Tribological Properties of Ni/Cu/Ni Coating on the Ti-6Al-4V Alloy after Annealing at Various Temperatures
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials Synthesis
2.2. Characterization after the Thermal Diffusion
2.3. Tribological Properties
3. Results and Discussion
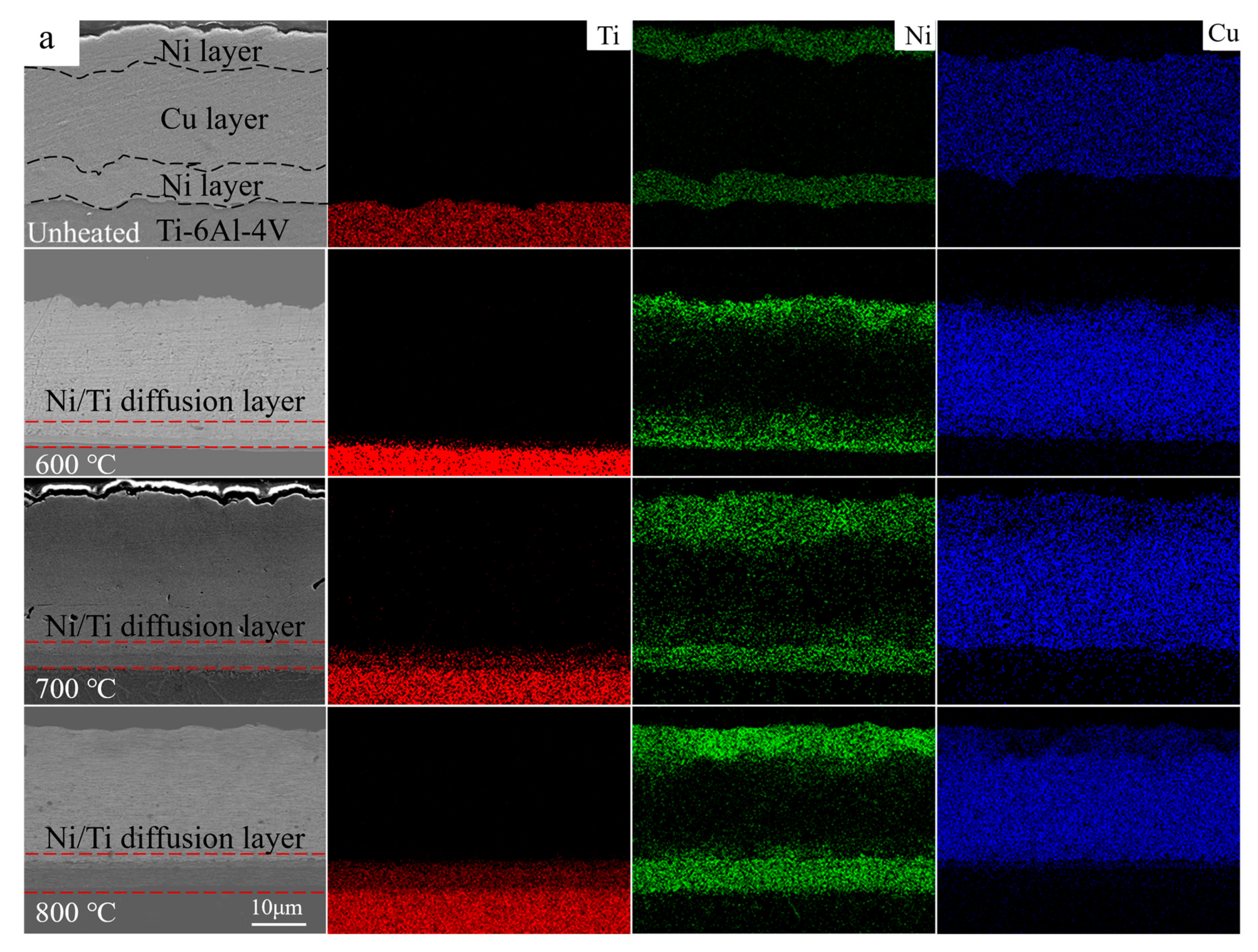
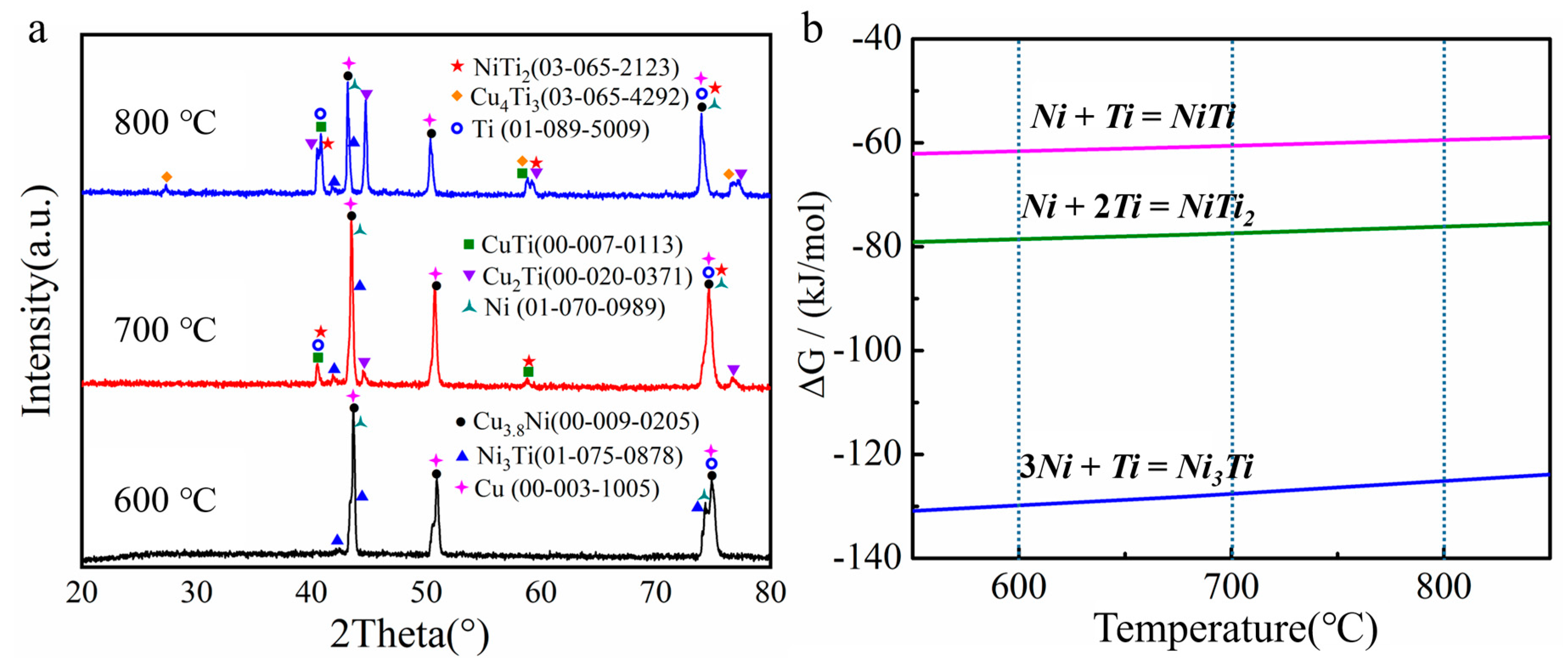
3.1. The Diffusion Behaviors and Phases Transitions
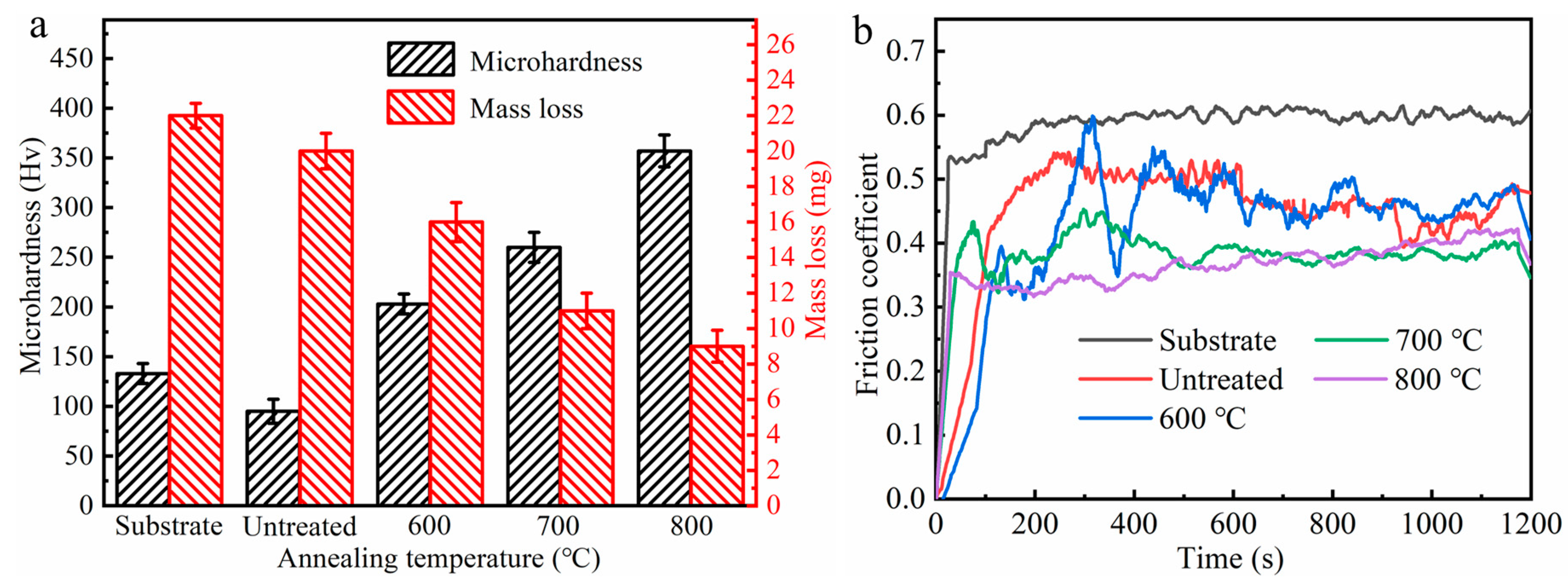
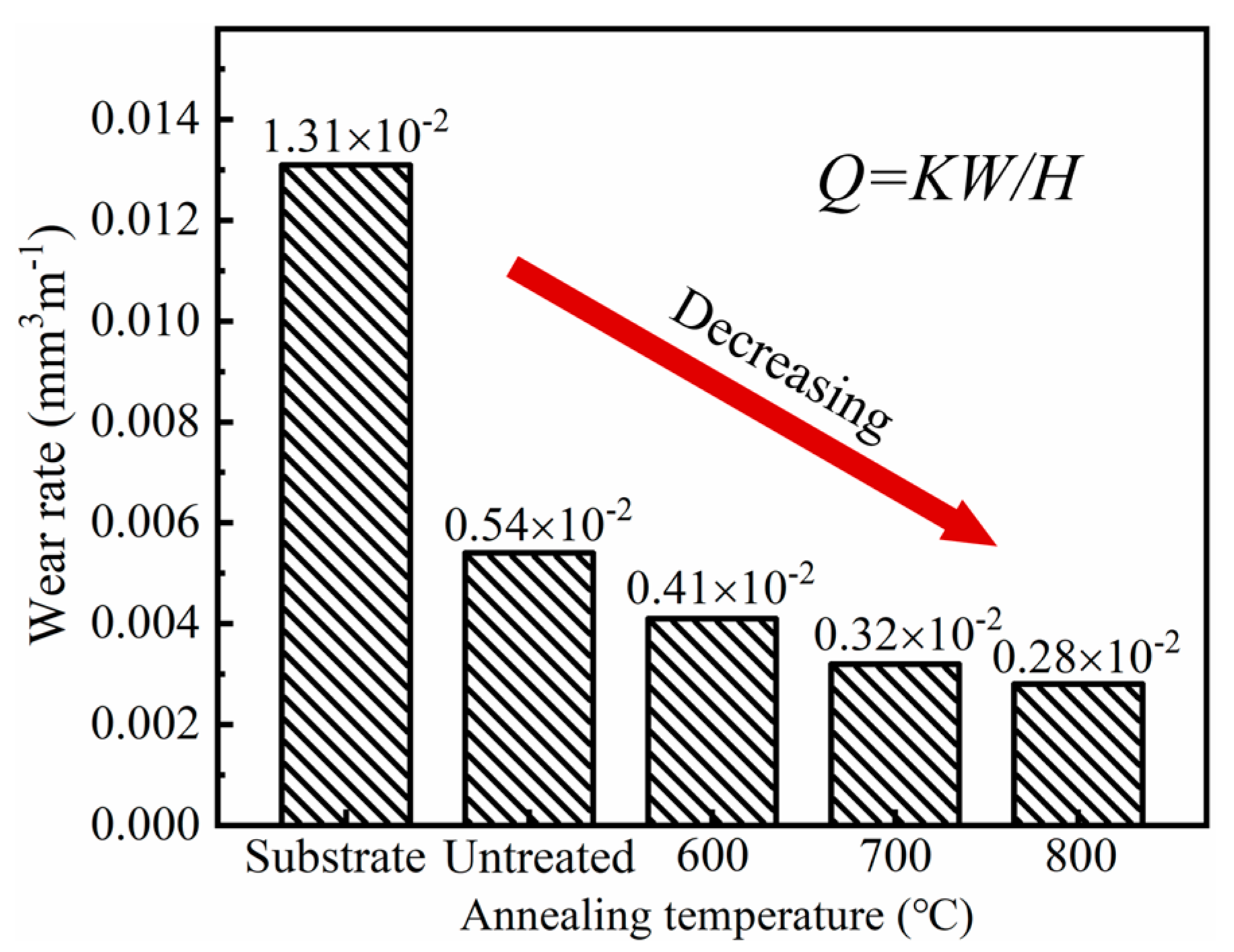
3.2. Tribological Properties of the Ni/Cu/Ni Coating on the Ti-6Al-4V Alloy
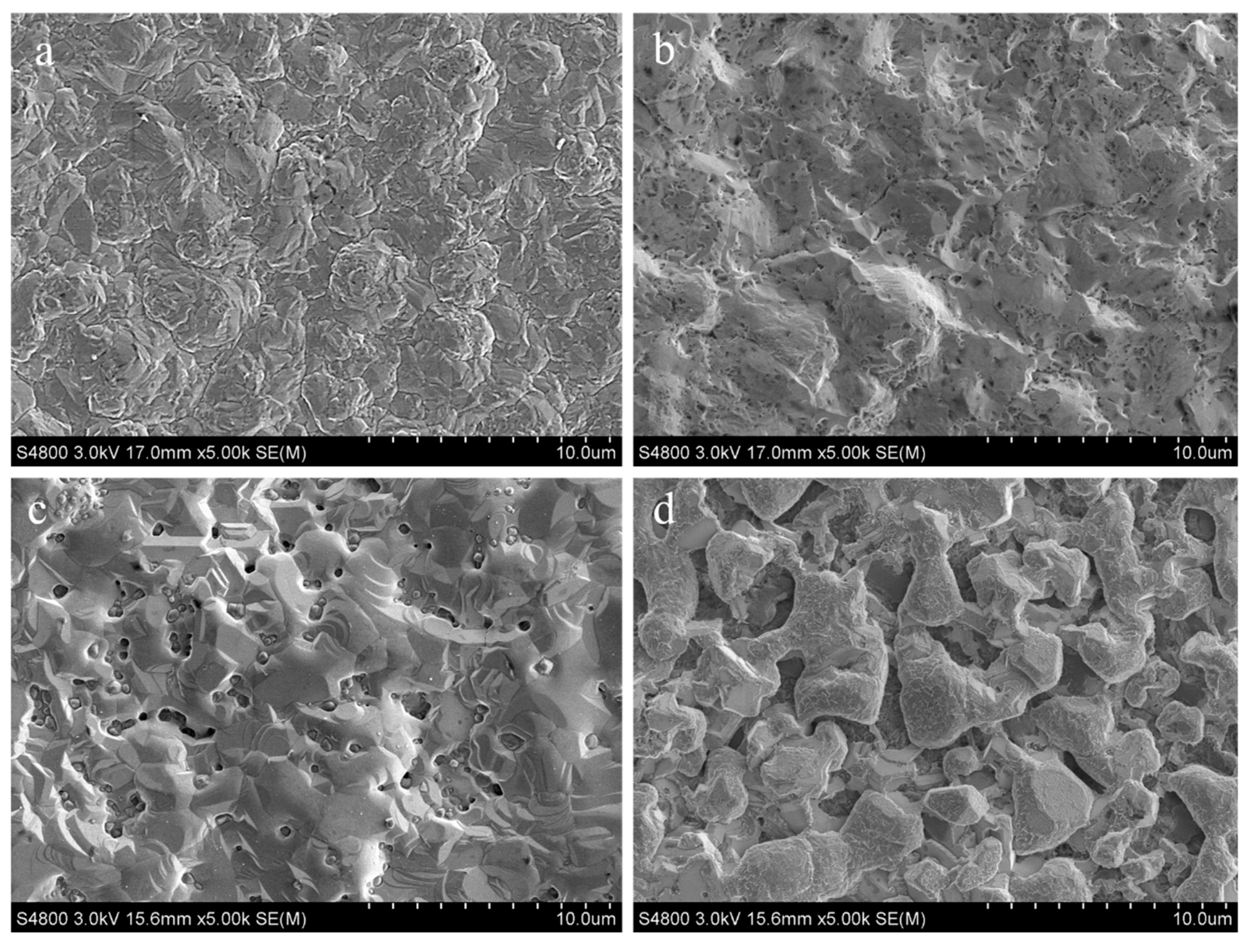
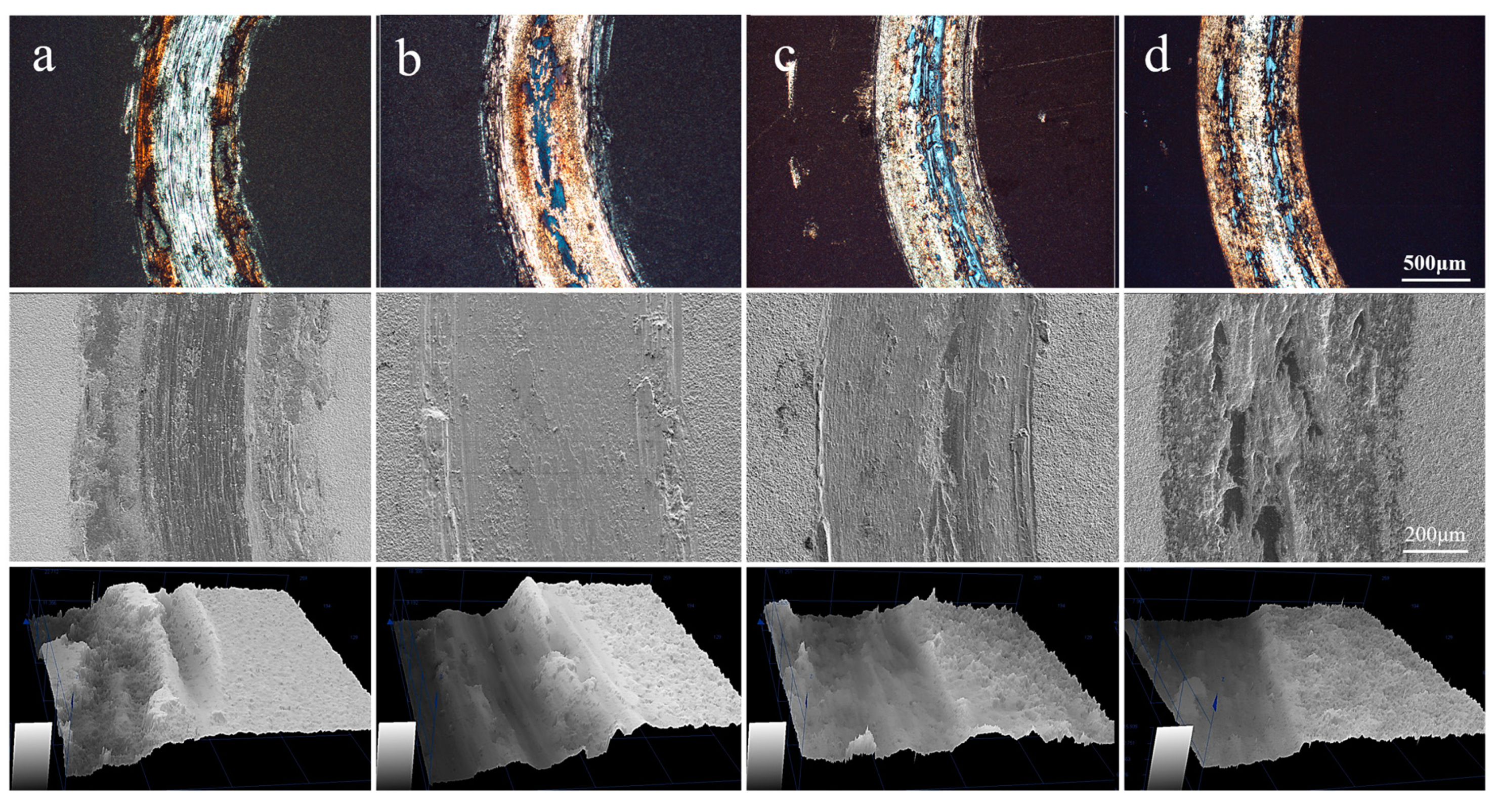
3.3. Wear Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, F.; Wang, D. Study on the Effects of Heat-treatment on Mechanical Properties of TC4 Titanium Alloy Sheets for Aviation Application. Titan. Ind. Progress. 2017, 45, 56–60. [Google Scholar]
- Chen, Y.; Liu, S.; Zhao, Y.; Liu, Q.; Zhu, L.; Song, X.; Zhang, Y.; Hao, J. Diffusion behavior and mechanical properties of Cu/Ni coating on TC4 alloy. Vaccum 2017, 143, 150–157. [Google Scholar] [CrossRef]
- Shen, Z.C.; Xie, F.Q.; Wu, X.Q.; Yao, X.F. Properties of Coating on TC4 Titanium Alloy by Copper Electroplating. China Surf. Eng. 2012, 25, 45–49. [Google Scholar]
- Zhang, Y.; Zhang, H.; Wu, J.; Wang, X. Enhanced thermal conductivity in copper matrix composites reinforced with titanium-coated diamond particles. Scr. Mater. 2011, 65, 1097–1100. [Google Scholar] [CrossRef]
- Jiang, P.; He, X.; Li, X.; Yu, L.; Wang, H. Wear resistance of a laser surface alloyed Ti–6Al–4V alloy. Surf. Coat. Technol. 2000, 130, 24–28. [Google Scholar] [CrossRef]
- Aydın, K.; Kaya, Y.; Kahraman, N.; Aydin, K. Experimental study of diffusion welding/bonding of titanium to copper. Mater. Des. 2012, 37, 356–368. [Google Scholar] [CrossRef]
- Yao, X.; Xie, F.; Wang, Y.; Wu, X. Research on Tribological and Wear Properties of Cu Coating on TC4 Alloy. Rare Met. Mater. Eng. 2012, 41, 2135–2138. [Google Scholar]
- Petrovic, S.; Peruško, D.; Mitrić, M.; Kovac, J.; Dražić, G.; Gaković, B.; Homewood, K.; Milosavljevic, M. Formation of intermetallic phase in Ni/Ti multilayer structure by ion implantation and thermal annealing. Intermetallics 2012, 25, 27–33. [Google Scholar] [CrossRef]
- Wang, F.L.; Sheng, G.M.; Deng, Y.Q. Impulse pressuring diffusion bonding of titanium to 304 stainless steel using pure Ni interlayer. Rare Met. 2016, 35, 1–6. [Google Scholar] [CrossRef]
- Hu, L.; Xue, Y.; Shi, F. Intermetallic formation and mechanical properties of Ni-Ti diffusion couples. Mater. Des. 2017, 130, 175–182. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Wang, Y.; Liu, X.; Tang, B. Microstructure and tribological behaviors of Ti6Al4V alloy treated by plasma Ni alloying. Appl. Surf. Sci. 2011, 257, 10267–10272. [Google Scholar] [CrossRef]
- Xia, Y.X.; Li, D. Effects of Post Heat-treatment of Electroplating on Plating Adhesion for TC4 Titanium Alloys. Rare Met. Mater. Eng. 2001, 30, 390–391. [Google Scholar]
- Shen, Q.; Xiang, H.; Luo, G.; Wang, C.; Li, M.; Zhang, L. Microstructure and mechanical properties of TC4/oxygen-free copper joint with silver interlayer prepared by diffusion bonding. Mater. Sci. Eng. A 2014, 596, 45–51. [Google Scholar] [CrossRef]
- Bastin, G.F.; Rieck, G.D. Diffusion in the titanium-nickel system: I. occurrence and growth of the various intermetallic compounds. Met. Mater. Trans. A 1974, 5, 1817–1826. [Google Scholar] [CrossRef]
- Puente, A.P.Y.; Dunand, D. Synthesis of NiTi microtubes via the Kirkendall effect during interdiffusion of Ti-coated Ni wires. Intermetallics 2018, 92, 42–48. [Google Scholar] [CrossRef]
- Fan, H.J.; Gösele, U.; Zacharias, M. Formation of Nanotubes and Hollow Nanoparticles Based on Kirkendall and Diffusion Processes: A Review. Small 2007, 3, 1660–1671. [Google Scholar] [CrossRef]
- He, P.; Liu, D. Mechanism of forming interfacial intermetallic compounds at interface for solid state diffusion bonding of dissimilar materials. Mater. Sci. Eng. A 2006, 437, 430–435. [Google Scholar] [CrossRef]
- Van Dal, M.; Pleumeekers, M.; Kodentsov, A.; Van Loo, F. Intrinsic diffusion and Kirkendall effect in Ni–Pd and Fe–Pd solid solutions. Acta Mater. 2000, 48, 385–396. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Q.; Sun, D.; Han, X. Co-effect of heat and direct current on growth of intermetallic layers at the interface of Ti–Ni diffusion couples. J. Alloy. Compd. 2011, 509, 1201–1205. [Google Scholar] [CrossRef]
- Lin, C.-M.; Kai, W.-Y.; Su, C.-Y.; Tsai, C.-N.; Chen, Y.-C. Microstructure and mechanical properties of Ti-6Al-4V alloy diffused with molybdenum and nickel by double glow plasma surface alloying technique. J. Alloy. Compd. 2017, 717, 197–204. [Google Scholar] [CrossRef]
- Simões, S.; Viana, F.; Ramos, A.S.; Vieira, M.T.; Vieira, M.F. Reaction zone formed during diffusion bonding of TiNi to Ti6Al4V using Ni/Ti nanolayers. J. Mater. Sci. 2013, 48, 7718–7727. [Google Scholar] [CrossRef]
- Wu, M.F.; Yang, M.; Zhang, C.; Yang, P. Research on the liquid phase spreading and microstructure of Ti/Cu eutectic reaction. Trans. China Weld. Inst. 2005, 26, 68–71. [Google Scholar]
- Akbarpour, M.; Javadhesari, S.M. Wear performance of novel nanostructured Ti-Cu intermetallic alloy as a potential material for biomedical applications. J. Alloy. Compd. 2017, 699, 882–886. [Google Scholar] [CrossRef]
- Campo, K.N.; Lima, D.D.D.; Lopes, E.S.N.; Caram, R. Erratum to: On the selection of Ti–Cu alloys for thixoforming processes: Phase diagram and microstructural evaluation. J. Mater. Sci. 2016, 51, 9912–9913. [Google Scholar] [CrossRef] [Green Version]
- Semboshi, S.; Iwase, A.; Takasugi, T. Surface hardening of age-hardenable Cu–Ti alloy by plasma carburization. Surf. Coat. Technol. 2015, 283, 262–267. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Y.; Lin, N.; Huang, X.; Hang, R.; Fan, A.; Tang, B. Microstructure, antibacterial properties and wear resistance of plasma Cu–Ni surface modified titanium. Surf. Coat. Technol. 2013, 232, 515–520. [Google Scholar] [CrossRef]
- Zhou, X.; Shen, Y. Surface morphologies, tribological properties, and formation mechanism of the Ni–CeO2 nanocrystalline coatings on the modified surface of TA2 substrate. Surf. Coat. Technol. 2014, 249, 6–18. [Google Scholar] [CrossRef]
- Fan, D.; Liu, X.; Huang, J.; Fu, R.; Chen, S.; Zhao, X. An ultra-hard and thick composite coating metallurgically bonded to Ti–6Al–4V. Surf. Coat. Technol. 2015, 278, 157–162. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, L.J.; Zhang, F.X.; Zhang, H.X. Thermal stability and hardening behavior in super elastic Ni-rich Nitinol alloys with Al addition. Mater. Sci. Eng. A 2017, 708, 514–522. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Y.; Xue, Q.; Liu, H.; Xu, T. Microstructure and tribological properties of electro deposited Ni–Co alloy deposits. Appl. Surf. Sci. 2005, 242, 326–332. [Google Scholar] [CrossRef]
- Ranganatha, S.; Venkatesha, T.; Vathsala, K. Development of electroless Ni–Zn–P/nano-TiO2 composite coatings and their properties. Appl. Surf. Sci. 2010, 256, 7377–7383. [Google Scholar] [CrossRef]
- Cai, Q.; Liu, W.; Ma, Y.; Zhu, W.; Pang, X. Effect of joining temperature on the microstructure and strength of W–steel HIP joints with Ti/Cu composite interlayer. J. Nucl. Mater. 2018, 507, 198–207. [Google Scholar] [CrossRef]
- Wang, N.; Chen, Y.-N.; Zhang, L.; Li, Y.; Liu, S.-S.; Zhan, H.-F.; Zhu, L.-X.; Zhu, S.-D.; Zhao, Y.-Q. Isothermal Diffusion Behavior and Surface Performance of Cu/Ni Coating on TC4 Alloy. Materials 2019, 12, 3884. [Google Scholar] [CrossRef] [PubMed]
- Dipak, T.W.; Chinmaya, K.P.; Ritesh, P. NiTi coating on Ti-6Al-4V alloy by TIG cladding process for improvement of wear resistance: Microstructure evolution and mechanical performances. J. Mater. Process. Tech. 2018, 262, 551–561. [Google Scholar]



| Electrolyte Composition | Operating Condition | |
|---|---|---|
| Nickel plating | nickel sulfate hexahydrate (NiSO4·6H2O): 180 g/L sodium sulfate (Na2SO4): 70 g/L magnesium sulfate (MgSO4): 30 g/L sodium chloride (NaCl): 30 g/L boric acid (H3BO3): 30 g/L | voltage: 3 V temperature: 25 °C duration: 10 min |
| copper plating | copper sulfate pentahydrate (CuSO4·5H2O): 210 g/L sodium chloride (NaCl): 20 mg/L sulfuric acid(H2SO4): 70 g/L | voltage: 0.65 V temperature: 25 °C duration: 20 min |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Wang, N.; Zhu, L.; Wu, G.; Li, L.; Yang, M.; Zhang, L.; Chen, Y. Tribological Properties of Ni/Cu/Ni Coating on the Ti-6Al-4V Alloy after Annealing at Various Temperatures. Materials 2020, 13, 847. https://doi.org/10.3390/ma13040847
Luo J, Wang N, Zhu L, Wu G, Li L, Yang M, Zhang L, Chen Y. Tribological Properties of Ni/Cu/Ni Coating on the Ti-6Al-4V Alloy after Annealing at Various Temperatures. Materials. 2020; 13(4):847. https://doi.org/10.3390/ma13040847
Chicago/Turabian StyleLuo, Jinheng, Nan Wang, Lixia Zhu, Gang Wu, Lifeng Li, Miao Yang, Long Zhang, and Yongnan Chen. 2020. "Tribological Properties of Ni/Cu/Ni Coating on the Ti-6Al-4V Alloy after Annealing at Various Temperatures" Materials 13, no. 4: 847. https://doi.org/10.3390/ma13040847




