Preparation of Hybrid Nanoparticle Nucleating Agents and Their Effects on the Crystallization Behavior of Poly(ethylene terephthalate)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.2.1. Preparation of Silicon Chloride
2.2.2. Preparation of SiO2-diethylene glycol/SiO2-triethylene glycol/SiO2-tetraethylene glycol
2.2.3. Preparation of SiO2-diethylene glycol-LMPET/SiO2-triethylene glycol-LMPET/SiO2-tetraethylene glycol-LMPET
2.2.4. Preparation of PET/PET-3, PET/PET-4, PET/PET-5
2.3. Characterizations
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fielding-Russell, G.S.; Pillai, P.S. A Study of the Crystallization of Γ-Irradiated Polyethylene Terephthalate Using Differential Scanning Calorimetry. Kolloid-Zeitschriftand ZeitschriftfürPolymere 1972, 250, 2–8. [Google Scholar] [CrossRef]
- Scheirs, J.; Long, T.E. Modern Polyesters: Chemistry and Technology of Polyesters and Copolyesters; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Ge, C.; Shi, L.; Yang, H.; Tang, S. Nonisothermal Melt Crystallization Kinetics of Poly(Ethylene Terephthalate)/Barite Nanocomposites. Polym. Compos. 2010, 30, 1504–1514. [Google Scholar] [CrossRef]
- Rahman, M.H.; Nandi, A.K. On the Crystallization Mechanism of Poly(Ethylene Terepthalate) in Its Blends with Poly(Vinylidene Fluoride). Polymer 2002, 43, 6863–6870. [Google Scholar] [CrossRef]
- Ke, Y.C.; Tian, B.W.; Xia, Y.F. The Nucleation, Crystallization and Dispersion Behavior of Pet–Monodisperse SiO2 Composites. Polymer 2007, 48, 3324–3336. [Google Scholar] [CrossRef]
- Yang, H.; Caydamli, Y.; Fang, X.; Tonelli, A.E. Crystallization Behaviors of Modified Poly(Ethylene Terephthalate) and Their Self-Nucleation Ability. Macromol. Mater. Eng. 2015, 300, 403–413. [Google Scholar] [CrossRef]
- Moyer, D.C. Society of Plastics Engineers. In Proceedings of the Global Plastics Environmental Conference 2010: Sustainability and Recycling, Orlando, FL, USA, 8–10 March 2010. [Google Scholar]
- Belke, R.E.; Cabasso, I. Poly(Vinylidene Fluoride)/Poly(Vinyl Acetate) Miscible Blends: 1. Thermal Analysis and Spectroscopic (FTIR) Characterization. Polymer 1988, 29, 1831–1842. [Google Scholar] [CrossRef]
- Zweifel, H. Plastics Additives Handbook; Hanser Publications: Cincinnati, OH, USA, 1987. [Google Scholar]
- Iyer, K.A.; Torkelson, J.M. Importance of Superior Dispersion versus Filler Surface Modification Inproducing Robust Polymer Nanocomposites: The Example of Polypropylene/Nanosilica Hybrids. Polymer 2015, 68, 147–157. [Google Scholar] [CrossRef]
- Xanthos, M.; Baltzis, B.C.; Hsu, P.P. Effects of Carbonate Salts on Crystallization Kinetics and Properties of Recycled Poly(Ethylene Terephthalate). J. Appl. Polym. Sci. 2015, 64, 1423–1435. [Google Scholar] [CrossRef]
- Aoyama, S.; Park, Y.T.; Ougizawa, T.; Macosko, C.W. Melt Crystallization of Poly(Ethylene Terephthalate): Comparing Addition ofGraphene vs. Carbon Nanotubes. Polymer 2014, 55, 2077–2085. [Google Scholar] [CrossRef]
- Borjanović, V.; Bistričić, L.; Pucić, I.; Mikac, L.; Slunjski, R.; Jakšić, M.; McGuire, G.; Stanković, A.T.; Shenderova, O. Proton-Radiation Resistance of Poly(Ethylene Terephthalate)–Nanodiamond–GrapheneNanoplateletNanocomposites. J. Mater. Sci. 2016, 51, 1000–1016. [Google Scholar] [CrossRef]
- Han, K.Q.; Mu-Huo, Y.U. Crystalline Behavior of Pet/Nano-Meter TiO2 Composite. Chin. Plast. Ind. 2004, 11, 014. [Google Scholar]
- Georgiev, G.; Cebe, P.; Capel, M. Effects of Nematic Polymer Liquid Crystal on Crystallization and Structure of Pet/Vectra Blends. J. Mater. Sci. 2005, 40, 1141–1152. [Google Scholar] [CrossRef]
- Sun, W.; Wang, Y.; Tang, J.; Huang, Z.; Liu, J.; Huang, L.; Wang, Y.; Jiao, J.; Wang, W.; Zheng, H. Effect of Nano-SiO2 Modified by Block Oligomer on the Crystallization Property of Pet. Mater. Rev. 2015, 29, 84–88. [Google Scholar]
- Antoniadis, G.; Paraskevopoulos, K.M.; Bikiaris, D.; Chrissafis, K. Non-Isothermal Crystallization Kinetic of Poly(Ethylene Terephthalate)/Fumed Silica (Pet/SiO2 ) Prepared by in Situ Polymerization. Thermochim. Acta 2010, 510, 103–112. [Google Scholar] [CrossRef]
- Cai, D.; Zhang, Y.; Chen, Y. Effect of Organic Modification of Sioon Non-Isothermal Crystallization of Pet in Pet/Sionanocomposites. Iran. Polym. J. 2007, 16, 851–859. [Google Scholar]
- Lu, H.; Wang, H.; Zheng, A.; Xiao, H. Hybrid Poly(Ethylene Terephthalate)/Silica Nanocomposites Prepared by in-Situ Polymerization. Polym. Compos. 2010, 28, 42–46. [Google Scholar] [CrossRef]
- Wang, N.; Qiao, S.R.; Yang, B. Study on the Dispersivity of Nano-SiO2 in Pet and Crystalline Properties of Pet/Nanometer SiO2 Composites. Mater. Rev. 2006, 200–203. [Google Scholar]
- Chen, K.; Zhao, Y.; Yuan, X. Grafting of Poly(Lauryl Acrylate) onto Nano-Silica by ‘Click Chemistry’. Chem. Res. Chin. Univ. 2014, 30, 339–342. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.Y.; Tang, J.G.; Yang, B. Preparation and Properties of Pet-Peg-SiO2 Co-Polymerized Hybrid Nanoblocks. J. Mater. Eng. 2008, 36, 101–105. [Google Scholar] [CrossRef]
- Badia, J.D.; Strömberg, E.; Karlsson, S.; Ribes-Greus, A. The Role of Crystalline, Mobile Amorphous and Rigid Amorphous Fractions in the Performance of Recycled Poly (Ethylene Terephthalate) (Pet). Polym. Degrad. Stab. 2012, 97, 98–107. [Google Scholar] [CrossRef]
- Cashman, K.V. Relationship between Plagioclase Crystallization and Cooling Rate in Basaltic Melts. Contrib. Mineral. Petrol. 1993, 113, 126–142. [Google Scholar] [CrossRef]
- Criado, J.M.; Ortega, A. Non-Isothermal Crystallization Kinetics of Metal Glasses: Simultaneous Determination of Both the Activation Energy and the Exponent N of the Jma Kinetic Law. Acta Metall. 1987, 35, 1715–1721. [Google Scholar] [CrossRef]
- Mandelkern, L. Crystallization of Polymers; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Jeziorny, A. Parameters Characterizing the Kinetics of the Non-Isothermal Crystallization of Poly(Ethylene Terephthalate) Determined by D.S.C. Polymer 1978, 19, 1142–1144. [Google Scholar] [CrossRef]
- Dosière, M. Crystallization of Polymers; McGraw-Hill: New York, NY, USA, 1964. [Google Scholar]
- Li, J.; Liang, Q.; Lu, A.; Luo, S.; Liu, T.; Liu, Y.; Shi, D.; Liu, T. The Crystallization Behaviors of Copolymeric Flame-Retardant Poly(Ethylene Terephthalate) with Organophosphorus. Polym. Compos. 2013, 34, 867–876. [Google Scholar] [CrossRef]
- Wellen, R.M.R.; Rabello, M.S. The Kinetics of Isothermal Cold Crystallization and Tensile Properties of Poly(Ethylene Terephthalate). J. Mater. Sci. 2005, 40, 6099–7104. [Google Scholar] [CrossRef]






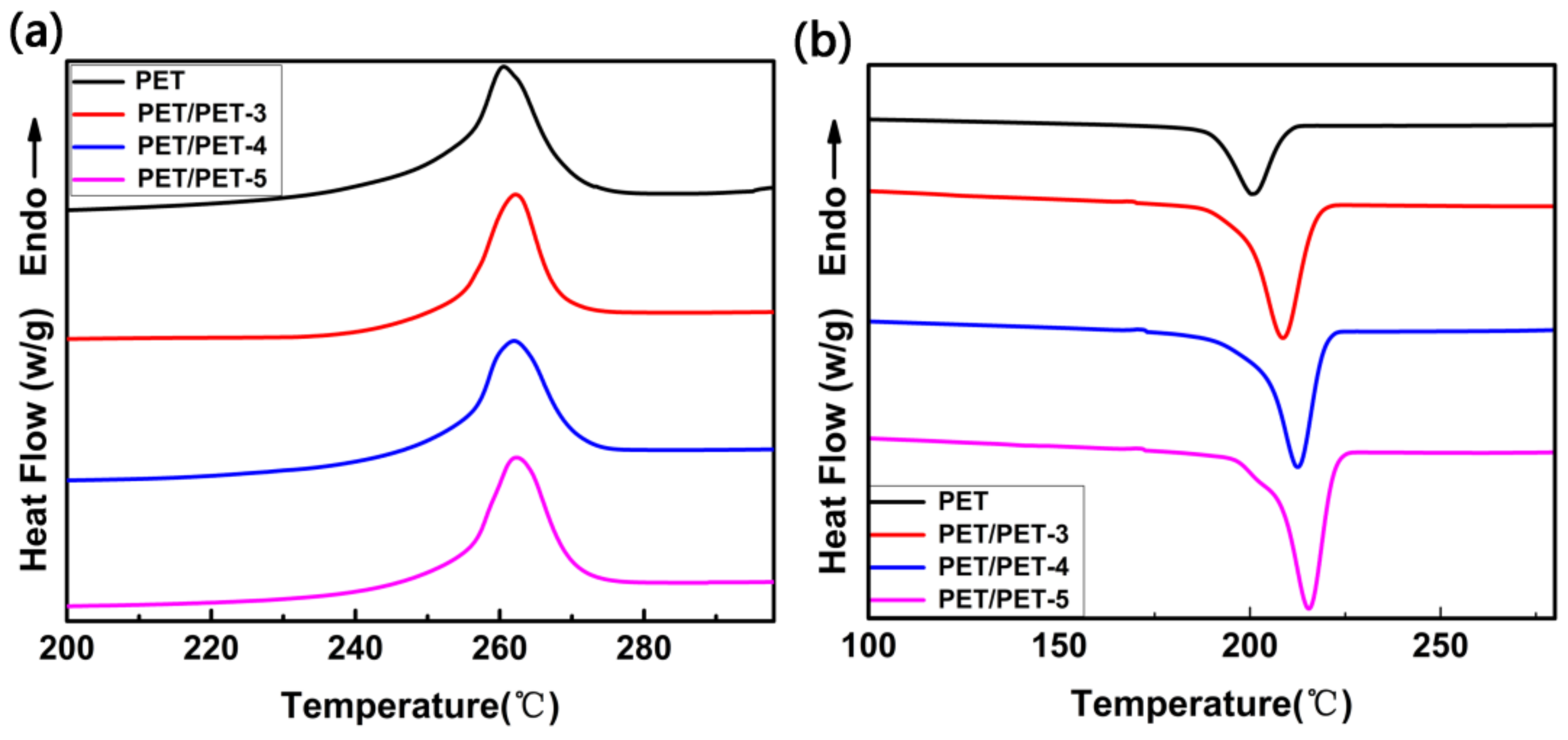



| Sample | Tc (°C) | (J·g−1) | ∆Hc (J·g−1) | Xc (%) |
|---|---|---|---|---|
| PET | 199.2 | 32.1 | 40.1 | 23 |
| PET/PET-3 | 208.8 | 37.1 | 42.3 | 27 |
| PET/PET-4 | 212.5 | 38.9 | 43.8 | 29 |
| PET/PET-5 | 216.1 | 40.9 | 45.5 | 30 |
| Samples | β (°C/min) | n | Zc |
|---|---|---|---|
| PET | −5 | 3.9 | 0.24 |
| −10 | 3.3 | 0.79 | |
| −15 | 3.2 | 0.87 | |
| −20 | 3.1 | 1.01 | |
| PET/PET-3 | −5 | 2.9 | 0.52 |
| −10 | 3.2 | 0.83 | |
| −15 | 3.1 | 0.98 | |
| −20 | 2.9 | 1.10 | |
| PET/PET-4 | −5 | 2.9 | 0.57 |
| −10 | 3.0 | 0.90 | |
| −15 | 3.1 | 1.04 | |
| −20 | 3.1 | 1.13 | |
| PET/PET-5 | −5 | 3.1 | 0.68 |
| −10 | 3.0 | 0.97 | |
| −15 | 3.3 | 1.08 | |
| −20 | 3.2 | 1.23 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Z.; Wang, Y.; Wang, J.; Wang, S.; Zhuang, H.; Liu, J.; Huang, L.; Wang, Y.; Wang, W.; Belfiore, L.A.; et al. Preparation of Hybrid Nanoparticle Nucleating Agents and Their Effects on the Crystallization Behavior of Poly(ethylene terephthalate). Materials 2018, 11, 587. https://doi.org/10.3390/ma11040587
Han Z, Wang Y, Wang J, Wang S, Zhuang H, Liu J, Huang L, Wang Y, Wang W, Belfiore LA, et al. Preparation of Hybrid Nanoparticle Nucleating Agents and Their Effects on the Crystallization Behavior of Poly(ethylene terephthalate). Materials. 2018; 11(4):587. https://doi.org/10.3390/ma11040587
Chicago/Turabian StyleHan, Zhenzhen, Yao Wang, Jiuxing Wang, Shichao Wang, Hongwei Zhuang, Jixian Liu, Linjun Huang, Yanxin Wang, Wei Wang, Laurence A. Belfiore, and et al. 2018. "Preparation of Hybrid Nanoparticle Nucleating Agents and Their Effects on the Crystallization Behavior of Poly(ethylene terephthalate)" Materials 11, no. 4: 587. https://doi.org/10.3390/ma11040587





