The Coupling Effect of O2 and H2S on the Corrosion of G20 Steel in a Simulating Environment of Flue Gas Injection in the Xinjiang Oil Field
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Solution
2.2. Corrosion Experiment in Autoclave
2.3. Evaluation of Corrosion Rates
2.4. Characterization of Corrosion Products
3. Results
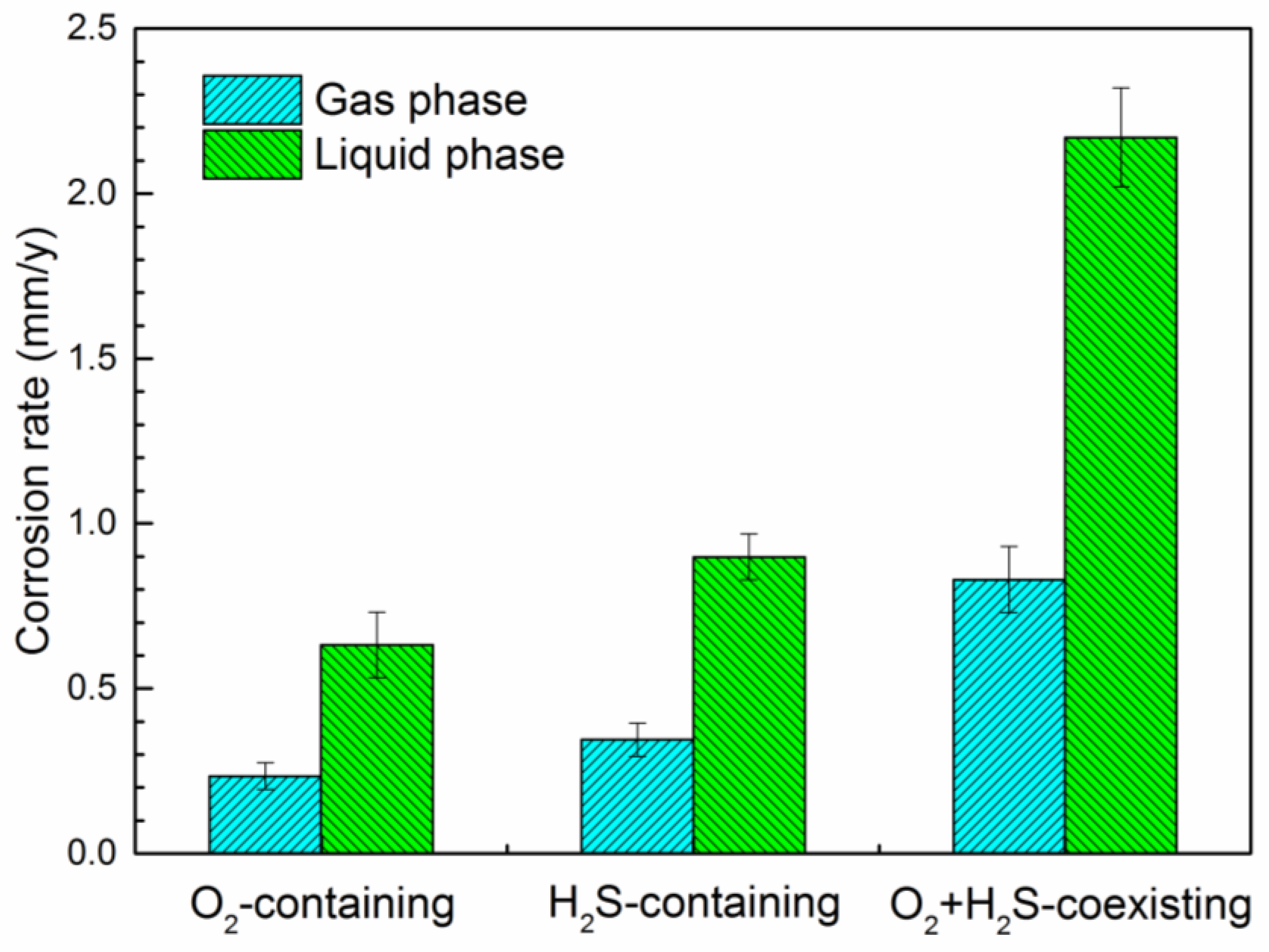
3.1. Corrosion Rates
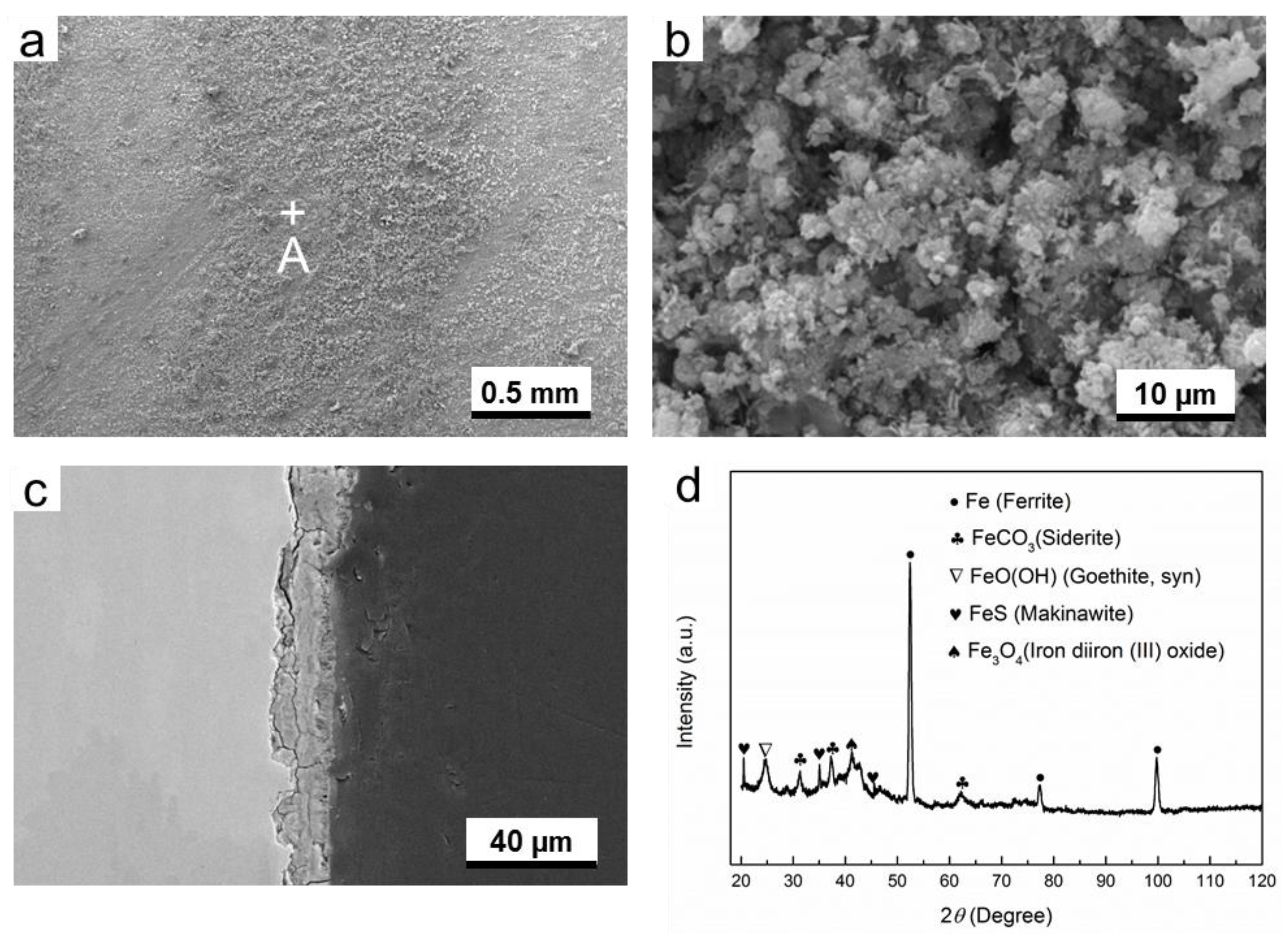
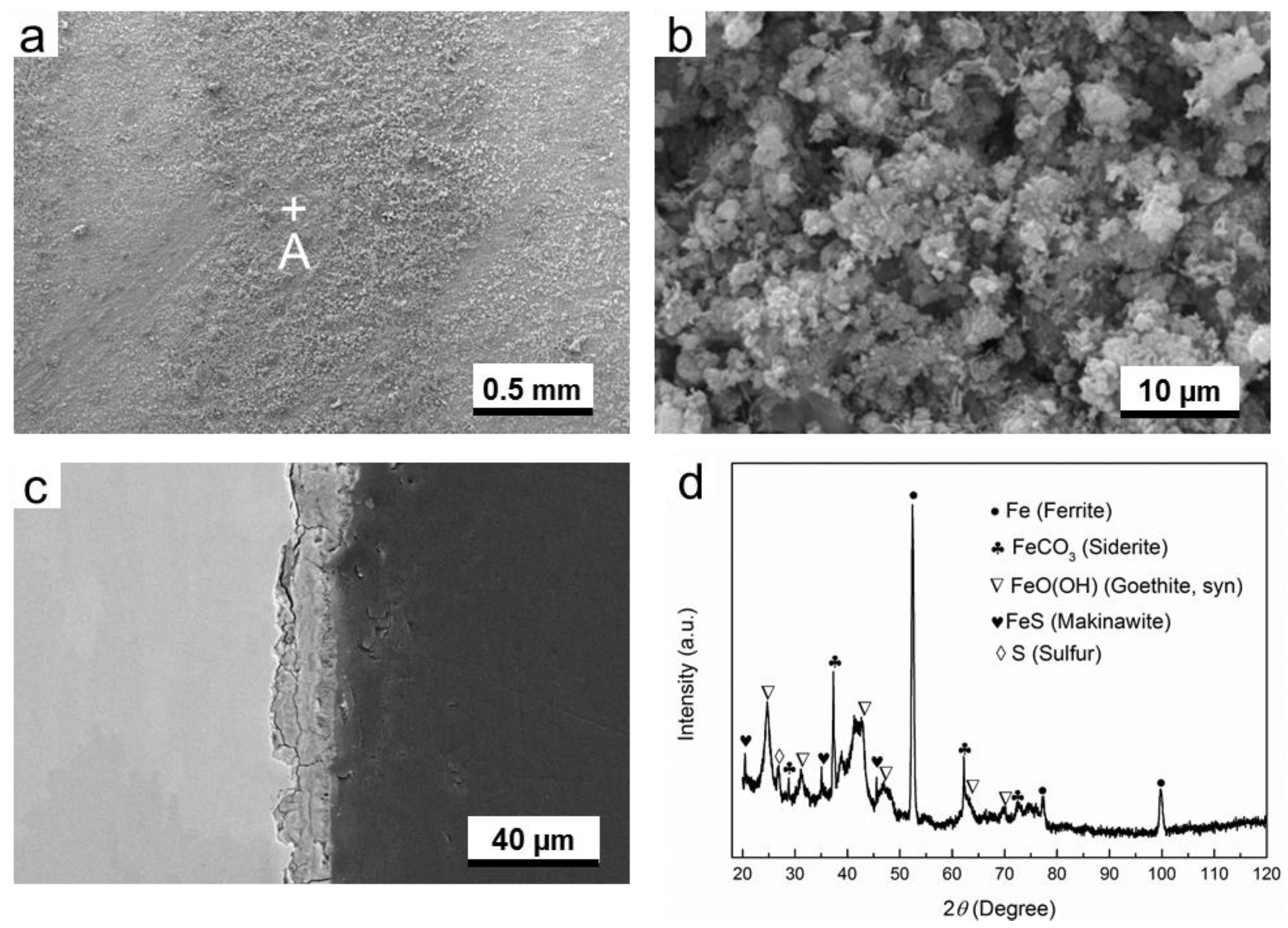
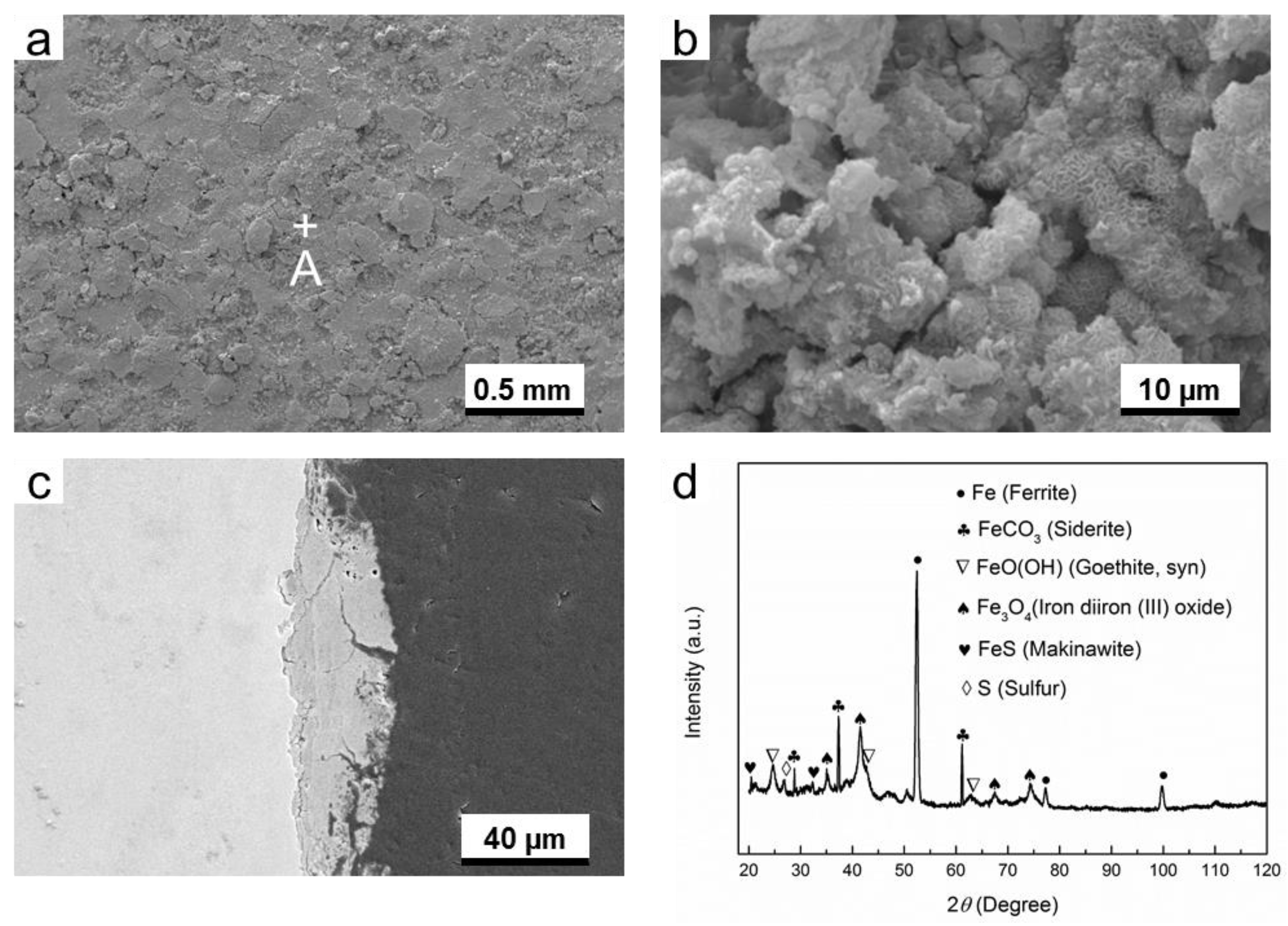
3.2. Microstucture and Composition of Corrosion Products
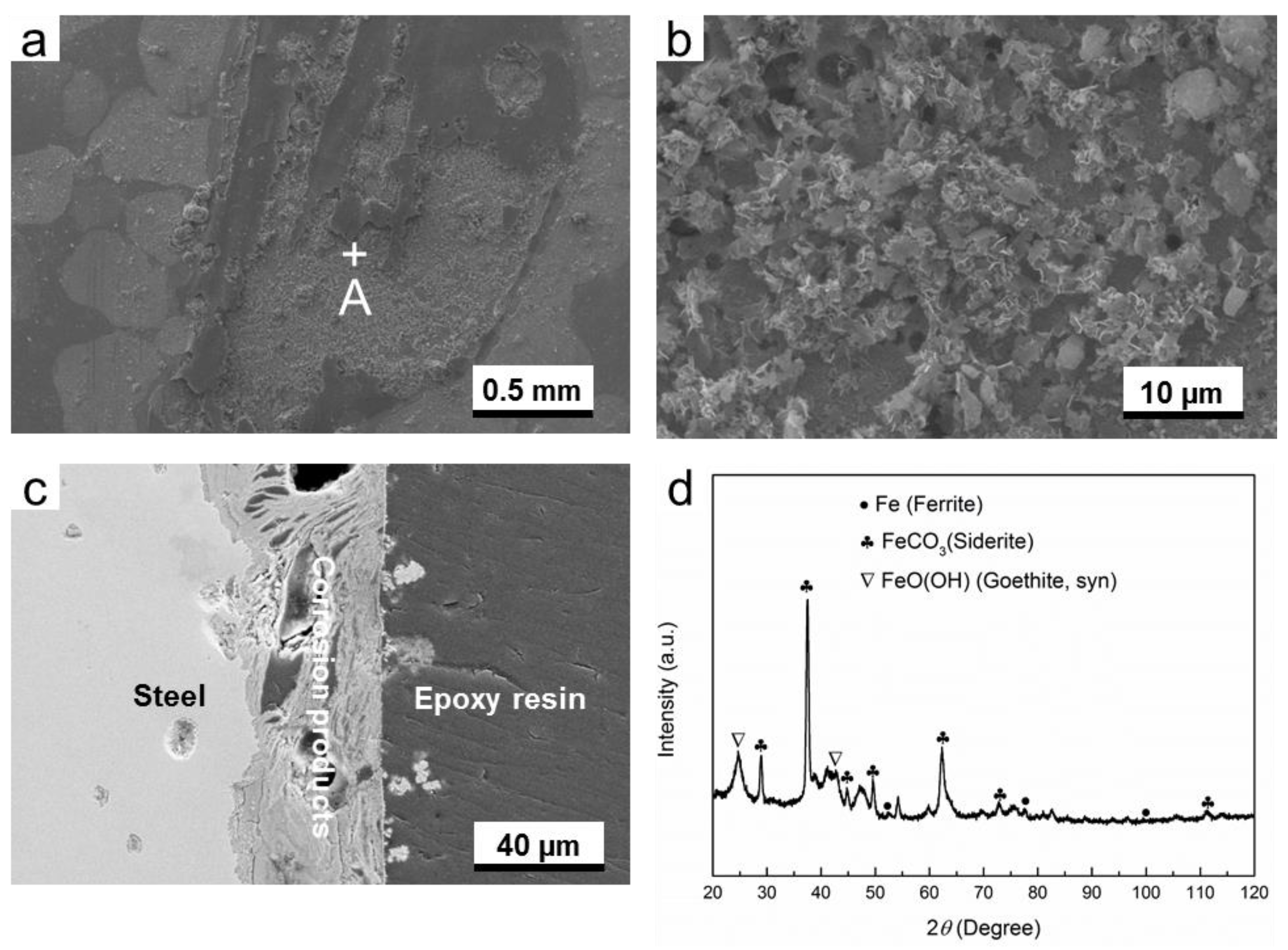
3.2.1. O2-Containing Environment
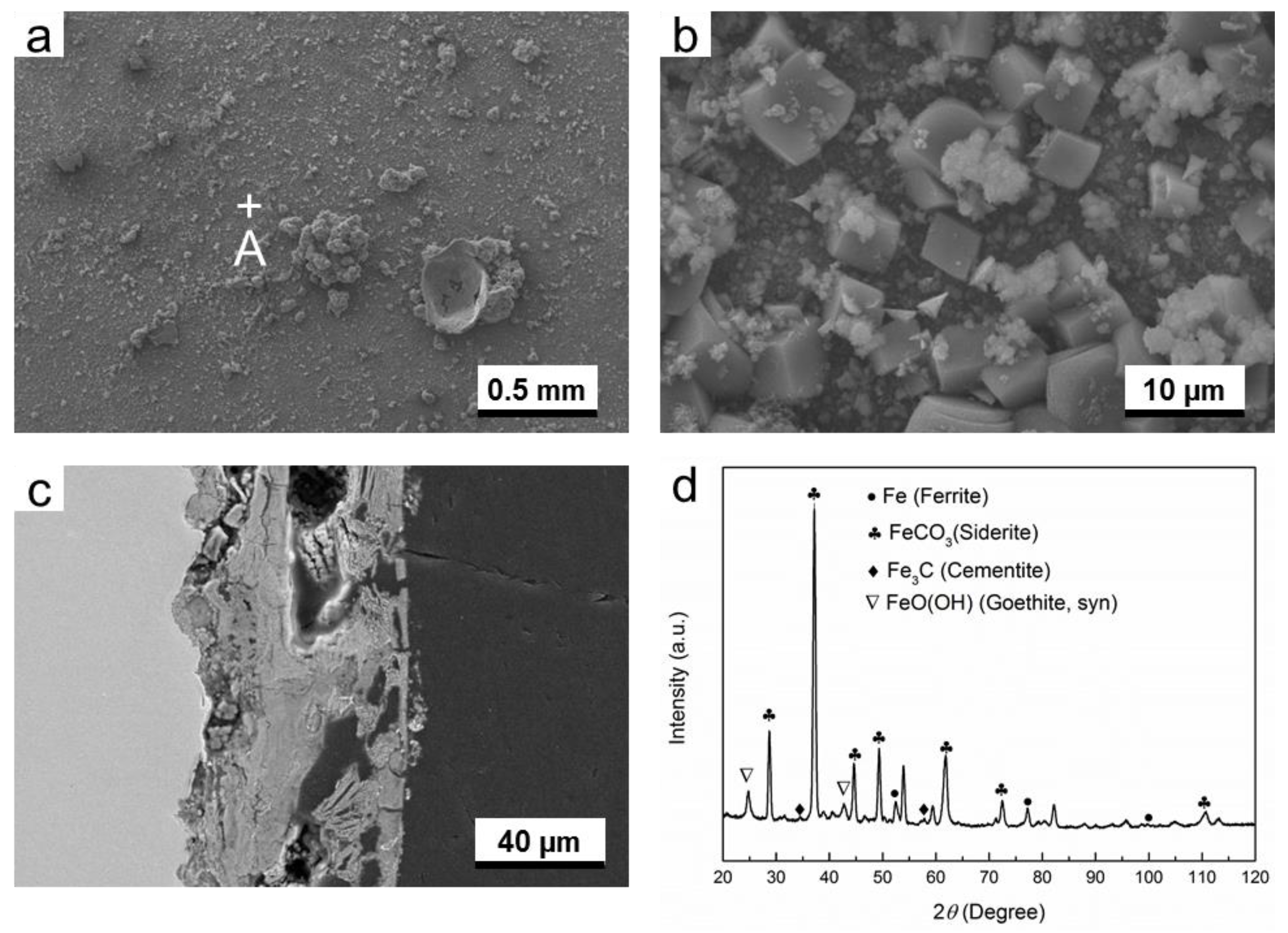
3.2.2. H2S-Containing Environment
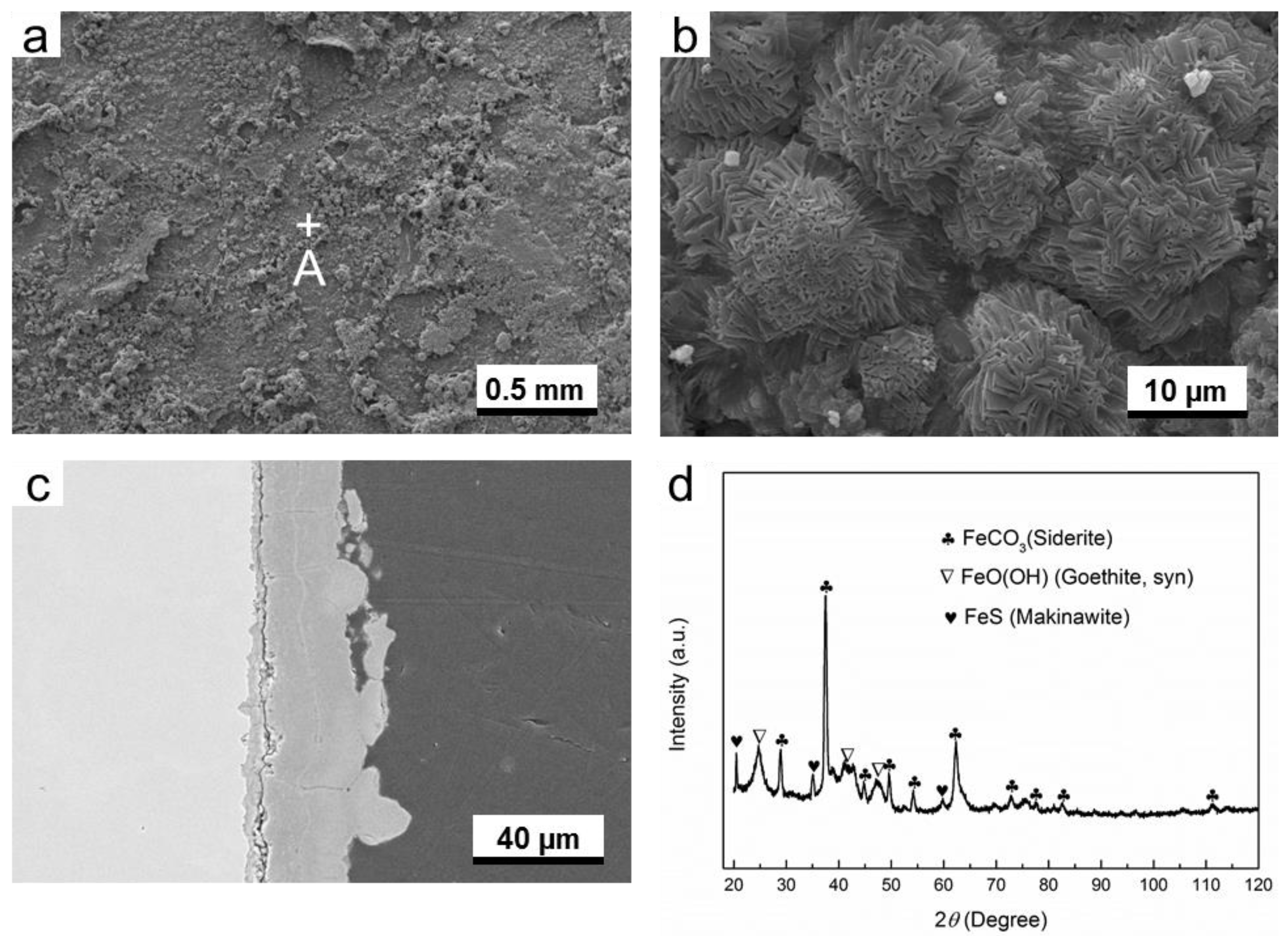
3.2.3. O2-H2S-Coexisting Environment
4. Discussion
4.1. Corrosion Reactions and the Formation Mechanisms of Corrosion Products in Flue Gas Injection Environment
4.2. The Coupling Effect of O2 and H2S on the Flue Gas Injection Corrosion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dong, M.; Huang, S. Flue gas injection for heavy oil recovery. J. Can. Petrol. Technol. 2002, 41, 44–50. [Google Scholar] [CrossRef]
- Yee, C.T.; Stroich, A. Flue gas injection into a mature SAGD steam chamber at Dover project (formerly UTF). In Proceedings of the Canadian International Petroleum Conference, Calgary, AB, Canada, 11–13 June 2002. [Google Scholar]
- Kahyarian, A.; Singer, M.; Nesic, S. Modeling of uniform CO2 corrosion of mild steel in gas transportation systems: A review. J. Nat. Gas Sci. Eng. 2016, 29, 530–549. [Google Scholar] [CrossRef]
- De Warrd, C.; Lotz, U.; Milliams, D.E. Predictive model for CO2 corrosion engineering in wet natural gas pipelines. Corrosion 1991, 47, 976–985. [Google Scholar] [CrossRef]
- Nesic, S.; Lunde, L. Carbon dioxide corrosion of carbon steel in two-phase flow. Corrosion 1994, 50, 717–727. [Google Scholar] [CrossRef]
- Gao, M.; Pang, X.; Gao, K. The growth mechanism of CO2 corrosion product films. Corros. Sci. 2011, 53, 557–568. [Google Scholar] [CrossRef]
- Li, W.; Pots, B.F.M.; Zhong, X.; Nesic, S. Inhibition of CO2 corrosion of mild steel-steel of mechanical effects of highly turbulent disturbed flow. Corros. Sci. 2017, 126, 208–226. [Google Scholar] [CrossRef]
- Heuer, J.K.; Stubbins, J.F. An XPS characterization of FeCO3 films from CO2 Corrosion. Corros. Sci. 1999, 41, 1231–1243. [Google Scholar] [CrossRef]
- Choi, Y.S.; Nesic, S.; Young, D. Effect of impurities on the corrosion behavior of CO2 transmission pipeline steel in supercritical CO2-water environments. Environ. Sci. Technol. 2010, 44, 9233–9238. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Sun, C.; Zhang, G.; Li, X.; Zhao, W.; Jiang, T.; Liu, H.; Cheng, X.; Wang, Y. Effect of O2 and H2S impurities on the corrosion behavior of X65 steel in water-saturated supercritical CO2 system. Corros. Sci. 2016, 107, 31–40. [Google Scholar] [CrossRef]
- Lin, X.; Liu, W.; Wu, F.; Xu, C.; Dou, J.; Lu, M. Effect of O2 on corrosion of 3Cr in high temperature and high pressure CO2-O2 environment. Appl. Surf. Sci. 2015, 329, 104–115. [Google Scholar] [CrossRef]
- Abelev, E.; Sellberg, J.; Ramanarayanan, T.A.; Bernasek, S.L. Effect of H2S on Fe corrosion in CO2-saturated brine. J. Mater. Sci. 2009, 44, 6767–6781. [Google Scholar] [CrossRef]
- Choi, Y.S.; Nesic, S.; Ling, S. Effect of H2S on the CO2 corrosion of carbon steel in acidic solutions. Electrochim. Aata 2011, 56, 1752–1760. [Google Scholar] [CrossRef]
- Sun, C.; Sun, J.; Wang, Y.; Lin, X.; Li, X.; Cheng, X.; Liu, H. Synergistic effect of O2, H2S and SO2 impurities on the corrosion behavior of X65 steel in water-saturated supercritical CO2 system. Corros. Sci. 2016, 107, 193–203. [Google Scholar] [CrossRef]
- Brown, J.; Graver, B.; Gulbrandsen, E.; Dugstad, A.; Morland, B. Update of DNV recommended practice RP-J202 with focus on CO2 corrosion with impurities. Energy Procedia 2014, 63, 2432–2441. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Y.; Choi, Y.-S.; Yang, Y.; Nesic, S. Corrosion of carbon steel in MDEA-based CO2 capture plants under regenerator conditons: Effect of O2 and H2S heat-stable salts. Corrosion 2015, 71, 30–37. [Google Scholar] [CrossRef]
- Clarke, S.G. The use of inhibitors (with special reference to antimony) in the selective removal of metallic coatings and rust. Trans. Electrochem. Soc. 1936, 69, 131–144. [Google Scholar] [CrossRef]
- Jin, P.; Bota, G.; Robbins, W.; Nesic, S. Analysis of oxide scales formed in the naphthenic acid corrosion of carbon steel. Energy Fuel 2016, 30, 6853–6862. [Google Scholar] [CrossRef]
- Zhang, G.A.; Zeng, Y.; Guo, X.P.; Jiang, F.; Shi, D.Y.; Chen, Z.Y. Electrochemical corrosion behavior of carbon steel under dynamic high pressure H2S/CO2 environment. Corros. Sci. 2012, 65, 37–47. [Google Scholar] [CrossRef]
- Gao, S.; Brown, B.; Young, D.; Singer, M. Formation of iron oxide and iron sulfide at high temperature and their effects on corrosion. Corros. Sci. 2018, 135, 167–176. [Google Scholar] [CrossRef]
- Fujinami, M.; Ujihira, Y. Chemical state analysis of corrosion products on a steel in H2S-N2 and H2S-O2-N2 environment by means of conversion electron mössbauer spectroscopy. Appl. Surf. Sci. 1984, 17, 276–284. [Google Scholar] [CrossRef]
- De Waard, C.; Milliams, D.E. Carbonic acid corrosion of steel. Corrosion 1975, 31, 177–181. [Google Scholar] [CrossRef]
- Nesic, S.; Postlethwaite, J.; Olsen, S. An electrochemical model for prediction of corrosion of mild steel in aqueous carbon dioxide solutions. Corrosion 1996, 52, 280–294. [Google Scholar] [CrossRef]
- Zheng, Y.; Brown, B.; Nesic, S. Electrochemical study and modeling of H2S corrosion of mild steel. Corrosion 2014, 70, 351–365. [Google Scholar] [CrossRef]
- Zheng, Y.; Ning, J.; Brown, B.; Nesic, S. Electrochemical model of mild steel corrosion in a mixed H2S/CO2 aqueous environment in the absence of protective corrosion product layers. Corrosion 2015, 71, 316–325. [Google Scholar] [CrossRef]
- Li, W.; Brown, B.; Young, D.; Nesic, S. Investigation of pseudo-passivation of mild steel in CO2 corrosion. Corrosion 2014, 70, 294–302. [Google Scholar] [CrossRef]
- Yamashita, M.; Konishi, H.; Kozakura, T.; Mizuki, J.; Uchida, H. In situ observation of initial rust formation process on carbon steel under Na2SO4 and NaCl solution films with wet/dry cycles using synchrotron radiation X-rays. Corros. Sci. 2005, 47, 2492–2498. [Google Scholar] [CrossRef]
- Ning, J.; Zheng, Y.; Young, D.; Brown, B.; Nesic, S. Thermodynamic study of hydrogen sulfide corrosion of mild steel. Corrosion 2014, 70, 375–389. [Google Scholar] [CrossRef]
- Ning, J.; Zheng, Y.; Young, D.; Brown, B.; Nesic, S. A Thermodynamic Model for the Prediction of Mild Steel Corrosion Products in an Aqueous Hydrogen Sulfide Environment. Corrosion 2015, 71, 945–960. [Google Scholar] [CrossRef]
- Craig, B.D. The nature of iron sulfides formed on steel in an H2S-O2 environment. Corrosion 1979, 35, 136–138. [Google Scholar] [CrossRef]
- Bourdoiseau, J.A.; Jeannin, M.; Sabot, R.; Rémazeiles, C.; Refait, P. Characterisation of macknawite by Raman spectroscopy: Effects of crystallization, drying and oxidation. Corros. Sci. 2008, 50, 3247–3255. [Google Scholar] [CrossRef]
- Smith, S.N.; Pacheco, J.L. Prediction of corrosion in slightly sour environments. In Proceedings of the NACE International’s Annual Conference and Exposition, Denver, CO, USA, 7–11 April 2002. [Google Scholar]
- Steudel, R. Mechanism for the formation of elemental sulfur from aqueous sulfide in chemical and microbiological desulfurization processed. Ind. Eng. Chem. Res. 1996, 35, 1417–1423. [Google Scholar] [CrossRef]
- Dugstad, A.; Halseid, M.; Morland, B. Experimental techniques used for corrosion testing in dense phase CO2 with flue gas impurities. In Proceedings of the NACE International’s Annual Conference and Exposition, San Antonio, TX, USA, 9–13 March 2014. [Google Scholar]
- Fang, H.; Young, D.; Nesic, S. Corrosion of Mild Steel in the Presence of Elemental Sulfur. In Proceedings of the NACE International’s Annual Conference and Exposition, New Orleans, LA, USA, 16–20 March 2008. [Google Scholar]
- Macdonald, D.D.; Roberts, B.; Hyne, J.B. The corrosion of carbon steel by wet elemental sulfur. Corros. Sci. 1978, 18, 411–425. [Google Scholar] [CrossRef]
- Boden, P.J.; Maldonado-Zagal, S.B. Hydrolysis of elemental sulfur in water and its effects on the corrosion of mild steel. Br. Corros. J. 1982, 17, 116–120. [Google Scholar] [CrossRef]


| Ions | Concentration (mg/L) |
|---|---|
| Ca2+ | 125 |
| Mg2+ | 35 |
| Cl− | 3940 |
| HCO3− | 1894 |
| SO42− | 134 |
| Na+ + K+ | 3126 |
| No. | Temperature (°C) | Pressure (MPa) | CO2 (MPa) | O2 (MPa) | H2S (ppmv) | Gas/Liquid Phase |
|---|---|---|---|---|---|---|
| 1 | 60 | 15 | 2.25 | 0.21 | 0 | Gas |
| 2 | 60 | 15 | 2.25 | 0 | 600 | Gas |
| 3 | 60 | 15 | 2.25 | 0.21 | 600 | Gas |
| 4 | 60 | 15 | 2.25 | 0.21 | 0 | Liquid |
| 5 | 60 | 15 | 2.25 | 0 | 600 | Liquid |
| 6 | 60 | 15 | 2.25 | 0.21 | 600 | Liquid |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, X.; Wang, Y.; Liang, J.; Chen, L.; Song, X. The Coupling Effect of O2 and H2S on the Corrosion of G20 Steel in a Simulating Environment of Flue Gas Injection in the Xinjiang Oil Field. Materials 2018, 11, 1635. https://doi.org/10.3390/ma11091635
Zhong X, Wang Y, Liang J, Chen L, Song X. The Coupling Effect of O2 and H2S on the Corrosion of G20 Steel in a Simulating Environment of Flue Gas Injection in the Xinjiang Oil Field. Materials. 2018; 11(9):1635. https://doi.org/10.3390/ma11091635
Chicago/Turabian StyleZhong, Xiankang, Yanran Wang, Jianjun Liang, Long Chen, and Xiaoqin Song. 2018. "The Coupling Effect of O2 and H2S on the Corrosion of G20 Steel in a Simulating Environment of Flue Gas Injection in the Xinjiang Oil Field" Materials 11, no. 9: 1635. https://doi.org/10.3390/ma11091635




