Solid-State Synthesis and Photocatalytic Activity of Polyterthiophene Derivatives/TiO2 Nanocomposites
Abstract
: Poly(3,4-propylenedioxy-2,2′:5′,2″-terthiophene)/TiO2 and poly(3,4-(2,2-dimethylenepropylenedioxy)-2,2′:5′,2″-terthiophene)/TiO2 nanocomposites were synthesized by a simple solid-state method. Additionally, the poly(3,4-propylenedioxy thiophene)/TiO2 and poly(3,4-2,2-dimethylenepropylenedioxythiophene)/TiO2 nanocomposites were synthesized in a similar manner for comparison. The structure and morphology were characterized by Fourier transform infrared (FTIR), ultraviolet-visible (UV-Vis) absorption spectroscopy, X-ray diffraction (XRD) and transmission electron microscopy (TEM). The photocatalytic activities of the nanocomposites were examined through the degradation processes of a methylene blue (MB) solution under UV light and sunlight irradiation. The results of FTIR and UV-Vis spectra showed that the composites were successfully synthesized by solid-state method and the poly(3,4-propylenedioxy-2,2′:5′,2″-terthiophene)/TiO2 and poly(3,4-(2,2-dimethylenepropylenedioxy)-2,2′:5′,2″-terthiophene)/TiO2 nanocomposite had a higher oxidation degree and conjugation length than others. The results also indicated that the TiO2 had no effect on the crystallinity of composites, but was well embedded in the polymer matrix. Additionally, the highest degradation efficiency of 90.5% occurred in the case of the poly(3,4-propylenedioxy-2,2′:5′,2″-terthiophene)/TiO2 nanocomposite.1. Introduction
The use of semiconducting materials in environmental decontamination as photocatalysts has attracted a great deal of attention in recent years [1]. When semiconducting materials are exposed to light with energy larger than their band gaps, electron-hole pairs can be created, which can initiate photocatalytic reactions [2]. Most photocatalytic systems are based on TiO2, which is chemically stable and has a long life-time of electron-hole pairs generated by optical excitation. However, the band gap of TiO2 is ~3.2 eV, which is in the ultraviolet regime, and therefore, visible light, which comprises most of the solar light, cannot be absorbed by TiO2 [3]. To overcome this problem, some strategies have been investigated, including noble metal deposition, the doping of metal or nonmetal ions, mixing with another metal oxide, surface photosensitization with dye and preparing composites with a conducting polymer [4–6]. Conducting polymers have attracted considerable attention, because of their interesting semiconducting, electronic and optical properties [7]. Among conducting polymers, polyaniline and polythiophene are widely used for the fabricating of conducting polymer/TiO2 hybrid materials [8,9]. Polythiophene and its derivatives show many advantages in combining with TiO2, and the large internal interface area in the polythiophene/TiO2 composite enables an efficient separation of charge, which is very important for photovoltaic application [10,11]. At the present time, numbers of reports have been published on the preparation of polythiophene and its derivatives/TiO2 composites, including original in situ photopolymerization [12], electrochemical polymerization [13] and the chemical solution method [14]. Although polythiophene has become the focus of considerable interest, polythiophene suffers from the occurrence of undesired α,β- and β,β’-couplings during polymerization, which deteriorate their electronic and optical properties [15,16]. As derivatives of polythiophene, polyterthiophene-type conjugated polymers have rapidly gained considerable attention, due to the preexistence of α,α’-linkages in their monomers, which makes the whole polyterthiophene-type chain grow regularly and leads to very interesting electronic, electrochromic and optical properties [17–19]. Additionally, they can be prepared by electrochemical and chemical methods. Recently, we have demonstrated a novel room-temperature solid-state oxidative method for the polymerization of ethylenedioxy-substituted terthiophene, and we have found that solid-state polymerization was an effective method for the polymerization of the terthiophene-type monomer [20].
In this paper, we selected 3,4-propylenedioxy-2,2′:5′,2″-terthiophene and 3,4-(2,2-dimethylenepropylenedioxy)-2,2′:5′,2″-terthiophene as monomers for the preparation of the polyterthiophene derivatives/TiO2 nanocomposites. As these monomers have α,α’-linkages, it can be deduced that the resulting polymers may grow regularly and will have a high absorption coefficient and a wide absorption wavelength in the visible part of the spectrum, which will allow the TiO2 photocatalyst to harvest incident light efficiently in composite photocatalyst. Furthermore, these monomers are in a solid state, and they can be polymerized by the solid-state method. In the present work, with the aim of the above consideration, the room-temperature solid-state polymerization method was applied for the oxidative polymerization of the above terthiophene monomers, 3,4-propylenedioxy-2,2′:5′,2″-terthiophene (TPT) and 3,4-(2,2-dimethylenepropylenedioxy)-2,2’:5’,2″-terthiophene (TMPT), for the preparation of the poly(3,4-propylenedioxy-2,2′:5′,2″-terthiophene)/TiO2 (poly(TPT)/TiO2) and poly(3,4-(2,2-dimethylenepropylene-dioxy)-2,2′:5′,2″-terthiophene)/TiO2 (poly(TMPT)/TiO2) nanocomposites. Additionally, the poly(3,4-propy-lenedioxythiophene)/TiO2 (PProDOT/TiO2) and poly(3,4-2,2-dimethylene-propylenedioxythiophene)/TiO2 (PProDOT-Me2/TiO2) nanocomposites were synthesized in a similar manner for comparison. The structure and properties of the nanocomposites were investigated by FTIR, UV-Vis and X-ray diffraction. In addition, the photocatalytic activities of the nanocomposite were evaluated by the degradation processes of a methylene blue (MB) solution under UV light and sunlight irradiation.
2. Results and Discussion
2.1. Fourier Transform Infrared (FTIR) Spectra
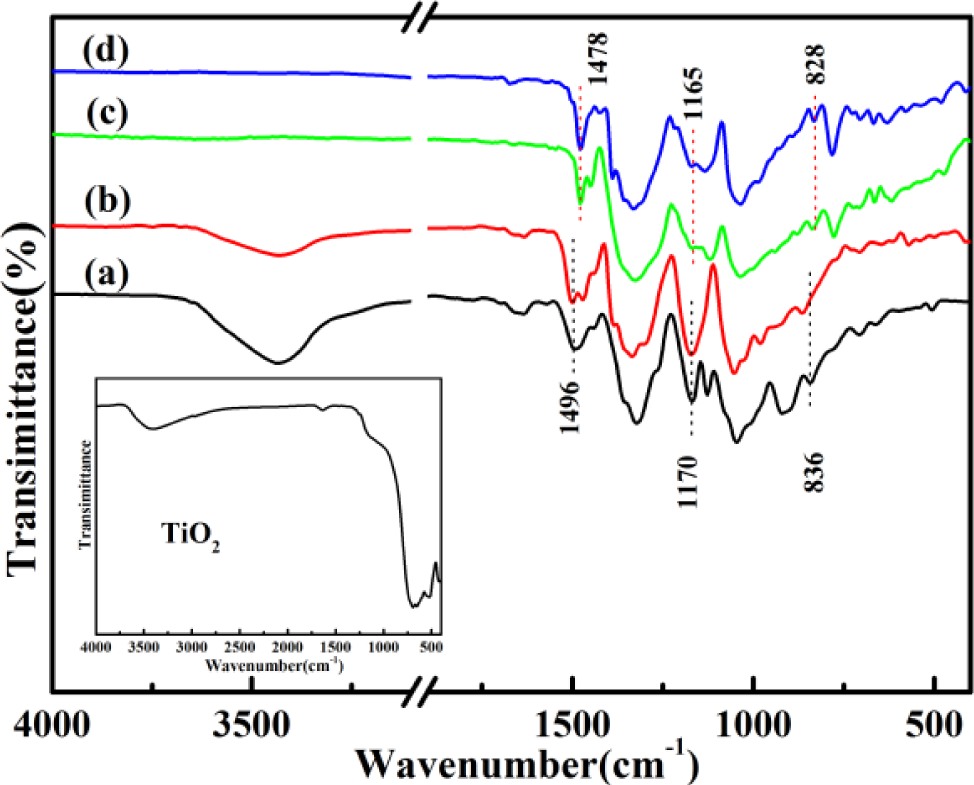
Figure 1 gives the FTIR spectra of solid-state synthesized PProDOT/TiO2, PProDOT-Me2/TiO2, poly(TPT)/TiO2, poly(TMPT)/TiO2 and nano-TiO2. As can be seen in Figure 1, the bands for PProDOT/TiO2 and PProDOT-Me2/TiO2 are as follows: ~1650, ~1490, ~1320, ~1167, ~1125, ~1045, ~920, ~838 and ~706 cm−1. The bands at ~1650, ~1490 and ~1320 cm−1 are assigned to the characteristic bands of the thiophene ring [20]. The bands appearing at ~1167, ~1125 and ~1045 cm−1 are associated with the C–O–C bending vibration in the propylene oxide group [21,22], while the bands at ~916, ~838 and ~706 cm−1 are the characteristic bands of the stretching vibrations of the C–S–C bond in the thiophene ring [23–25], which are similar to the previously reported IR spectrum of PPorDOT [26,27]. Furthermore, comparing the FTIR spectra of poly(TPT)/TiO2 and poly(TMPT)/TiO2 with that of PProDOT/TiO2 and PProDOT-Me2/TiO2, one can see that the positions of the main IR bands of poly(TPT)/TiO2 and poly(TMPT)/TiO2 nanocomposites are nearly identical with that of PProDOT/TiO2 and PProDOT-Me2/TiO2. However, the bands of the poly(TPT)/TiO2 and poly(TMPT)/TiO2 nanocomposites at ~1496, ~1170 and ~832 cm−1 are shifted slightly from their corresponding position to the lower wavenumber compared with that of the PProDOT/TiO2 and PProDOT-Me2/TiO2 nanocomposites, which indicates that the higher oxidation degree and stronger interaction between the TiO2 with polymer occur in the case of the poly(TPT)/TiO2 and poly(TMPT)/TiO2 nanocomposites, as compared to the PProDOT/TiO2 and PProDOT-Me2/TiO2 nanocomposites [28]. In addition, a close look will reveal that the characteristic peak of TiO2 at ~3410 cm−1 is appearing in the case of PProDOT/TiO2 and PProDOT-Me2/TiO2 nanocomposites. However, there is no characteristic peak corresponding to the TiO2 in the poly(TPT)/TiO2 and poly(TMPT)/TiO2 nanocomposites in Figure 1, implying that the TiO2 was enwrapped by the polymer [29].
2.2. Ultraviolet-Visible (UV-Vis) Absorption Spectra
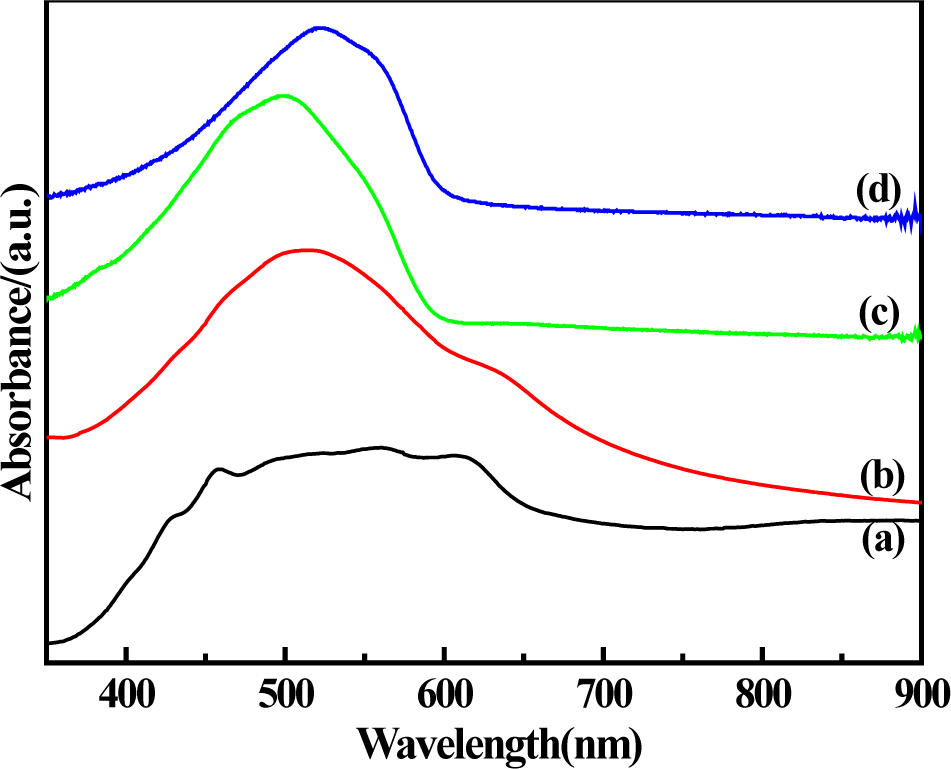
The UV-Vis spectra of solid-state synthesized PProDOT/TiO2, PProDOT-Me2/TiO2, poly(TPT)/TiO2 and poly(TMPT)/TiO2 in N-methylpyrrolidone(NMP) are shown in Figure 2. As shown in Figure 2, PProDOT/TiO2 displays a broad peak raging from ~430 nm to 610 nm with several shoulders at ~430, ~450, ~500, ~560 and ~610 nm, while the PProDOT-Me2/TiO2, poly(TPT)/TiO2 and poly(TMPT)/TiO2 display relatively sharp peaks raging from ~460 nm to ~610 nm with several shoulders at ~460, ~500, ~554 and ~610 nm. The absorption peaks and shoulders at ~430–560 nm are assigned to the π → π* transition of the thiophene ring [30–33], while the peaks at ~610 nm are the p-doping peaks of the polythiophene molecular chains [34]. Comparing with the absorption spectra of others, more shoulders appear in the PProDOT/TiO2 nanocomposite, which can be considered as the absorption peaks arising from conjugated segments having different conjugation lengths [35]. Furthermore, the peak of the PProDOT-Me2/TiO2 nanocomposite at ~500 nm is red shifted to ~523 nm in the poly(TMPT)/TiO2 nanocomposite. Generally, the red shift of the absorption spectrum shows the increase of the conjugated chain length [36]. Moreover, the peak of poly(TPT)/TiO2 appears at a relatively lower wavelength compared to the poly(TMPT)/TiO2, which is mostly originated from the strong interaction between the TiO2 particles and the poly(TMPT) [37].
2.3. X-ray Diffraction Patterns and Energy Dispersive X-ray Spectroscopy (EDX) Analysis
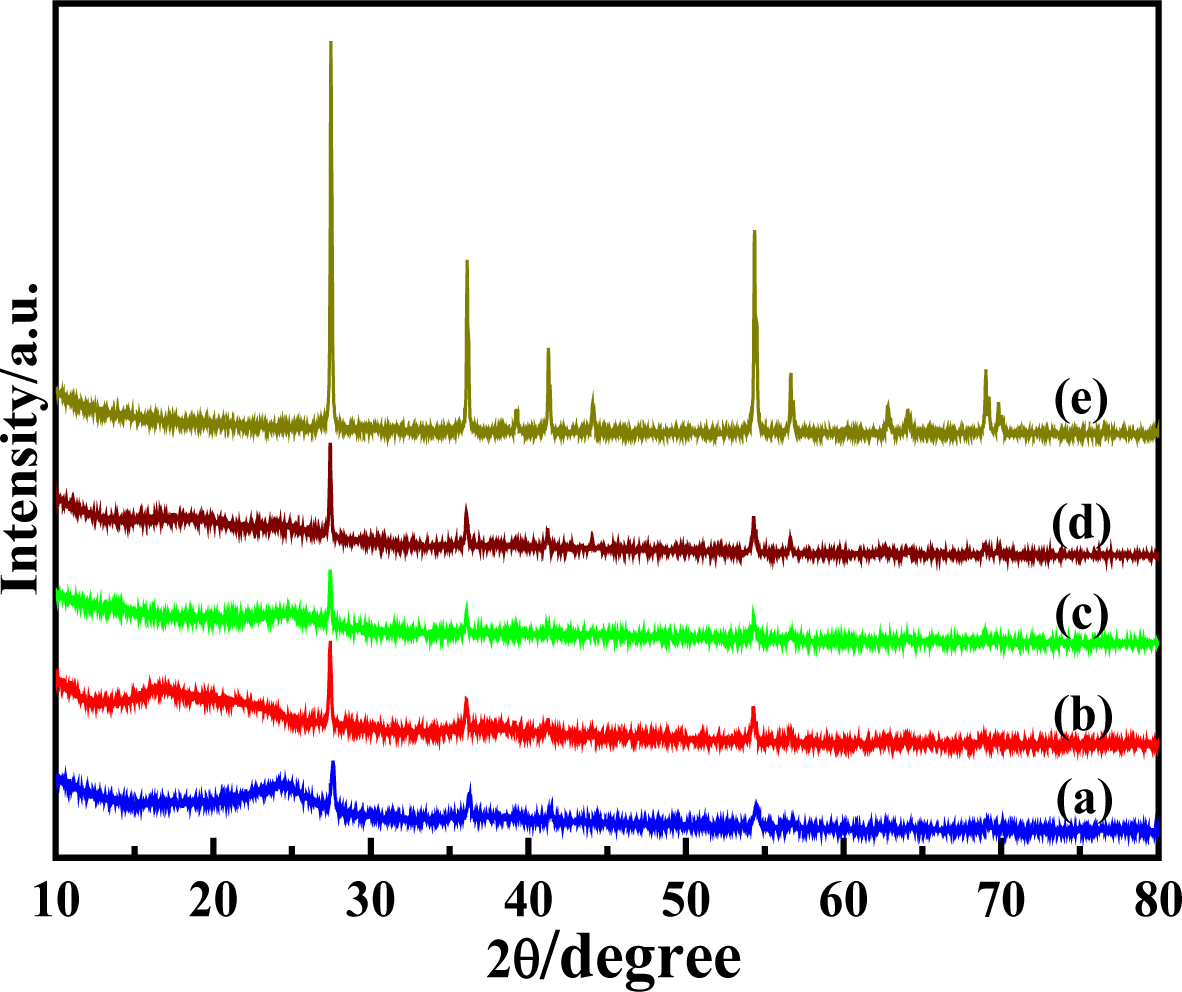
Figure 3 shows the XRD patterns of solid-state synthesized PProDOT/TiO2, PProDOT-Me2/TiO2, poly(TPT)/TiO2, poly(TMPT)/TiO2 and nano-TiO2, respectively. As seen from Figure 3, the composites possess rather broad diffraction peaks, suggesting a small degree of crystallinity, but an amorphous structure, which is similar to other thiophene derivatives [38], and its diffraction peaks are located at about 2θ ~ 24.4° (associated with the intermolecular π → π* stacking or assigned to the (020) reflection) [39] and 2θ ~ 37.5° (assigned to the (111) reflection [40,41]). The XRD patterns of composites shows the presence of the characteristic diffraction peaks of rutile TiO2 (~27.6°, 36.1°, 41.3° 54.4°, 56.7° and 69.5°), suggesting the successful incorporation of rutile TiO2. Figure 3 implies that the poly(TMPT)/TiO2 nanocomposite has lower crystallinity than others. This can be associated with the introduction of dimethyl substituents in the PProDOT or poly(TPT/TiO2), which may decrease the crystallinity with its separation effect on the polymer main chains [42]. However, the intensity of the characteristic diffraction peaks of TiO2 is lower in the case of the PProDOT/TiO2 and poly(TPT)/TiO2 nanocomposites than that of PProDOT-Me2/TiO2 and poly(TMPT)/TiO2, suggesting that the TiO2 particles are uniformly embedded in the polymer matrix of PProDOT/TiO2 and poly(TPT)/TiO2 [43].
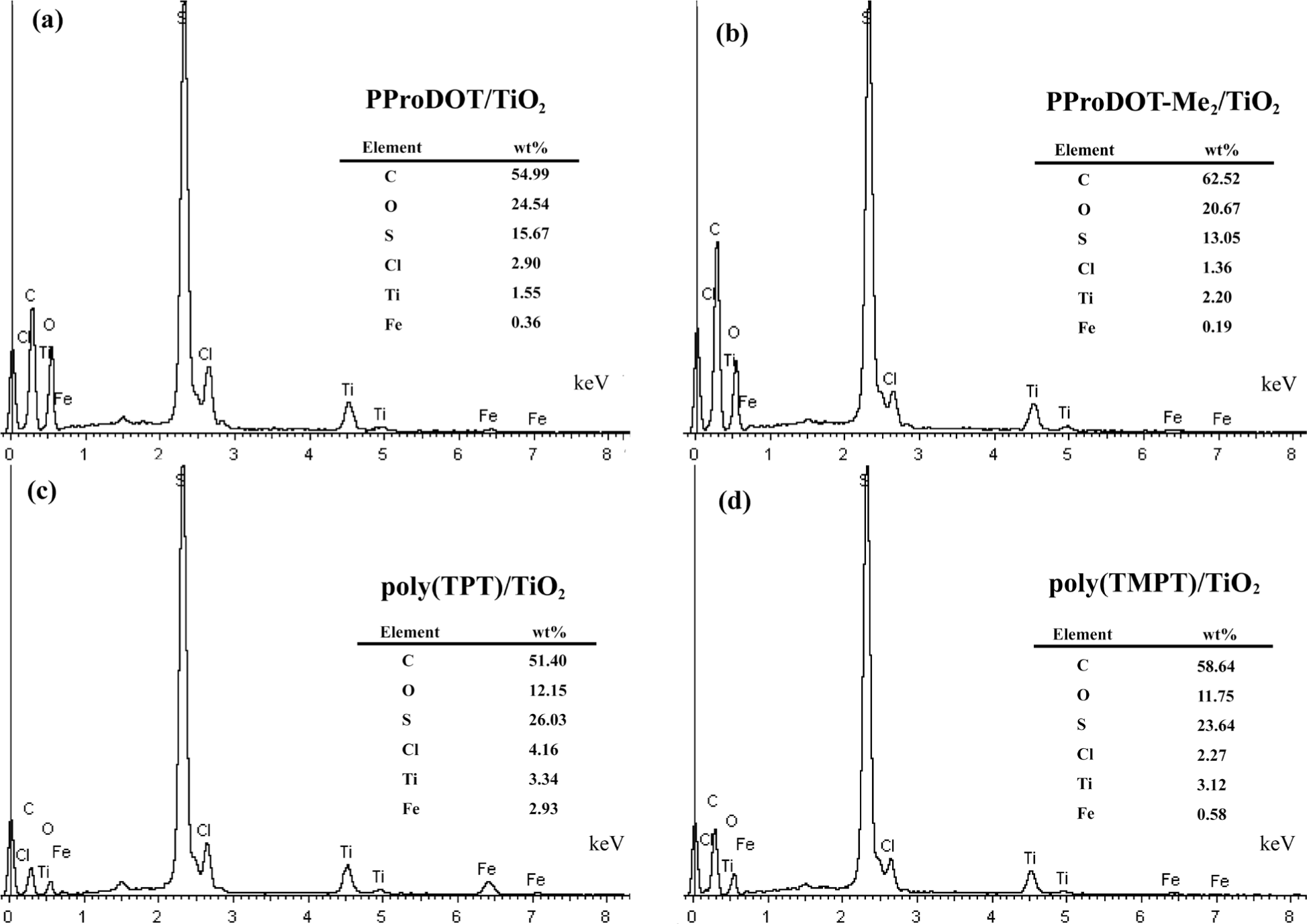
The energy dispersive X-ray spectroscopy (EDX) analysis on each composite is presented in Figure 4. As shown in Figure 4, the elements of C, O, S, Cl, Ti and Fe are observed. Additionally, the quantitative analysis of the result of EDX indicates that the wt% of Ti in each nanocomposite is 1.55%, 2.20%, 3.34% and 3.12%, respectively, which implies that poly(TPT)/TiO2 and poly(TMPT)/TiO2 have a higher wt% of TiO2 than that of PProDOT/TiO2 and PProDOT-Me2/TiO2.
2.4. Morphology
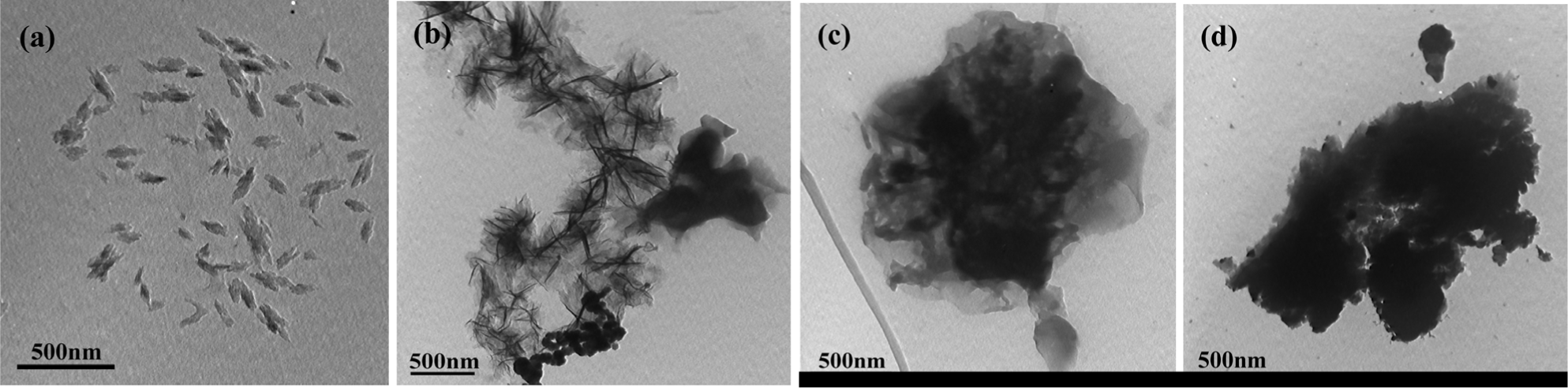
Figure 5 represents the transmission electron microscopy (TEM) images of solid-state synthesized PProDOT/TiO2, PProDOT-Me2/TiO2, poly(TPT)/TiO2 and poly(TMPT)/TiO2. As shown in Figure 5, all the nanocomposites display a sponge-like morphology, except from the PProDOT/TiO2 nanocomposite. Additionally, the TiO2 particles (dark-shaded nanoparticles) are found to be entrapped in polymer (light shaded) matrix. These results reveal that the TiO2 particles are not simply mixed up or blended with the polymer, suggesting that the TiO2 particles are embedded in the polymer matrix, which is quite in accordance with the results of the XRD analysis.
2.5. Photocatalytic Properties
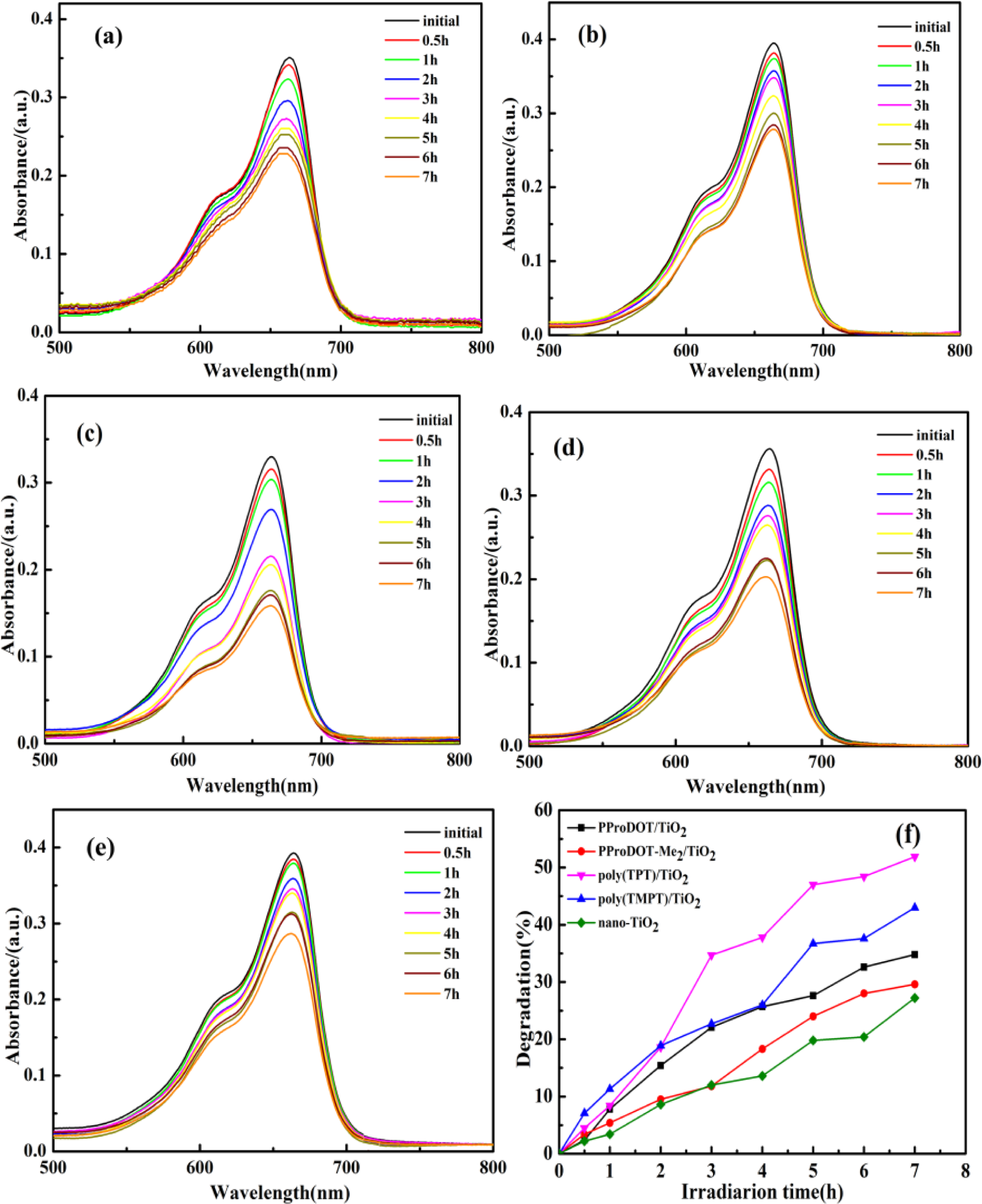
Figure 6 shows the photocatalytic degradation of methylene blue (MB) dye with the presence of the PProDOT/TiO2, PProDOT-Me2/TiO2, poly(TPT)/TiO2 and poly(TMPT)/TiO2 nanocomposites and pure nano-TiO2 as catalysts under UV light at different irradiations time, while Figure 7 indicates the photocatalytic degradation of MB dye in the presence of the above composites and the pure nano-TiO2 as the catalyst under natural sunlight at different irradiations time. Generally, the adsorption/desorption equilibrium is an important preliminary step in the photocatalytic degradation process. Therefore, prior to irradiation, the MB solution was magnetically stirred in the dark for 30 min to ensure the establishment of the adsorption/desorption equilibrium. As can be seen in Figures 6 and 7, the diminished characteristic band of MB dye at ~660 nm after 7 h under UV light and natural sunlight indicates that the MB has been degraded by all the nanocomposites and pure nano-TiO2. Additionally, the degradation efficiency of each nanocomposite is 32.5%, 29.6%, 51.5% and 44.4% under UV light for PProDOT/TiO2, PProDOT-Me2/TiO2, poly(TPT)/TiO2 and poly(TMPT)/TiO2, respectively, while the degradation efficiency of each nanocomposite is 79.6%, 62.8%, 90.5% and 84.6% under natural sunlight for PProDOT/TiO2, PProDOT-Me2/TiO2, poly(TPT)/TiO2 and poly(TMPT)/TiO2, respectively. In addition, the degradation efficiency of nano-TiO2 under UV light and natural sunlight is 27% and 11.6%, respectively. It is easy to see that the degradation efficiency of the MB in the presence of poly(TPT)/TiO2 and poly(TMPT)/TiO2 is higher than that of PProDOT/TiO2 and PProDOT-Me2/TiO2. Meanwhile, the degradation efficiency increased up to two and three times compared to PProDOT/TiO2 and PProDOT-Me2/TiO2, respectively, under the same conditions, which suggests that the poly(TPT)/TiO2 and poly(TMPT)/TiO2 nanocomposites are more effective photocatalysts for the degradation of MB than the PProDOT/TiO2 and PProDOT-Me2/TiO2 nanocomposites.
Based on previous reports, the photocatalytic degradation of MB under UV or visible light irradiation is mainly related to the generation of reactive hydroxy and hydroperoxy radicals [44–46]. Herrmann et al. investigated the TiO2/UV photocatalytic degradation of MB in aqueous heterogeneous suspensions, and a detailed degradation pathway was determined by a careful identification of intermediate products, in particular aromatics, whose successive hydroxylations led to the aromatic ring opening. Furthermore, they found that TiO2/UV-based photocatalysis was simultaneously able to oxidize the MB with an almost complete mineralization of carbon and of nitrogen and sulfur heteroatoms into CO2, NH4+, NO3− and SO42−, respectively [47]. According to the reports, the hydroxy and hydroperoxy radicals produced by the action of photocatalyst are capable of oxidizing MB molecules at the surface layer of photocatalyst. MB molecules tend to be adsorbed by the surface of the catalyst. This process can be enhanced by controlling the surface charge of the photocatalyst. Moreover, MB is a cationic dye, and a negatively charged photocatalyst surface accelerates the adsorption of the MB and, consequently, the photo degradation process [44,45].
As can be seen in Figure 7, almost complete degradation of the MB has been achieved 7 h under natural light irradiation using nanocomposites as the photocatalyst, except from the PProDOT-Me2/TiO2 nanocomposites. In addition, the degradation efficiency of both poly(TPT)/TiO2 and poly(TMPT)/TiO2 are higher than that of PProDOT/TiO2 and PProDOT-Me2/TiO2. To summarize, the poly(TPT)/TiO2 and poly(TMPT)/TiO2 nanocomposites had higher degradation efficiencies than the PProDOT/TiO2 and PProDOT-Me2/TiO2 nanocomposites under both light sources. Moreover, the degradation efficiency of all nanocomposites under sunlight are much higher than under UV light, which indicates that the nanocomposites are more effective photocatalysts for the degradation of MB under the higher intensity of sunlight irradiation than UV irradiation.
Figure 8 shows the schematic mechanism of MB dye degradation to explain the photocatalytic activity of the nanocomposite catalyst under sunlight. According to the previous report, TiO2 particles can absorb UV light to create electrons (e−) in the conduction band and holes (h+) in the valence band [48], respectively. If the electrons and holes cannot be captured in time, they will recombine with each other within a few nanoseconds, which will reduce the photocatalytic efficiency of TiO2. However, in the case of composites, due to the existence of the interface between polymer and TiO2, separated electrons and holes have little possibility to recombine again. This ensures higher charge separation efficiency and better photo-oxidation capacity for the nanocomposite. In addition, the polymer can absorb the visible light and produces an electron (e−) that transfers to the conduction band of TiO2 [48,49]. The amount of •OH and O2•− formed in the case of composites is more than that with TiO2 alone as a photocatalyst. Moreover, MB molecules can transfer from solution to the catalyst’s surface and be adsorbed with an offset face-to-face orientation via π-π conjugation between MB and aromatic regions of the polymer, and therefore, the adsorptivity of MB on polymer increases compared to that of MB on bare TiO2, which makes the composites have a higher efficiency in the photodegradation of MB compared to TiO2 alone as a photocatalyst.
3. Experimental Section
3.1. Materials
2-(tributylstannyl)-thiophene, N-bromo-succinimide (NBS), 3,4-propylenedioxythiophene (ProDOT), 3,4-(2,2-dimethylenepropylenedioxy)thiophene(ProDOT-Me2) and anhydrous iron(III) chloride were obtained from Aldrich (Tokyo, Japan) and used as received. Pd (PPh3)4 was synthesized according to the literature [50,51]. The nano-TiO2 (rutile, lipophilic, with an average size of 100 nm, Shanghai Aladdin Reagent Company, Shanghai, China) and all other chemicals were used as received without further purification.
3.2. Preparation of Monomers
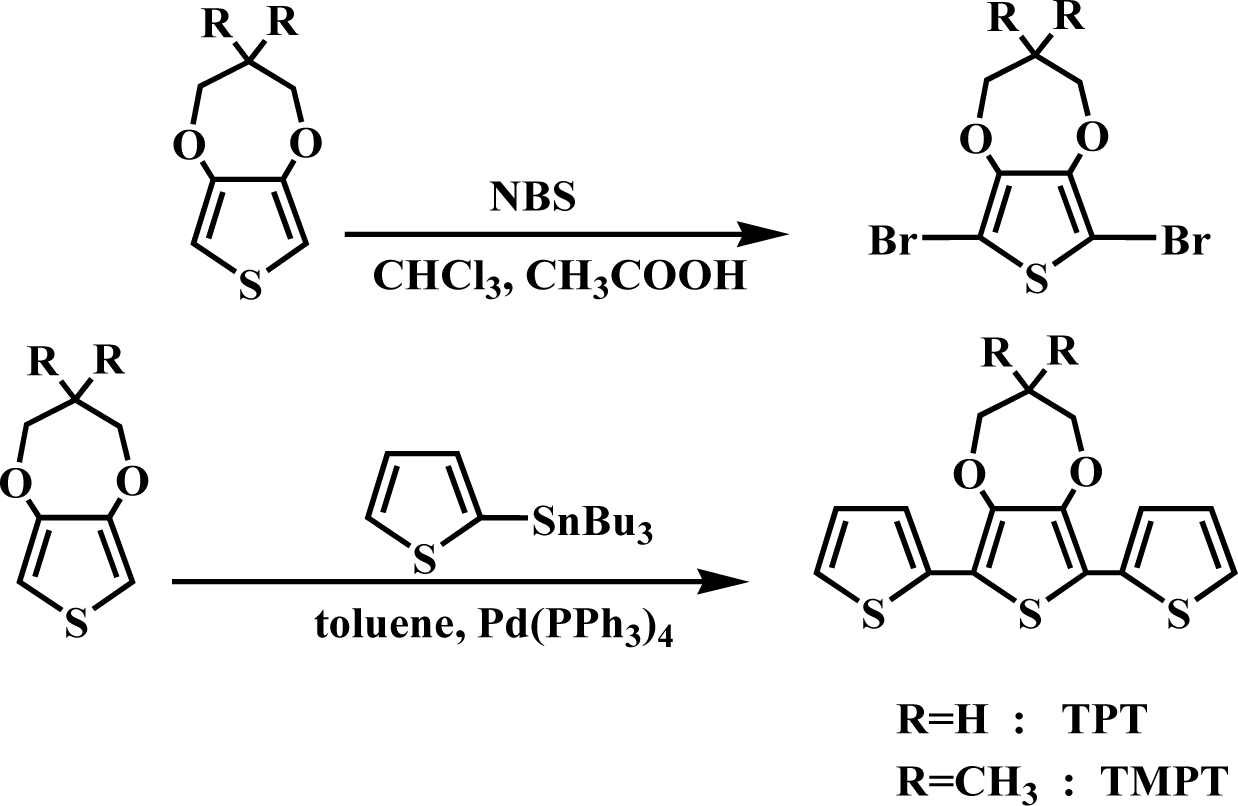
3,4-propylenedioxy-2,2′:5′,2″-terthiophene(TPT) and 3,4-(2,2-dimethylenepropylene-dioxy)-2,2′:5′,2″-terthiophene(TMPT) were synthesized according to the procedure given in [42], as shown in Scheme 1.
3.3. Preparation of Composites
A typical solid-state synthesis procedure of the composite was as follows: a mixture of 0.3 g (0.9 mmol) 3,4-propylenedioxy-2,2′:5′,2″-terthiophene (TPT) and 15 mg TiO2 in 3 mL chloroform were ultrasonicated for 30 min to facilitate the monomer in adsorbing onto the surface of TiO2. After ultrasonication, the mixture was allowed to evaporate the chloroform, then the mixture was put in a mortar, and 0.61 g (3.6 mmol) anhydrous iron(III) chloride (FeCl3) were added to this mixture, then ground for 1 h. Then, the mixture was washed with chloroform, ethanol and distilled water, respectively, until the filtrate was colorless, finally drying the powder under vacuum at 60 °C for 48 h. The obtained composite was denoted as poly(TPT)/TiO2. Poly(TMPT)/TiO2 was synthesized in a similar manner by keeping the ratio of [TMPT]/[FeCl3] at 1:4.
The other composites, poly(3,4-propylenedioxythiophene)/TiO2 nanocomposite(PProDOT/TiO2) and poly(3,4-(2,2-dimethylenepropylenedioxy)thiophene)/TiO2 composite(PProDOT/TiO2), were obtained in a similar manner by keeping the ratio of [monomer]/[FeCl3] at 1:4.
3.4. Structure Characterization
The Fourier transform infrared (FTIR) spectra of the composite were obtained by using a BRUKERQEUINOX-55 Fourier transform infrared spectrometer (Billerica, MA, USA) (frequency range: 4000–500 cm−1). The UV-Vis spectra and photocatalytic activity of the samples were recorded on a UV-Visible spectrophotometer (UV4802, Unico, Franksville, WI, USA). X-ray powder diffraction (XRD) patterns were obtained by using a Bruker AXS D8 diffractometer (Bruker AXS Co., Karlsruhe, Germany), with a monochromatic Cu-Ka radiation source (λ = 0.15418). The scan range (2θ) was 5°–80°. Transmission electron microscopy (TEM) experiments were carried out in a Hitachi 2600 electron microscope (Tokyo, Japan). The samples for TEM measurements were prepared by placing a few drops of sample ethanol suspension on copper supports.
3.5. Measurement of Photocatalytic Activities
The photocatalytic activities of nanocomposites were performed using MB as degraded materials in quartz tubes under UV light and natural sunlight irradiation. FSL MW1-Y15 (Royal Dutch Philips Electronics Ltd., Amsterdam, The Netherlands) was used as the irradiation source (λ = 254 nm) located in a light infiltrated chamber. Before irradiation, the suspension was stirred magnetically for 30 min in dark conditions until an adsorption-desorption equilibrium was established. Then, the suspensions were irradiated by light sources. Under natural sunlight investigations, all experiments were done inside a laboratory in an open atmosphere in the month of September. The photodegradation efficiency (R, %) was calculated by use of the following equation: R = [C0 − C/C0] (where C0 represents the concentration of the dye before illumination and C denotes the concentration of dye after a certain irradiation time, respectively).
4. Conclusions
In this paper, the polyterthiophene derivatives/TiO2 nanocomposites, such as poly(3,4-propylenedioxy-2,2′:5′,2″-terthiophene)/TiO2 nanocomposite (poly(TPT)/TiO2) and poly(3,4-(2,2-dimethylenepropylenedioxy)-2,2′:5′,2″-terthiophene)/TiO2 nanocomposite (poly(TMPT)/TiO2), were synthesized by a simple solid-state method. The poly(3,4-propylenedioxythiophene)/TiO2 nanocomposite (PProDOT/TiO2) and poly(3,4-2,2-dimethylenepropylenedioxythiophene)/TiO2 nanocomposite (PProDOT-Me2/TiO2) were synthesized by the same method for comparison. The results showed that the higher oxidation degree and the strong interaction between TiO2 and polymer occurred in the case of poly(TPT)/TiO2 and poly(TMPT)/TiO2 compared with that of PProDOT/TiO2 and PProDOT-Me2/TiO2. This phenomenon mainly resulted from the linear growth tendency of terthiophene-type monomers, which made the whole polyterthiophene-type chain grow regularly and led to an enhancement of the electronic and optical properties of the nanocomposites. The results also indicated that TiO2 had no effect on the crystallinity of the polymer, but TiO2 can be embedded in the polymer matrix, which implied that the solid-state method can be an effective method for preparing such composite materials. Furthermore, the UV-Vis photodegradation efficiency of MB by these composites showed that the poly(TPT)/TiO2 and poly(TMPT)/TiO2 nanocomposites had higher degradation efficiency than that of the PProDOT/TiO2 and PProDOT-Me2/TiO2 nanocomposites, which resulted from the positive effect of the higher oxidation degree of the polyterthiophene derivatives/TiO2 nanocomposites, as well as the strong interaction between TiO2 and the polyterthiophene derivatives than that of PProDOT/TiO2 and PProDOT-Me2/TiO2. The photodegradation studies suggested that the polyterthiophene derivatives/TiO2 nanocomposites were more effective photocatalysts for the degradation of MB under UV and sunlight irradiation. All nanocomposites were more effective photocatalysts as compared to bare TiO2, because the polymers acted as light-harvesting species, and the polymers displayed a positive role in charge separation, except the adsorption effect of polymers for MB via π-π conjugation between MB and aromatic regions of the polymer.
Acknowledgments
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (No. 21064007, No. 21264014) and the Opening Project of Xinjiang Laboratory of Petroleum and Gas Fine Chemicals (XJDX0908-2011-05).
Author Contributions
Ruxangul Jamal designed the experiments, and edited the paper. Yakupjan Osman carried out carried out the experiments, Adalet Rahman, Ahmet Ali, and Yu Zhang advised about the scientific meanings of this study and edited the paper. Tursun Abdiryim directed, carried out the experiments and wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morán-pineda, M.; Castillo, S.; Asomoza, M.; Gómez, R. Al2O3-TiO2 sol-gel mixed oxides as suitable supports for the reduction of NO by CO. React. Kinet. Catal. Lett 2002, 76, 75–81. [Google Scholar]
- Park, E.J.; Jeong, B.; Jeong, M.-G.; Kim, Y.D. Synergetic effects of hydrophilic surface modification and N-doping for visible light response on photocatalytic activity of TiO2. Curr. Appl. Phys 2014, 14, 300–305. [Google Scholar]
- Patel, N.; Jaiswal, R.; Warang, T.; Scarduelli, G.; Dashora, A.; Ahuja, B.L.; Kothari, D.C.; Miotello, A. Efficient photocatalytic degradation of organic water pollutants using V–N-codoped TiO2 thin films. Appl. Catal. B Environ 2014, 150–151, 74–81. [Google Scholar]
- Mohamed, R. Characterization and catalytic properties of nano-sized Pt metal catalyst on TiO2-SiO2 synthesized by photo-assisted deposition and impregnation methods. J. Mater. Process. Technol 2009, 209, 577–583. [Google Scholar]
- Gu, D.-E.; Yang, B.-C.; Hu, Y.-D. A novel method for preparing V-doped titanium dioxide thin film photocatalysts with high photocatalytic activity under visible light irradiation. Catal. Lett 2007, 118, 254–259. [Google Scholar]
- Yan, X.; He, J.; Evans, D.G.; Duan, X.; Zhu, Y. Preparation, characterization and photocatalytic activity of Si-doped and rare earth-doped TiO2 from mesoporous precursors. Appl. Catal. B Environ 2005, 55, 243–252. [Google Scholar]
- Lock, J.P.; Lutkenhaus, J.L.; Zacharia, N.S.; Im, S.G.; Hammond, P.T.; Gleason, K.K. Electrochemical investigation of PEDOT films deposited via CVD for electrochromic applications. Synth. Met 2007, 157, 894–898. [Google Scholar]
- Zhang, L.; Liu, P.; Su, Z. Preparation of PANI–TiO2 nanocomposites and their solid-phase photocatalytic degradation. Polym. Degrad. Stab 2006, 91, 2213–2219. [Google Scholar]
- Uygun, A.; Turkoglu, O.; Sen, S.; Ersoy, E.; Yavuz, A.G.; Batir, G.G. The electrical conductivity properties of polythiophene/TiO2 nanocomposites prepared in the presence of surfactants. Curr. Appl. Phys 2009, 9, 866–871. [Google Scholar]
- Xu, S.H.; Li, S.Y.; Wei, Y.X.; Zhang, L.; Xu, F. Improving the photocatalytic performance of conducting polymer polythiophene sensitized TiO2 nanoparticles under sunlight irradiation. React. Kinet. Mech. Catal 2010, 101, 237–249. [Google Scholar]
- Xu, S.; Jiang, L.; Yang, H.; Song, Y.; Dan, Y. Structure and photocatalytic activity of polythiophene/TiO2 composite particles prepared by photoinduced polymerization. Chin. J. Catal 2011, 32, 536–545. [Google Scholar]
- Dehaudt, J.; Beouch, L.; Peralta, S.; Plesse, C.; Aubert, P.H.; Chevrot, C.; Goubard, F. Facile route to prepare film of poly(3,4-ethylene dioxythiophene)-TiO2 nanohybrid for solar cell application. Thin Solid Films 2011, 519, 1876–1881. [Google Scholar]
- Ma, L.; Li, Y.; Yu, X.; Yang, Q.; Noh, C.-H. Using room temperature ionic liquid to fabricate PEDOT/TiO2 nanocomposite electrode-based electrochromic devices with enhanced long-term stability. Sol. Energy Mater. Sol. Cells 2008, 92, 1253–1259. [Google Scholar]
- Muktha, B.; Mahanta, D.; Patil, S.; Madras, G. Synthesis and photocatalytic activity of poly (3-hexylthiophene)/TiO2 composites. J. Solid State Chem 2007, 180, 2986–2989. [Google Scholar]
- Yamamoto, T.; Sanechika, K.-I.; Yamamoto, A. Preparation and characterization of poly (thienylene)s. Bull. Chem. Soc. Jpn 1983, 56, 1497–1502. [Google Scholar]
- Roncali, J. Synthetic principles for bandgap control in linear π-conjugated systems. Chem. Rev 1997, 97, 173–206. [Google Scholar]
- Meerholz, K.; Heinze, J. Electrochemical solution and solid-state investigations on conjugated oligomers and polymers of the α-thiophene and the p-phenylene series. Electrochim. Acta 1996, 41, 1839–1854. [Google Scholar]
- Tsekouras, G.; Too, C.O.; Wallace, G.G. Effect of growth conditions on the photovoltaic efficiency of poly (terthiophene) based photoelectrochemical cells. Electrochim. Acta 2005, 50, 3224–3230. [Google Scholar]
- Zanardi, C.; Scanu, R.; Pigani, L.; Pilo, M.I.; Sanna, G.; Seeber, R.; Spano, N.; Terzi, F.; Zucca, A. Synthesis and electrochemical polymerisation of 3′-functionalised terthiophenes: Electrochemical and spectroelectrochemical characterisation. Electrochim. Acta 2006, 51, 4859–4864. [Google Scholar]
- Abdiryim, T.; Jamal, R.; Zhao, C.; Awut, T.; Nurulla, I. Structure and properties of solid-state synthesized poly(3′4′-ethylenedioxy-2,2′:5′,2″-terthiophene). Synth. Met 2010, 160, 325–332. [Google Scholar]
- Yang, Y.; Jiang, Y.; Xu, J.; Yu, J. Conducting polymeric nanoparticles synthesized in reverse micelles and their gas sensitivity based on quartz crystal microbalance. Polymer 2007, 48, 4459–4465. [Google Scholar]
- Kim, Y.S.; Oh, S.B.; Park, J.H.; Cho, M.S.; Lee, Y. Highly conductive PEDOT/silicate hybrid anode for ITO-free polymer solar cells. Sol. Energy Mater. Sol. Cells 2010, 94, 471–477. [Google Scholar]
- Cloutet, E.; Mumtaz, M.; Cramail, H. Synthesis of PEDOT latexes by dispersion polymerization in aqueous media. Mater. Sci. Eng. C 2009, 29, 377–382. [Google Scholar]
- Reddy, K.R.; Park, W.; Sin, B.C.; Noh, J.; Lee, Y. Synthesis of electrically conductive and superparamagnetic monodispersed iron oxide-conjugated polymer composite nanoparticles by in situ chemical oxidative polymerization. J. Colloid Interface Sci 2009, 335, 34–39. [Google Scholar]
- Shin, H.J.; Jeon, S.S.; Im, S.S. CNT/PEDOT core/shell nanostructures as a counter electrode for dye-sensitized solar cells. Synth. Met 2011, 161, 1284–1288. [Google Scholar]
- Kumar, N.A.; Choi, H.J.; Bund, A.; Baek, J.-B.; Jeong, Y.T. Electrochemical supercapacitors based on a novel graphene/conjugated polymer composite system. J. Mater. Chem 2012, 22, 12268–12274. [Google Scholar]
- Sindhu, S.; Siju, C.; Sharma, S.; Rao, K.; Gopal, E. Optical, electrochemical and morphological investigations of poly(3,4-propylenedioxythiophene)–sultone (PProDOT–S) thin films. Bull. Mater. Sci 2012, 35, 611–616. [Google Scholar]
- Abdiryim, T.; Ubul, A.; Jamal, R.; Xu, F.; Rahman, A. Electrochemical properties of the poly(3,4-ethylenedioxythiophene)/single-walled carbon nanotubes composite synthesized by solid-state heating method. Synth. Met 2012, 162, 1604–1608. [Google Scholar]
- Abdiryim, T.; Ubul, A.; Jamal, R.; Tian, Y.; Awut, T.; Nurulla, I. Solid-state synthesis and characterization of polyaniline/nano-TiO2 composite. J. Appl. Polym. Sci 2012, 126, 697–705. [Google Scholar]
- Kvarnström, C.; Neugebauer, H.; Blomquist, S.; Ahonen, H.; Kankare, J.; Ivaska, A. In situ spectroelectrochemical characterization of poly(3,4-ethylenedioxythiophene). Electrochim. Acta 1999, 44, 2739–2750. [Google Scholar]
- Han, M.G.; Foulger, S.H. Facile synthesis of poly(3,4-ethylenedioxythiophene) nanofibers from an aqueous surfactant solution. Small 2006, 2, 1164–1169. [Google Scholar]
- Fall, M.; Aaron, J.; Sakmeche, N.; Dieng, M.; Jouini, M.; Aeiyach, S.; Lacroix, J.; Lacaze, P. Electrochemical and spectroscopic properties of poly(3-methoxythiophene) electrosynthesized in an aqueous micellar medium. Synth. Met 1998, 93, 175–179. [Google Scholar]
- Yamamoto, T.; Shimizu, T.; Kurokawa, E. Doping behavior of water-soluble π-conjugated polythiophenes depending on pH and interaction of the polymer with DNA. React. Funct. Polym 2000, 43, 79–84. [Google Scholar]
- Abdiryim, T.; Zhao, C.; Jamal, R.; Ubul, A.; Shi, W.; Nurulla, I. The effect of solvents and organic acids on the p-doping behaviors of poly (3′,4′-Ethylenedioxy-2,2′:5′,2″-terthiophene). Polym. Sci. Ser. B 2012, 54, 413–419. [Google Scholar]
- Apperloo, J.J.; Janssen, R.; Nielsen, M.M.; Bechgaard, K. Doping in solution as an order-inducing tool prior to film formation of regio-irregular polyalkylthiophenes. Adv. Mater 2000, 12, 1594–1597. [Google Scholar]
- An, S.; Abdiryim, T.; Ding, Y.; Nurulla, I. A comparative study of the microemulsion and interfacial polymerization for polyindole. Mater. Lett 2008, 62, 935–938. [Google Scholar]
- Yang, J.; Zhao, X.; Shan, X.; Fan, H.; Yang, L.; Zhang, Y.; Li, X. Blue-shift of UV emission in ZnO/graphene composites. J. Alloys Compd 2013, 556, 1–5. [Google Scholar]
- Vacca, P.; Nenna, G.; Miscioscia, R.; Palumbo, D.; Minarini, C.; Sala, D.D. Patterned Organic and Inorganic Composites for Electronic Applications. J. Phys. Chem. C 2009, 113, 5777–5783. [Google Scholar]
- Kim, T.Y.; Park, C.M.; Kim, J.E.; Suh, K.S. Electronic, chemical and structural change induced by organic solvents in tosylate-doped poly(3,4-ethylenedioxythiophene)(PEDOT-OTs). Synth. Met 2005, 149, 169–174. [Google Scholar]
- Arbizzani, C.; Biso, M.; Manferrari, E.; Mastragostino, M. Methanol oxidation by pEDOT-pSS/PtRu in DMFC. J. Power Sources 2008, 178, 584–590. [Google Scholar]
- Cho, M.S.; Kim, S.Y.; Nam, J.D.; Lee, Y. Preparation of PEDOT/Cu composite film by in situ redox reaction between EDOT and copper(II) chloride. Synth. Met 2008, 158, 865–869. [Google Scholar]
- Abdiryim, T.; Jamal, R.; Ubul, A.; Nurulla, I. Solid-state synthesis of poly(3′,4′-dimethoxy-2,2′:5′,2″-terthiophene): Comparison with poly(terthiophene) and poly(3′,4′-ethylenedioxy-2,2′:5′,2″-terthiophene). Molecules 2012, 17, 8647–8660. [Google Scholar]
- Wang, F.; Min, S.X. TiO2/polyaniline composites: An efficient photocatalyst for the degradation of methylene blue under natural light. Chin. Chem. Lett 2007, 18, 1273–1277. [Google Scholar]
- Tschirch, J.; Dillert, R.; Bahnemann, D.; Proft, B.; Biedermann, A.; Goer, B. Photodegradation of methylene blue in water, a standard method to determine the activity of photocatalytic coatings? Res. Chem. Intermed 2008, 34, 381–392. [Google Scholar]
- Fu, P.-F.; Zhao, Z.; Peng, P.; Dai, X.-G. Photodegradation of methylene blue in a batch fixed bed photoreactor using activated carbon fibers supported TiO2 photocatalyst. Chin. J. Process Eng 2008, 8, 65–71. [Google Scholar]
- Kim, J.; Choi, W.; Park, H. Effects of TiO2 surface fluorination on photocatalytic degradation of methylene blue and humic acid. Res. Chem. Intermed 2010, 36, 127–140. [Google Scholar]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ 2001, 31, 145–157. [Google Scholar]
- Wang, S.; Qian, H.; Hu, Y.; Dai, W.; Zhong, Y.; Chen, J.; Hu, X. Facile one-pot synthesis of uniform TiO2–Ag hybrid hollow spheres with enhanced photocatalytic activity. Dalton Trans 2013, 42, 1122–1128. [Google Scholar]
- Zhu, S.; Wei, W.; Chen, X.; Jiang, M.; Zhou, Z. Hybrid structure of polyaniline/ZnO nanograss and its application in dye-sensitized solar cell with performance improvement. J. Solid State Chem 2012, 190, 174–179. [Google Scholar]
- Meng, H.; Perepichka, D.F.; Bendikov, M.; Wudl, F.; Pan, G.Z.; Yu, W.; Dong, W.; Brown, S. Solid-state synthesis of a conducting polythiophene via an unprecedented heterocyclic coupling reaction. J. Am. Chem. Soc 2003, 125, 15151–15162. [Google Scholar]
- Kanbara, T.; Miyazaki, Y.; Yamamoto, T. New π-conjugated heteroaromatic alternative copolymers with electron-donating thiophene or furan units and electron-withdrawing quinoxaline units: Preparation by palladium-catalyzed polycondensation and characterization of the copolymers. J. Polym. Sci. Part A Polym. Chem 1995, 33, 999–1003. [Google Scholar]


© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jamal, R.; Osman, Y.; Rahman, A.; Ali, A.; Zhang, Y.; Abdiryim, T. Solid-State Synthesis and Photocatalytic Activity of Polyterthiophene Derivatives/TiO2 Nanocomposites. Materials 2014, 7, 3786-3801. https://doi.org/10.3390/ma7053786
Jamal R, Osman Y, Rahman A, Ali A, Zhang Y, Abdiryim T. Solid-State Synthesis and Photocatalytic Activity of Polyterthiophene Derivatives/TiO2 Nanocomposites. Materials. 2014; 7(5):3786-3801. https://doi.org/10.3390/ma7053786
Chicago/Turabian StyleJamal, Ruxangul, Yakupjan Osman, Adalet Rahman, Ahmat Ali, Yu Zhang, and Tursun Abdiryim. 2014. "Solid-State Synthesis and Photocatalytic Activity of Polyterthiophene Derivatives/TiO2 Nanocomposites" Materials 7, no. 5: 3786-3801. https://doi.org/10.3390/ma7053786



