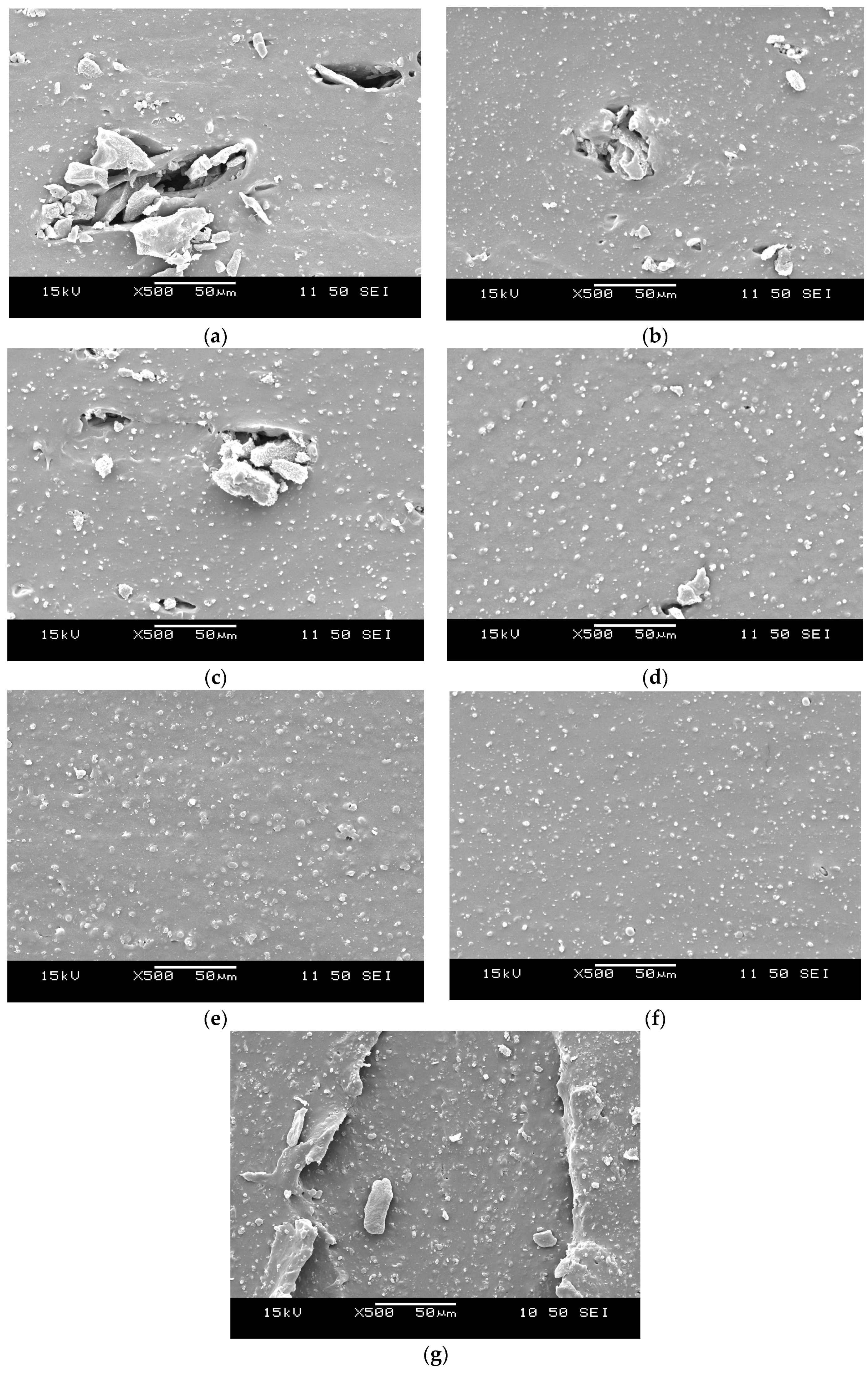
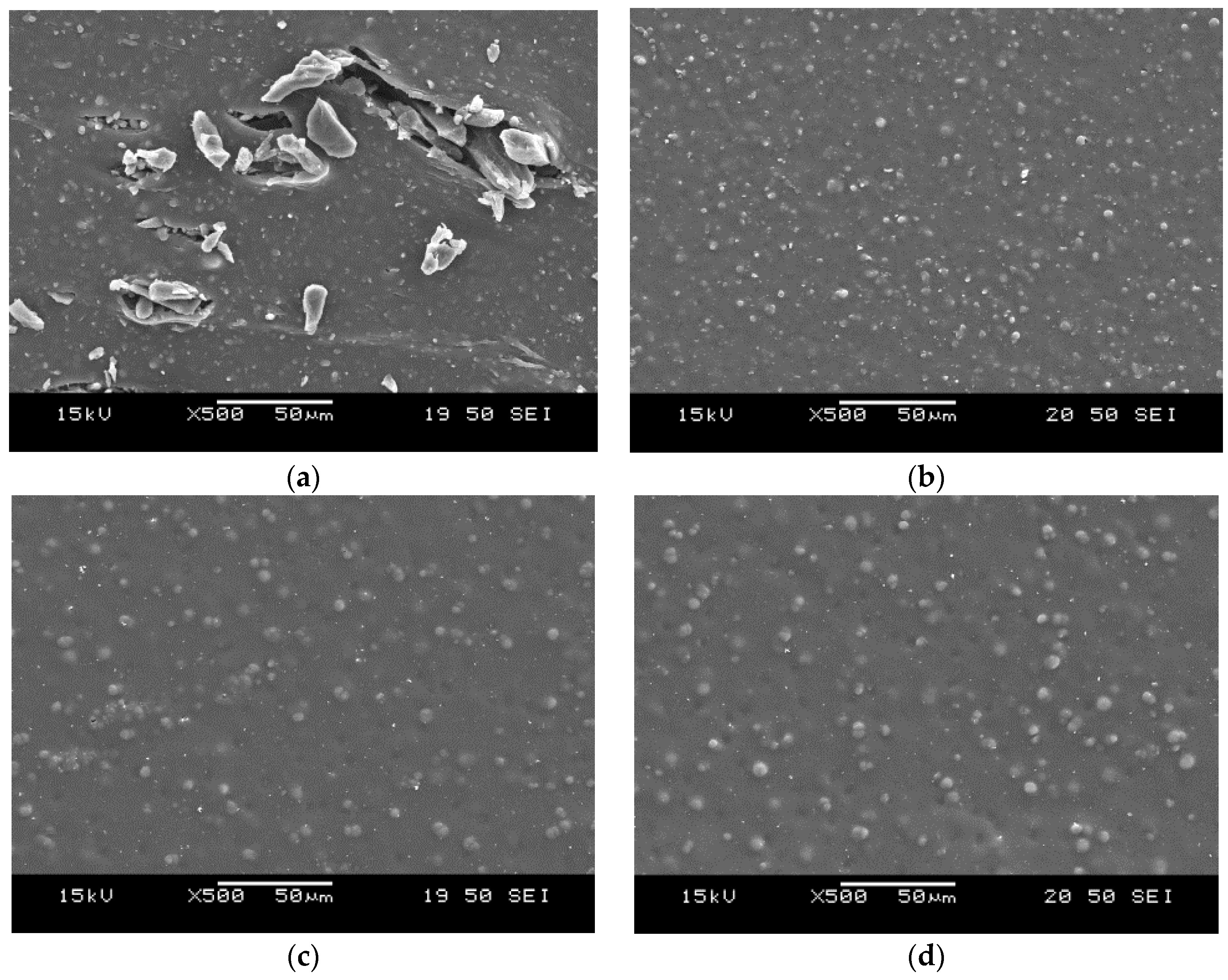
3.5. Morphology
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brunelle, D.J. Cyclic oligomer chemistry. J. Polym. Sci. Polym. Chem. 2008, 46, 1151–1164. [Google Scholar] [CrossRef]
- Abt, T.; Sánchez-Soto, M. A Review of the recent advances in cyclic butylene terephthalate technology and its composites. CRC Crit. Rev. Sol. State Mater. Sci. 2016, in press. [Google Scholar] [CrossRef] [Green Version]
- Tripathy, A.R.; Elmoumni, A.; Winter, H.H.; MacKngith, W.J. Effects of catalyst and polymerization temperature on the in-situ polymerization of cyclic poly(butylene terephthalate) oligomers for composite applications. Macromolecules 2005, 38, 709–715. [Google Scholar] [CrossRef]
- Mohd Ishak, Z.A.; Karger-Kocsis, J. On the in-situ polymerization of cyclic butylene terephthalate oligomers: DSC and rheological studies. Polym. Eng. Sci. 2006, 46, 743–750. [Google Scholar] [CrossRef]
- Tripathy, A.R.; Bourgaz, E.; Kukureka, S.N.; MacKinght, W.J. Poly(butylene terephthalate) nanocomposites prepared by in-situ polymerization. Macromolecules 2003, 36, 8593–8595. [Google Scholar] [CrossRef]
- Jiang, Z.; Siengchin, S.; Zhou, L.-M.; Steeg, M.; Karger-Kocsis, J.; Man, H.C. Poly(butylene terephthalate)/silica nanocomposites prepared from cyclic butylene terephthalate. Compos. A Appl. Sci. Manuf. 2009, 40, 273–278. [Google Scholar] [CrossRef]
- Abt, T.; Bou, J.J.; Sánchez-Soto, M. Isocyanate toughening of pCBT/organoclay nanocomposites with exfoliated structure and enhanced mechanical properties. Express Polym. Lett. 2014, 8, 953–966. [Google Scholar] [CrossRef]
- Parton, H.; Baets, J.; Lipnik, P.; Goderis, B.; Devaux, J.; Verpoest, I. Properties of poly(butylene-terephthalate) polymerized from cyclic oligomers and its composites. Polymer 2005, 46, 9871–9880. [Google Scholar] [CrossRef]
- Mohd Ishak, Z.A.; Leong, Y.W.; Steeg, M.; Karger-Kocsis, J. Mechanical properties of woven glass fabric reinforced in situ polymerized poly(butylene-terephthalate) composites. Compos. Sci. Technol. 2007, 67, 390–398. [Google Scholar] [CrossRef]
- Rösch, M. Verarbeitungshilfsmittel: Alles im Fluss (Processing aid: It’s all a matter of flow). Kunststoffe 2006, 96, 90–91. [Google Scholar]
- Karger-Kocsis, J.; Felhős, D.; Bárány, T.; Czigány, T. Hybrids of HNBR and in situ polymerizable cyclic butylene terephthalate (CBT) oligomers: Properties and dry sliding behavior. Express Polym. Lett. 2008, 2, 520–527. [Google Scholar] [CrossRef]
- Xu, D.; Karger-Kocsis, J.; Apostolov, A.A. Hybrids from HNBR and in situ polymerizable cyclic butylene terephthalate (CBT): Structure and rolling wear properties. Eur. Polym. J. 2009, 45, 1270–1281. [Google Scholar] [CrossRef]
- Xu, D.; Karger-Kocsis, J. Rolling and sliding wear properties of hybrid systems of uncured/cured HNBR and partly polymerized cyclic butylene terephthalate (CBT). Tribol. Int. 2010, 43, 289–298. [Google Scholar] [CrossRef]
- Halász, I.Z.; Bárány, T. Novel bifunctional additive for rubbers: Cyclic butylene terephthalate oligomer. Period. Polytech. Mech. Eng. 2015, 59, 182–188. [Google Scholar] [CrossRef]


| Abbreviation | Producer, Type | Main Properties |
|---|---|---|
| NBR | Lanxess, Perbunan® 3945F | Mooney viscosity (ML, 1 + 4, 100 °C): 45 ± 5 |
| SBR | Goodyear Chemical, Plioflex® 1502 | Mooney viscosity (ML, 1 + 4, 100 °C): 44 Bound styrene content: 23.5 m% |
| Rubber | Mixing Temperature [°C] | G’min [kPa] | G’max [kPa] | t0.9 [min] |
|---|---|---|---|---|
| NBR | 40 | 13.1 | 694.9 | 14.5 |
| 70 | 17.7 | 753.8 | 13.4 | |
| 100 | 23.4 | 759.4 | 13.6 | |
| 120 | 20.2 | 770.7 | 13.5 | |
| 140 | 26.8 | 897.2 | 13.5 | |
| 160 | 19.5 | 827.4 | 13.6 | |
| 170 | 20.9 | 762.9 | 14.3 | |
| SBR | 40 | 40.1 | 399.0 | 13.3 |
| 70 | 44.9 | 335.8 | 14.6 | |
| 100 | 44.2 | 409.2 | 14.7 | |
| 120 | 40.7 | 400.0 | 14.4 | |
| 140 | 28.9 | 308.3 | 14.6 | |
| 160 | 5.4 | 175.5 | 12.4 | |
| 170 | 5.3 | 147.9 | 22.4 |
| Rubber | Mixing Temperature [°C] | E’pl [MPa] | νc [mol/m3] | tanδmax [-] | Tg [°C] |
|---|---|---|---|---|---|
| NBR | 40 | 8.31 | 1137.0 | 1.40 | 1.2 |
| 70 | 8.22 | 1124.7 | 1.41 | 1.1 | |
| 100 | 8.77 | 1200.0 | 1.42 | 1.4 | |
| 120 | 8.20 | 1122.0 | 1.42 | 1.7 | |
| 140 | 8.21 | 1123.4 | 1.44 | 1.4 | |
| 160 | 8.64 | 1182.2 | 1.41 | 2.3 | |
| 170 | 9.35 | 1279.3 | 1.40 | 3.4 | |
| SBR | 40 | 5.95 | 814.1 | 1.17 | −27.9 |
| 70 | 6.09 | 833.3 | 1.21 | −28.3 | |
| 100 | 5.79 | 792.2 | 1.20 | −28.4 | |
| 120 | 6.39 | 874.3 | 1.19 | −27.8 | |
| 140 | 6.92 | 946.9 | 1.14 | −27.0 | |
| 160 | 7.21 | 986.5 | 1.08 | −24.9 | |
| 170 | 8.41 | 1150.7 | 0.87 | −23.3 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halász, I.Z.; Bárány, T. Phase Morphology and Mechanical Properties of Cyclic Butylene Terephthalate Oligomer-Containing Rubbers: Effect of Mixing Temperature. Materials 2016, 9, 722. https://doi.org/10.3390/ma9090722
Halász IZ, Bárány T. Phase Morphology and Mechanical Properties of Cyclic Butylene Terephthalate Oligomer-Containing Rubbers: Effect of Mixing Temperature. Materials. 2016; 9(9):722. https://doi.org/10.3390/ma9090722
Chicago/Turabian StyleHalász, István Zoltán, and Tamás Bárány. 2016. "Phase Morphology and Mechanical Properties of Cyclic Butylene Terephthalate Oligomer-Containing Rubbers: Effect of Mixing Temperature" Materials 9, no. 9: 722. https://doi.org/10.3390/ma9090722






