Study of Reactive Melt Processing Behavior of Externally Plasticized Cellulose Acetate in Presence of Isocyanate
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
| Plasticizer | Mn (g mol−1) | Ρ (g cm−3) | nD (–) | bp (1013 mbar) (°C) |
|---|---|---|---|---|
| GTA | 218.21 | 1.16 | 1.43 | 258 |
| TEC | 276.28 | 1.14 | 1.44 | 294 |
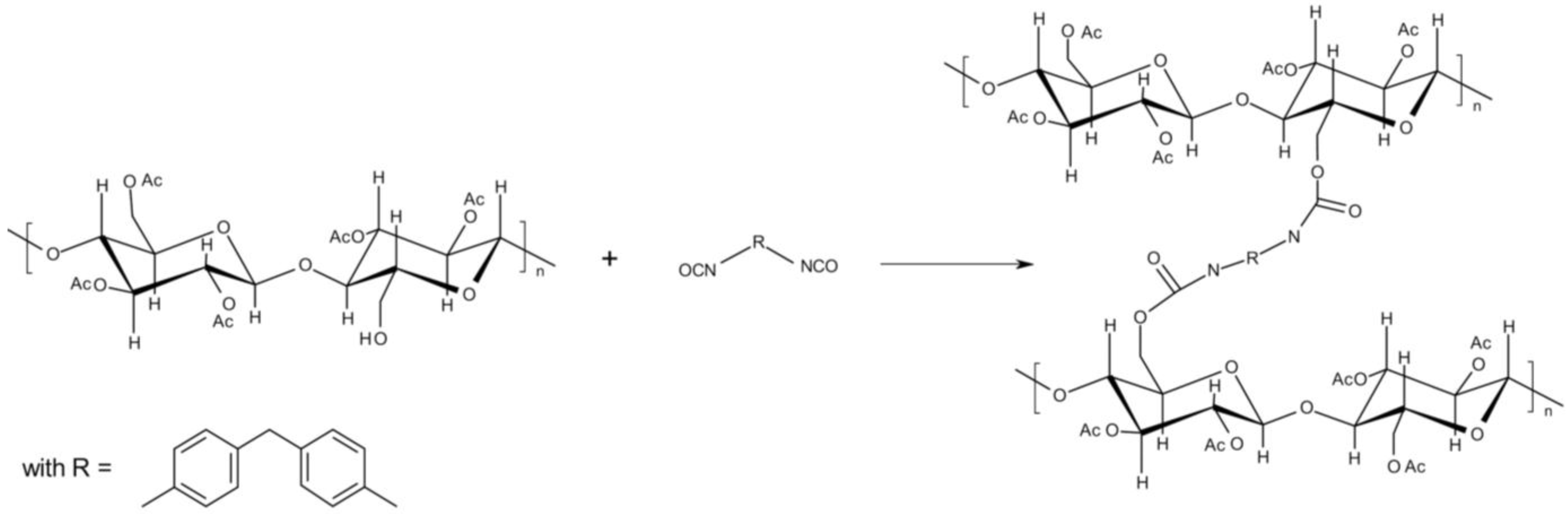
2.2. Reactive Melt Processing Conditions and Online Measurements
2.3. Viscosimetry and Further Investigations of the Products
3. Results and Discussion
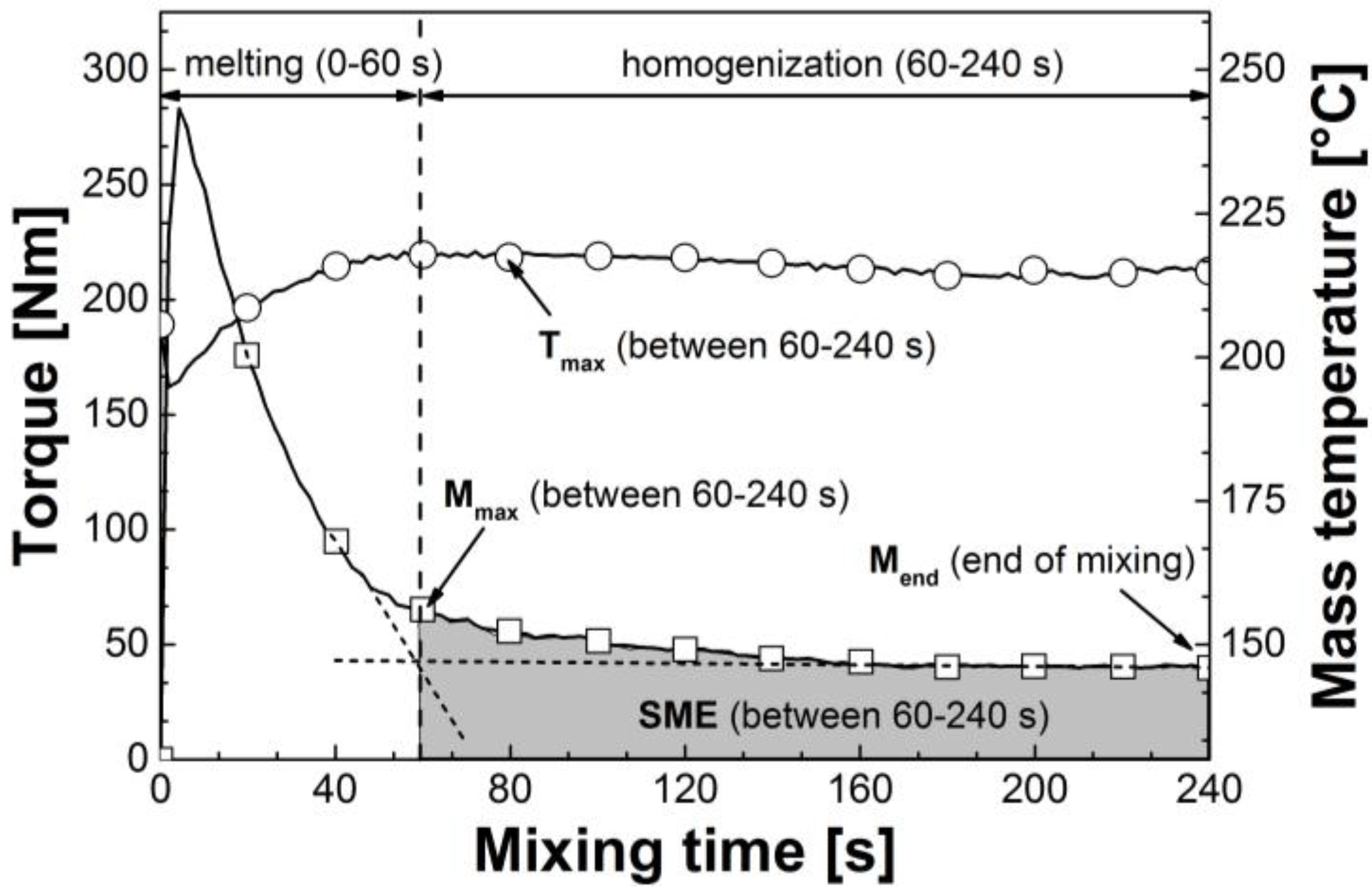
3.1. Reactive Melt Processing
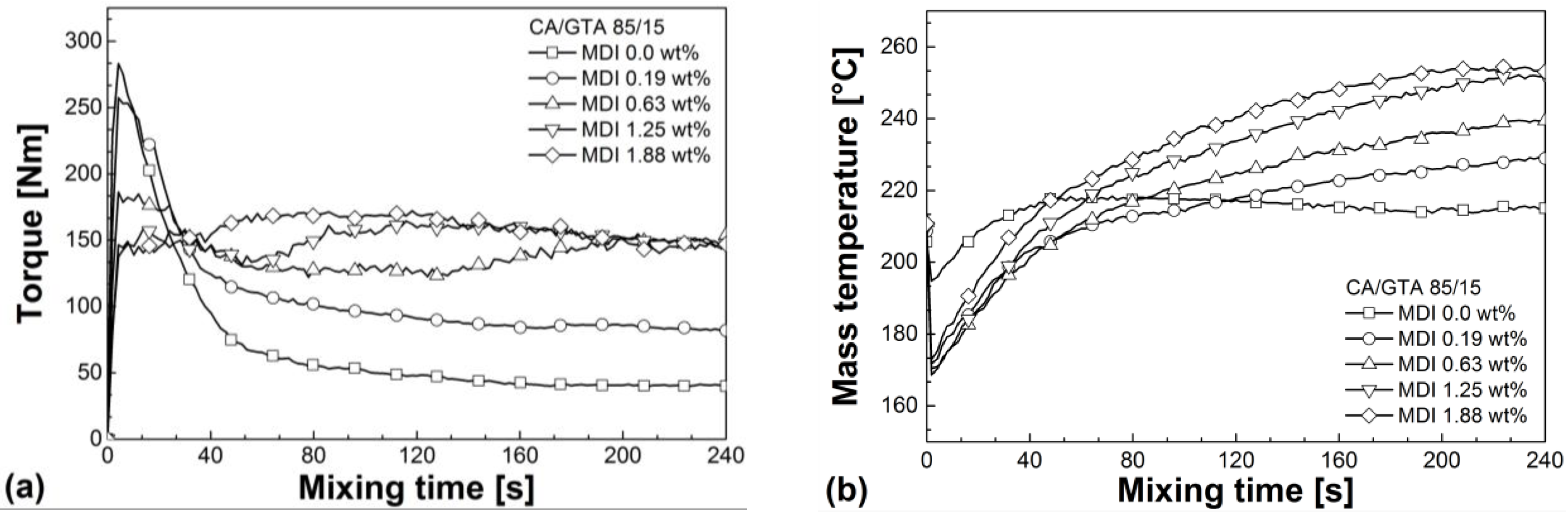
| Mixtures | Mmax (60–240 s) (Nm) | avg. M (60–240 s) (Nm) | Mend (Nm) | Tmax (60–240 s) (°C) | SME (60–240 s) (J g−1) |
|---|---|---|---|---|---|
| CA/GTA 85/15 | |||||
| + MDI 0 | 65.3 | 46.1 | 39.9 | 218.4 | 173.6 |
| + MDI 0.19 | 109.3 | 90.0 | 81.9 | 229.3 | 339.0 |
| + MDI 0.63 | 154.6 | 139.2 | 148.6 | 239.5 | 514.6 |
| + MDI 1.25 | 164.4 | 154.0 | 147.5 | 252.3 | 580.8 |
| + MDI 1.88 | 172.1 | 159.3 | 147.1 | 254.4 | 600.6 |
| CA/TEC 85/15 | |||||
| + MDI 0 | 105.6 | 87.2 | 70.5 | 224.7 | 308.6 |
| + MDI 0.19 | 111.8 | 89.9 | 83.2 | 228.9 | 338.3 |
| + MDI 0.63 | 116.0 | 104.5 | 107.0 | 233.6 | 393.8 |
| + MDI 1.25 | 151.6 | 131.5 | 142.1 | 243.6 | 477.3 |
| + MDI 1.88 | 175.6 | 158.6 | 159.1 | 248.0 | 597.7 |
3.2. Solubility Tests
| Mixtures | Acetone | DMF | DMSO | THF | Xy in acetone [%] |
|---|---|---|---|---|---|
| CA/GTA 85/15 | |||||
| + MDI 0 | + | + | + | + | 0 |
| + MDI 0.19 | + | + | + | + | 0 |
| + MDI 0.63 | − | − | − | − | 41 (1.81) |
| + MDI 1.25 | − | − | − | − | 52 (1.75) |
| + MDI 1.88 | − | − | − | − | 56 (2.18) |
| CA/TEC 85/15 | |||||
| + MDI 0 | + | + | + | + | 0 |
| + MDI 0.19 | + | + | + | + | 0 |
| + MDI 0.63 | − | − | − | − | 38 (0.31) |
| + MDI 1.25 | − | − | − | − | 45 (1.76) |
| + MDI 1.88 | − | − | − | − | 49 (1.47) |
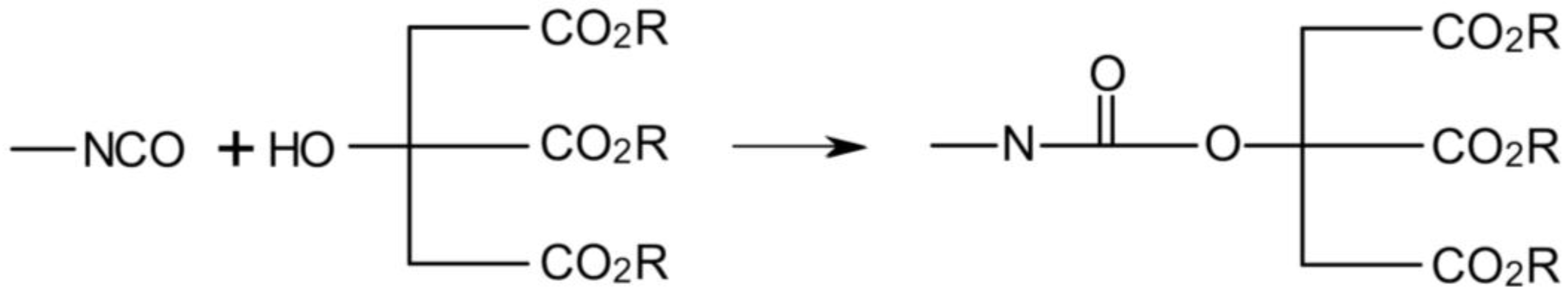
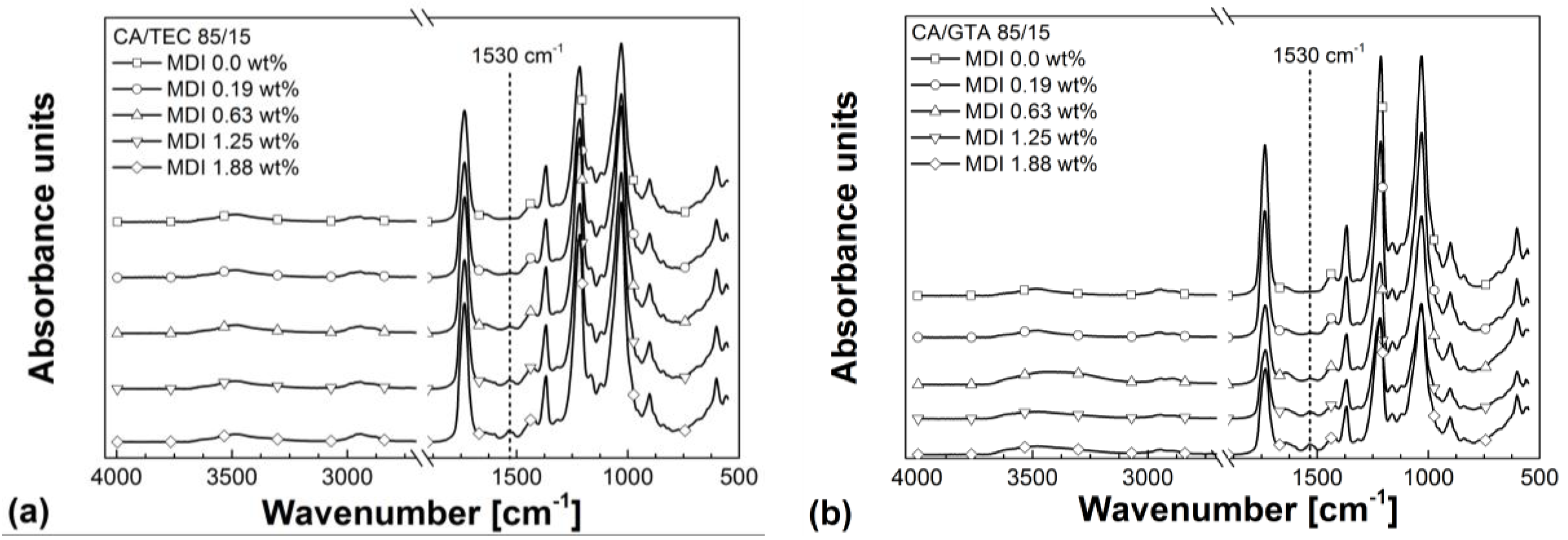
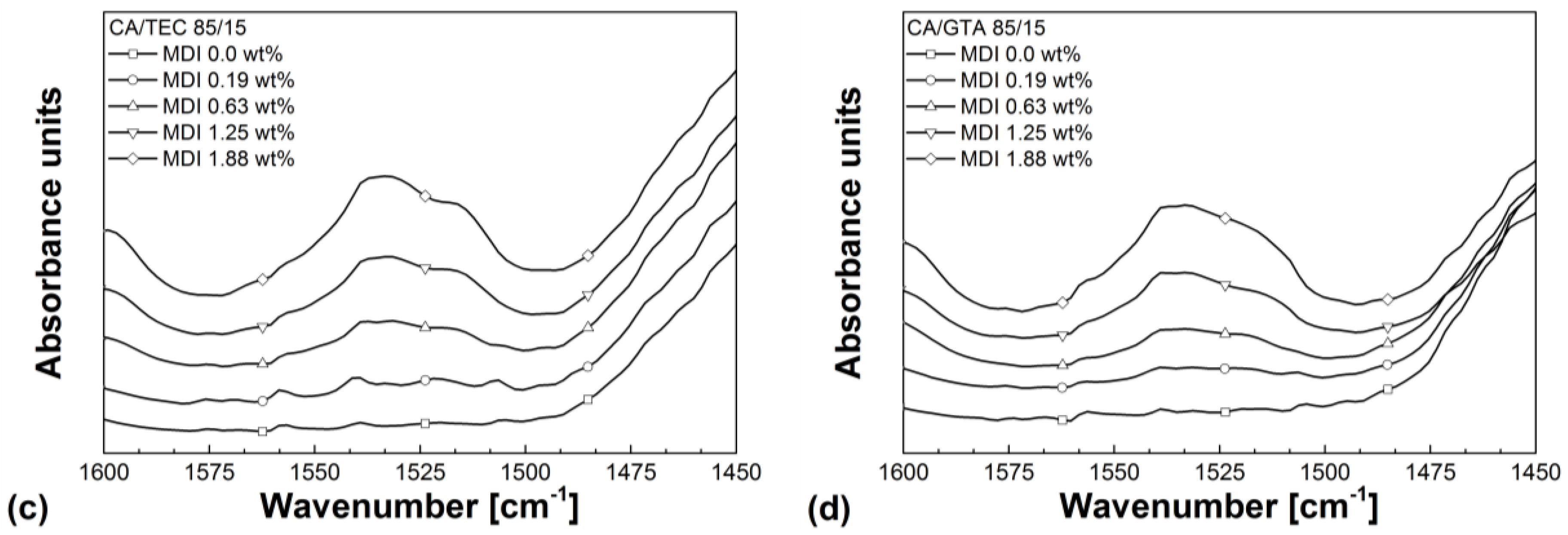
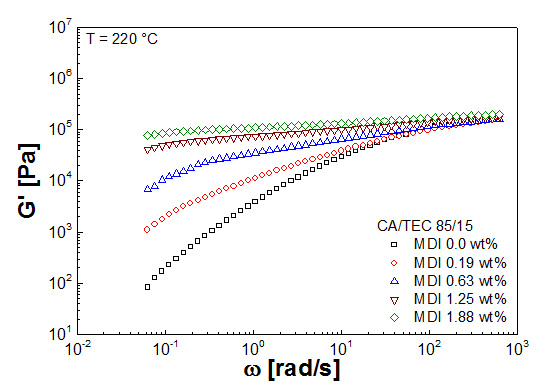
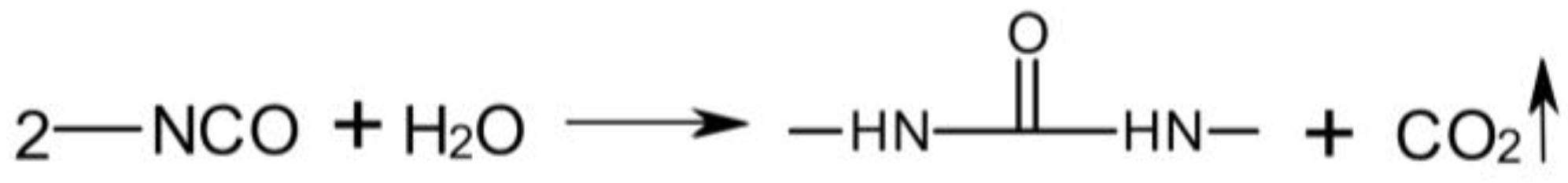
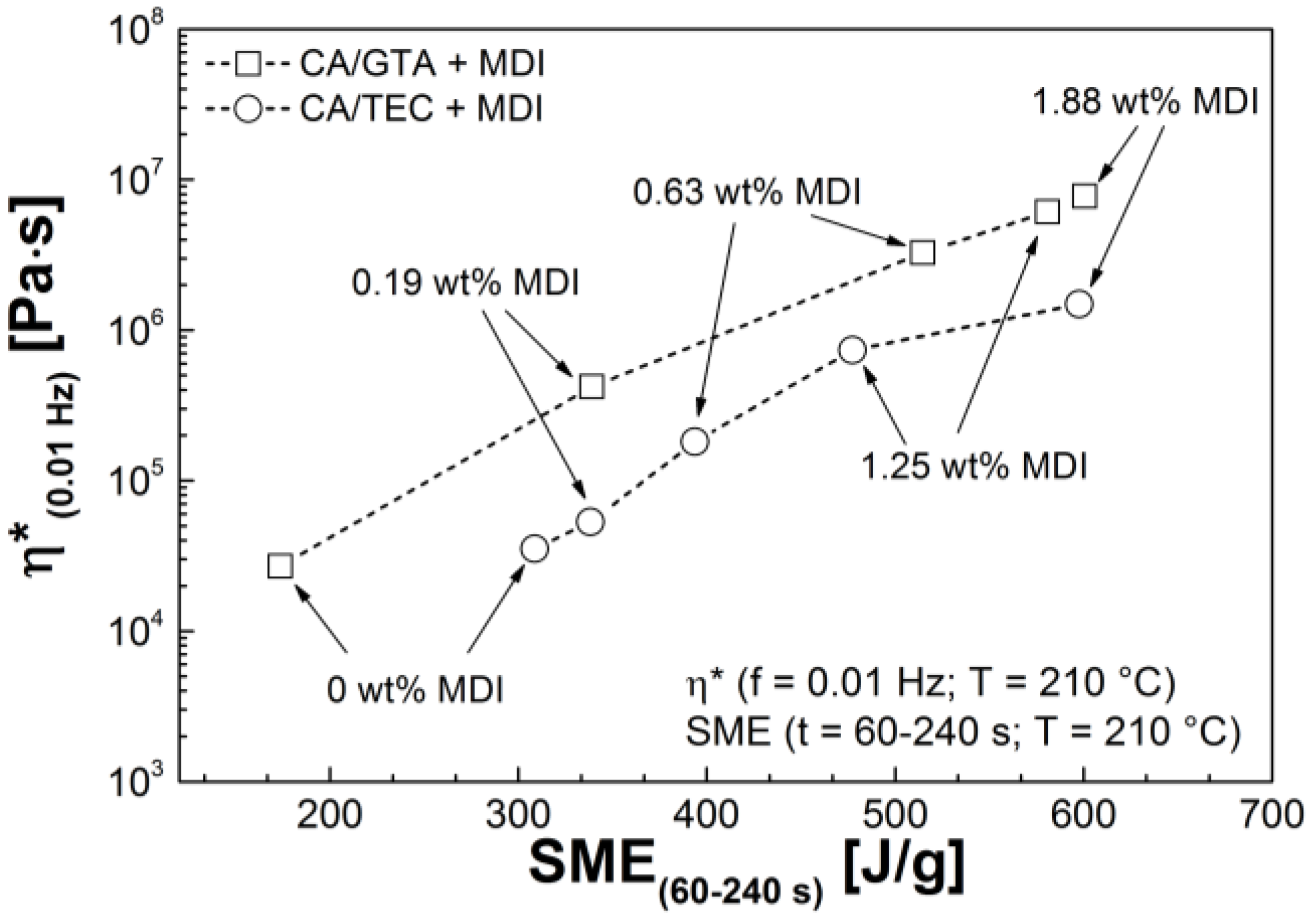
3.3. Rheological Properties
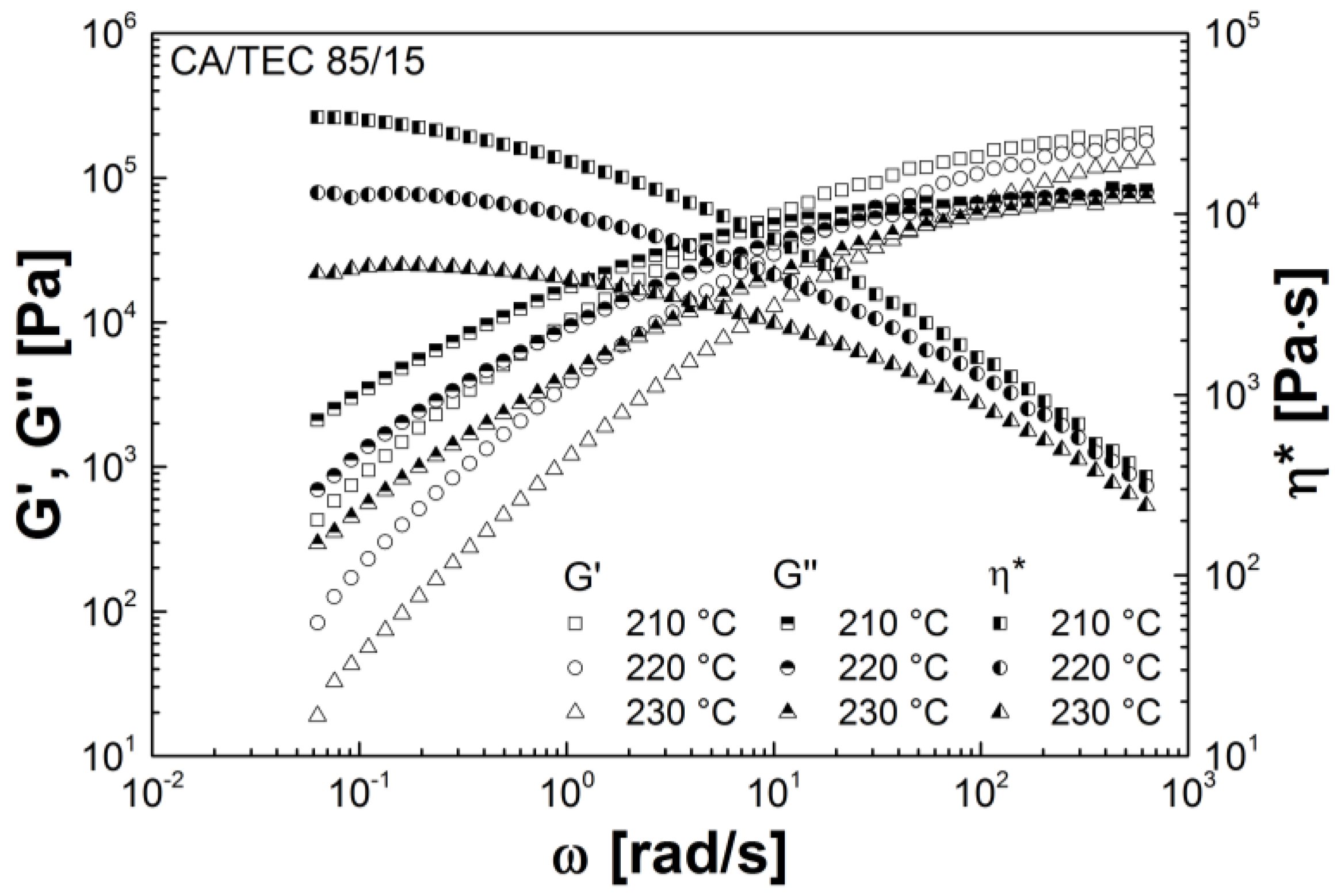
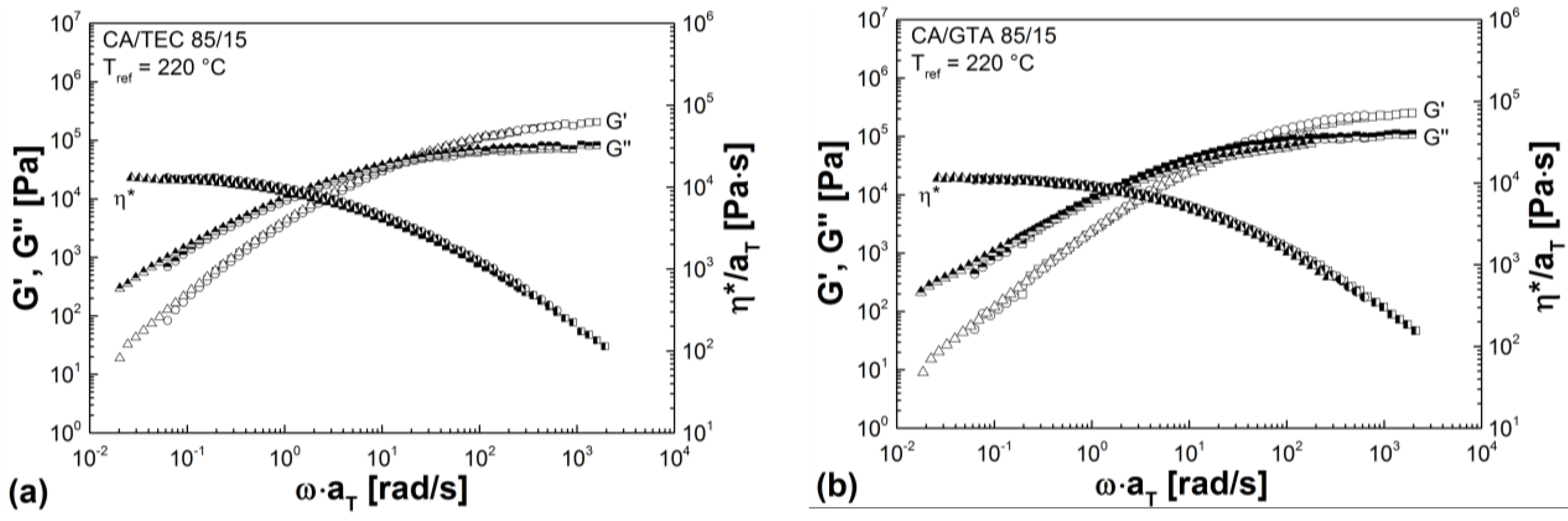
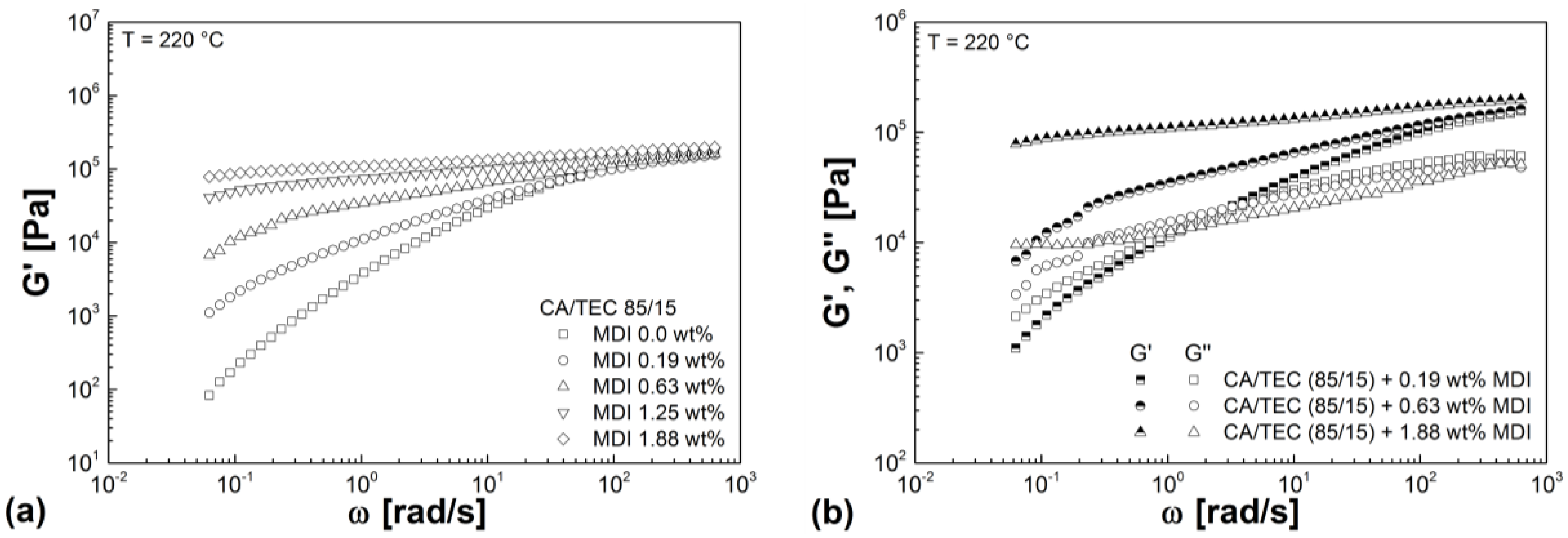
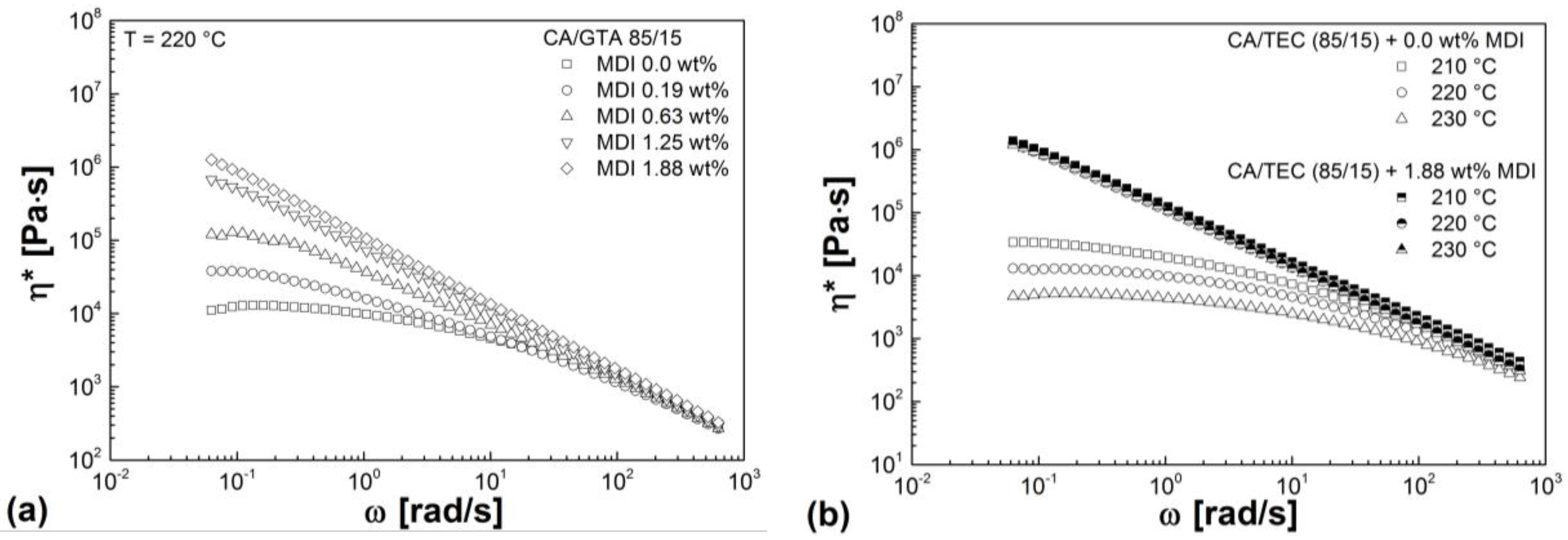
| Mixtures | G' (Pa) | G'' (Pa) | G* (Pa) | η* (Pa s) |
|---|---|---|---|---|
| CA/GTA 85/15 | ||||
| + MDI 0 | 5.7 × 101 | 5.1 × 102 | 5.1 × 102 | 1.1 × 104 |
| + MDI 0.19 | 1.4 × 104 | 2.2 × 104 | 2.6 × 104 | 4.2 × 105 |
| + MDI 0.63 | 2.0 × 105 | 3.8 × 104 | 2.1 × 105 | 3.3 × 106 |
| + MDI 1.25 | 3.9 × 105 | 5.3 × 104 | 3.9 × 105 | 6.2 × 106 |
| + MDI 1.88 | 4.8 × 105 | 6.6 × 104 | 4.9 × 105 | 7.8 × 106 |
| CA/TEC 85/15 | ||||
| + MDI 0 | 8.3 × 101 | 7.0 × 102 | 7.0 × 102 | 1.3 × 104 |
| + MDI 0.19 | 1.1 × 103 | 2.1 × 103 | 2.4 × 103 | 3.8 × 104 |
| + MDI 0.63 | 6.8 × 103 | 3.4 × 103 | 7.6 × 103 | 1.3 × 105 |
| + MDI 1.25 | 4.1 × 104 | 9.4 × 103 | 4.2 × 104 | 6.7 × 105 |
| + MDI 1.88 | 7.9 × 104 | 9.6 × 103 | 7.9 × 104 | 1.3 × 106 |
3.4. Thermal Properties
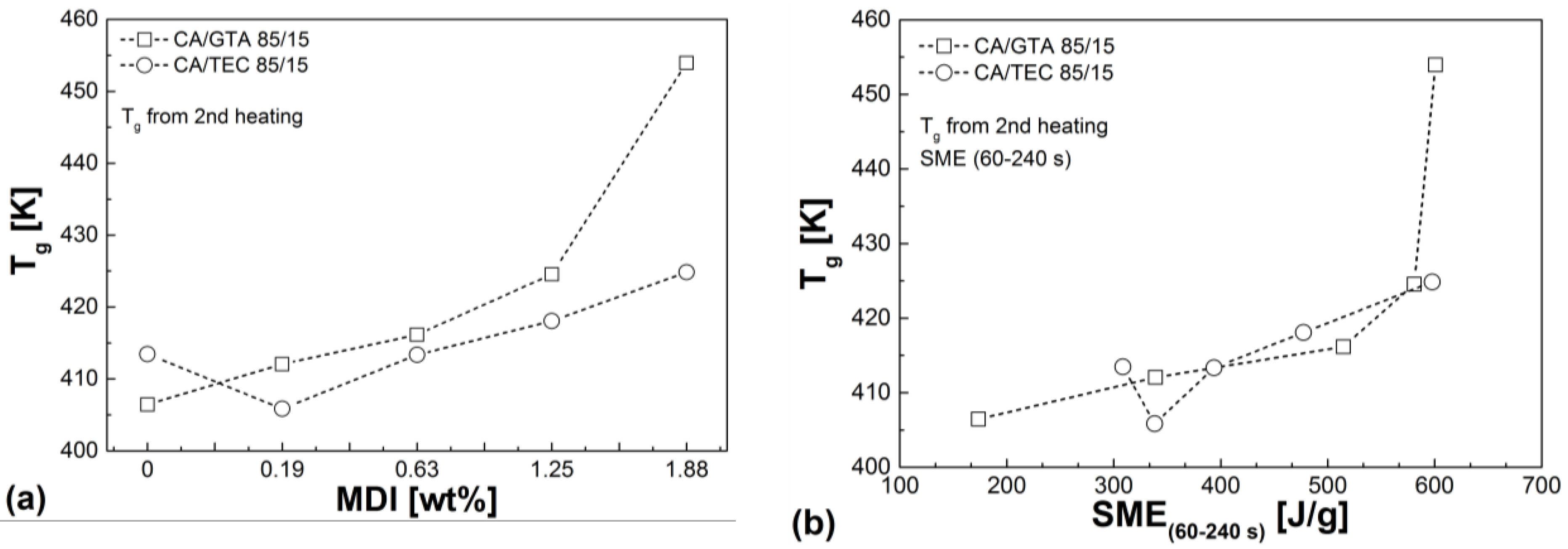
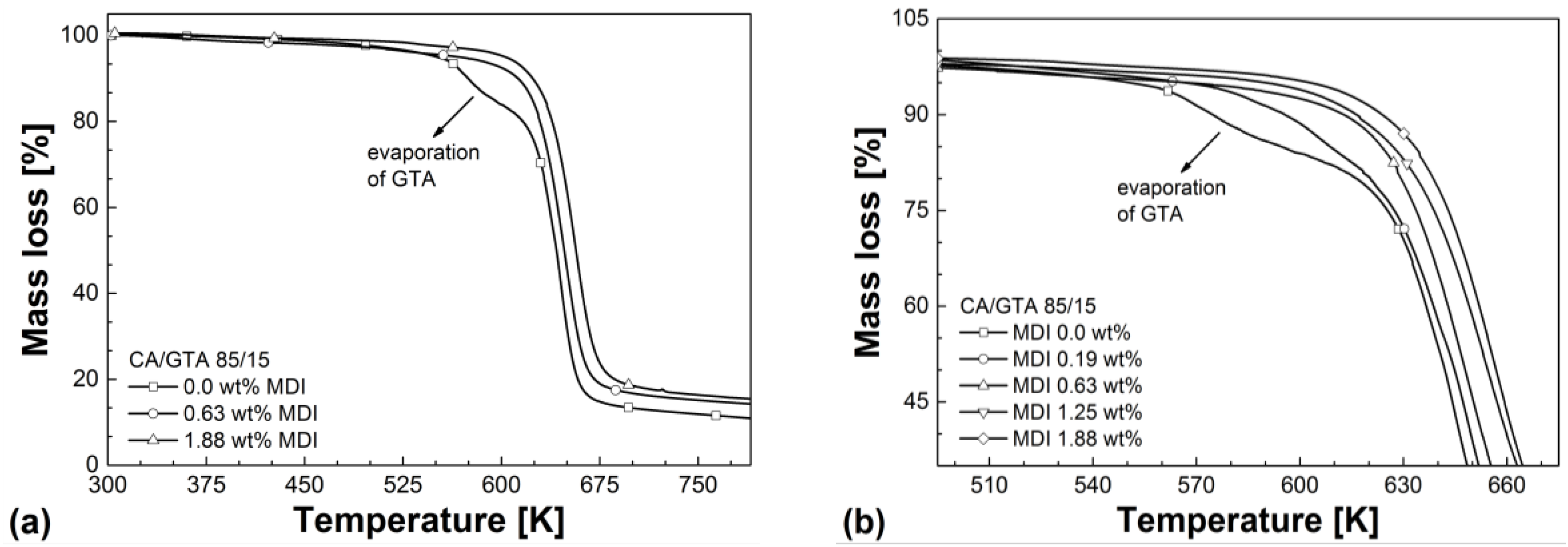
| Mixtures | Tg (2nd heating) (K) | Td (50% mass loss) (K) | Residue (%) | VST A50 (K) |
|---|---|---|---|---|
| CA/GTA 85/15 | ||||
| + MDI 0 | 406.5 | 642 | 10.1 | 394.9 |
| + MDI 0.19 | 412.1 | 644 | 10.6 | 401.2 |
| + MDI 0.63 | 416.2 | 648 | 13.9 | 410.5 |
| + MDI 1.25 | 424.6 | 654 | 15.2 | 420.1 |
| + MDI 1.88 | 454.0 | 657 | 15.7 | 448.7 |
| CA/TEC 85/15 | ||||
| + MDI 0 | 413.5 | 640 | 10.5 | 398.2 |
| + MDI 0.19 | 405.9 | 638 | 10.3 | 400.5 |
| + MDI 0.63 | 413.4 | 645 | 12.7 | 404.5 |
| + MDI 1.25 | 418,1 | 649 | 13.7 | 411,3 |
| + MDI 1.88 | 424,9 | 653 | 15.3 | 419,5 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schützenberger, P. Action de l'acide acétique anhydre sur la cellulose, l'amidon, les sucres, la mannite et ses congénères, les glucosides et certaines matières colorantes végétales. Compt. Rend. Hebd. Séances Acad. Sci. 1865, 61, 485–486. (In French) [Google Scholar]
- Mohanty, A.K.; Wibowo, A.; Misra, M.; Drzal, L.T. Development of renewable resource-based cellulose acetate bioplastic: Effect of process engineering on the performance of cellulosic plastics. Polym. Eng. Sci. 2003, 43, 1151–1161. [Google Scholar] [CrossRef]
- Fridman, O.A.; Sorokina, A.V. Criteria of efficiency of cellulose acetate plasticization. Polym. Sci. Ser. B 2006, 48, 233–236. [Google Scholar] [CrossRef]
- Fridman, O.A. Structural-relaxation mechanism of glassy-like polymers plasticization. Am. J. Polym. Sci. 2013, 3, 7–12. [Google Scholar]
- Zepnik, S.; Kabasci, S.; Kopitzky, R.; Radusch, H.-J.; Wodke, T. Extensional flow properties of externally plasticized cellulose acetate: Influence of plasticizer content. Polymers 2013, 5, 873–889. [Google Scholar] [CrossRef]
- Nishio, N. Material functionalization of cellulose and related polysaccharides via diverse microcompositions. Adv. Polym. Sci. 2006, 205, 97–151. [Google Scholar]
- Hamburger, C.J. The effect of chain growth retardation in the graft polymerization of styrene onto cellulose acetate. Ph.D. Thesis, Lawrence University, Appleton, WI, USA, 1 January 1967. [Google Scholar]
- Vidéki, B.; Klébert, S.; Pukánszky, B. External and internal plasticization of cellulose acetate with caprolactone: Structure and properties. J. Polym. Sci. B Polym. Phys. 2007, 45, 873–883. [Google Scholar] [CrossRef]
- Sobue, H.; Matsuzaki, K.; Komagata, H.; Ishida, A. Grafting of styrene on cellulose acetate films. J. Polym. Sci. C Polym. Symp. 1963, 2, 415–422. [Google Scholar] [CrossRef]
- Biermann, C.J.; Chung, J.B.; Narayan, R. Grafting of polystyrene onto cellulose acetate by nucleophilic displacement of mesylate groups using the polystyrylcarboxylate anion. Macromolecules 1987, 20, 954–957. [Google Scholar] [CrossRef]
- Billy, M.; da Costa, A.R.; Lochon, P.; Clément, R.; Dresch, M.; Etienne, S.; Hiver, J.M.; David, L.; Jonquières, A. Cellulose acetate graft copolymers with nano-structured architectures: Synthesis and characterization. Eur. Polym. J. 2010, 46, 944–957. [Google Scholar] [CrossRef]
- Kostrov, Y.A.; Litovchenko, G.D. Cross-linking of acetate fibres with blocked diisocyanate. Fibre Chem. 1969, 1, 439–443. [Google Scholar] [CrossRef]
- Ghatge, N.D.; Sabne, M.B.; Gujar, K.B.; Mahajan, S.S. Modified cellulose acetate membranes for desalination. J. Appl. Polym. Sci. 1984, 29, 1743–1748. [Google Scholar] [CrossRef]
- Mahajan, S.S.; Sabne, M.B.; Gujar, K.B.; Ghatge, N.D. Selectivity of isocyanate modified cellulose acetate membranes to sugars. Int. J. Polym. Mater. 1985, 11, 39–45. [Google Scholar] [CrossRef]
- Stiubianu, G.; Cazacu, M.; Nicolescu, A.; Hamciuc, V.; Vlad, S. Silicone-modified cellulose. Crosslinking of the cellulose acetate with 1,1,3,3-tetramethyldisiloxane by Pt-catalyzed dehydrogenative coupling. J. Polym. Res. 2010, 17, 837–845. [Google Scholar] [CrossRef]
- Nie, L.; Narayan, R. Grafting cellulose acetate with styrene maleic anhydride random copolymers for improved dimensional stability of cellulose acetate. J. Appl. Polym. Sci. 2003, 54, 601–617. [Google Scholar] [CrossRef]
- Yoshioka, M.; Hagiwara, N.; Shiraishi, N. Thermoplasticization of cellulose acetates by grafting of cyclic esters. Cellulose 1999, 6, 193–212. [Google Scholar] [CrossRef]
- Warth, H.; Mülhaupt, R.; Schätzle, J. Thermoplastic cellulose acetate and cellulose acetate compounds prepared by reactive processing. J. Appl. Polym. Sci. 1997, 64, 231–242. [Google Scholar]
- Klébert, S. Modification of Cellulose Acetate by Reactive Processing—Chemistry, Structure and Properties. Ph.D. Thesis, Budapest University of Technology and Economics, Budapest, Hungary, 13 December 2007. [Google Scholar]
- Klébert, S.; Nagy, L.; Domján, A.; Pukánszky, B. Modification of cellulose acetate with oligomeric polycaprolactone by reactive processing: Efficiency, compatibility, and properties. J. Appl. Polym. Sci. 2009, 113, 3255–3263. [Google Scholar] [CrossRef]
- Lee, S.H.; Shiraishi, N. Plasticization of cellulose diacetate by reaction with maleic anhydride, glycerol, and citrate esters during melt processing. J. Appl. Polym. Sci. 2001, 81, 243–250. [Google Scholar] [CrossRef]
- Teramoto, Y.; Yoshioka, M.; Shiraishi, N.; Nishio, Y. Plasticization of cellulose diacetate by graft copolymerization of ε-caprolactone and lactic acid. J. Appl. Polym. Sci. 2002, 84, 2621–2628. [Google Scholar] [CrossRef]
- Redl, A.; Morel, M.H.; Bonicel, J.; Guilbert, S.; Vergnes, B. Rheological properties of gluten plasticized with glycerol: Dependence on temperature, glycerol content and mixing conditions. Rheol. Acta 1999, 38, 311–320. [Google Scholar] [CrossRef]
- Vera-Graziano, R.; Hernandez-Sanchez, F.; Cauich-Rodriguez, J.V. Study of crosslinking density in polydimethylsiloxane networks by DSC. J. Appl. Polym. Sci. 1995, 55, 1317–1327. [Google Scholar] [CrossRef]
- Kunststoffe–Thermoplaste–Bestimmung der Vicat-Erweichungstemperatur (VST); DIN EN ISO 306:2014-03; Beuth Verlag: Berlin, Germany, 2014; pp. 1–20. (In German)
- Gao, Z.H.; Gu, J.Y.; Wang, X.-M.; Li, Z.G.; Bai, X.D. FTIR and XPS study of the reaction of phenyl isocyanate and cellulose with different moisture contents. Pigment Resin Technol. 2005, 34, 282–289. [Google Scholar] [CrossRef]
- Cailloux, J.; Santana, O.O.; Franco-Urquiza, E.; Bou, J.J.; Carrasco, F.; Gámez-Pérez, J.; Maspoch, M.L. Sheets of branched poly(lactic acid) obtained by one step reactive extrusion calendering process: Melt rheology analysis. Express Polym. Lett. 2013, 7, 304–318. [Google Scholar] [CrossRef]
- Hsieh, K.H.; Lin, B.Y.; Chiu, W.Y. Studies on diisocyanate-modified cellulose acetate membranes. Desalination 1989, 71, 97–105. [Google Scholar] [CrossRef]
- Botaro, V.R.; Gandini, A. Homogeneous chemical modification of cellulose acetate using different isocyanates. Polímeros 1998, 8, 64–71. [Google Scholar]
- Xing, Q.; Yates, K.; Vogt, C.; Qian, Z.; Frost, M.C.; Zhao, F. Increasing mechanical strength of gelatin hydrogels by divalent metal ion removal. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Mezger, T.G. Das Rheologie Handbuch: Für Anwender von Rotations-und Oszillations-Rheometern, 2nd ed.; Vincentz Network: Hanover, Germany, 2006; pp. 119–183. [Google Scholar]
- Chetty, A.; Kovács, J.; Sulyok, Zs.; Mészáros, Á.; Fekete, J.; Domján, A.; Szilágyi, A.; Vargha, V. A versatile characterization of poly(N-isopropylacrylamide-co-N,N'-methylene-bis-acryl-amide) hydrogels for composition, mechanical strength, and rheology. Express Polym. Lett. 2013, 7, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Winter, H.H.; Mours, M. Rheology of polymers near liquid–solid transitions. Adv. Polym. Sci. 1997, 134, 165–234. [Google Scholar]
- Wolff, F.; Kugler, C.; Münstedt, H. Viscoelastic properties of a silicone resin during crosslinking. Rheol. Acta 2011, 50, 917–924. [Google Scholar] [CrossRef]
- Niamlang, S.; Sirivat, A. Electromechanical responses of a crosslinked polydimethylsiloxane. Macromol. Symp. 2008, 264, 176–183. [Google Scholar] [CrossRef]
- Broedersz, C.P.; Kasza, K.E.; Jawerth, L.M.; Münster, S.; Weitz, D.A.; MacKintosh, F.C. Measurement of nonlinear rheology of cross-linked biopolymer gels. Soft Matter 2010, 6, 4120–4127. [Google Scholar] [CrossRef]
- Levita, G.; de Petris, S.; Marchetti, A.; Lazzeri, A. Crosslink density and fracture toughness of epoxy resins. J. Mater. Sci. 1991, 26, 2348–2352. [Google Scholar] [CrossRef]
- Lange, J.; Luisier, A.; Hult, A. Influence of crosslink density, glass transition temperature and addition of pigment and wax on the scratch resistance of an epoxy coating. J. Coating Technol. 1997, 69, 77–82. [Google Scholar] [CrossRef]
- Hale, A.; Macosko, C.W.; Bair, H.E. Glas transition temperature as a function of conversion in thermosetting polymers. Macromolecules 1991, 24, 2610–2621. [Google Scholar] [CrossRef]
- Chang, T.D.; Carr, S.H.; Brittain, J.O. Studies of epoxy resin systems: Part B: Effect of crosslinking on the physical properties of an epoxy resin. Polym. Eng. Sci. 1982, 22, 1213–1220. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Citric acid cross-linking of starch films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef]
- Ambrogi, V.; Brostow, W.; Carfagna, C.; Pannico, M.; Persico, P. Plasticizer migration from cross-linked flexible PVC: Effects on tribology and hardness. Polym. Eng. Sci. 2012, 52, 211–217. [Google Scholar] [CrossRef]
- Lakshmi, S.; Jayakrishnan, A. Photocross-linking of dithiocarbamate-substituted PVC reduces plasticizer migration. Polymer 1998, 39, 151–157. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erdmann, R.; Kabasci, S.; Kurek, J.; Zepnik, S. Study of Reactive Melt Processing Behavior of Externally Plasticized Cellulose Acetate in Presence of Isocyanate. Materials 2014, 7, 7752-7769. https://doi.org/10.3390/ma7127752
Erdmann R, Kabasci S, Kurek J, Zepnik S. Study of Reactive Melt Processing Behavior of Externally Plasticized Cellulose Acetate in Presence of Isocyanate. Materials. 2014; 7(12):7752-7769. https://doi.org/10.3390/ma7127752
Chicago/Turabian StyleErdmann, Rafael, Stephan Kabasci, Joanna Kurek, and Stefan Zepnik. 2014. "Study of Reactive Melt Processing Behavior of Externally Plasticized Cellulose Acetate in Presence of Isocyanate" Materials 7, no. 12: 7752-7769. https://doi.org/10.3390/ma7127752