A Study on Corrosion Inhibitor for Mild Steel in Ethanol Fuel Blend
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Immersion Test
2.3. Electrochemical Test
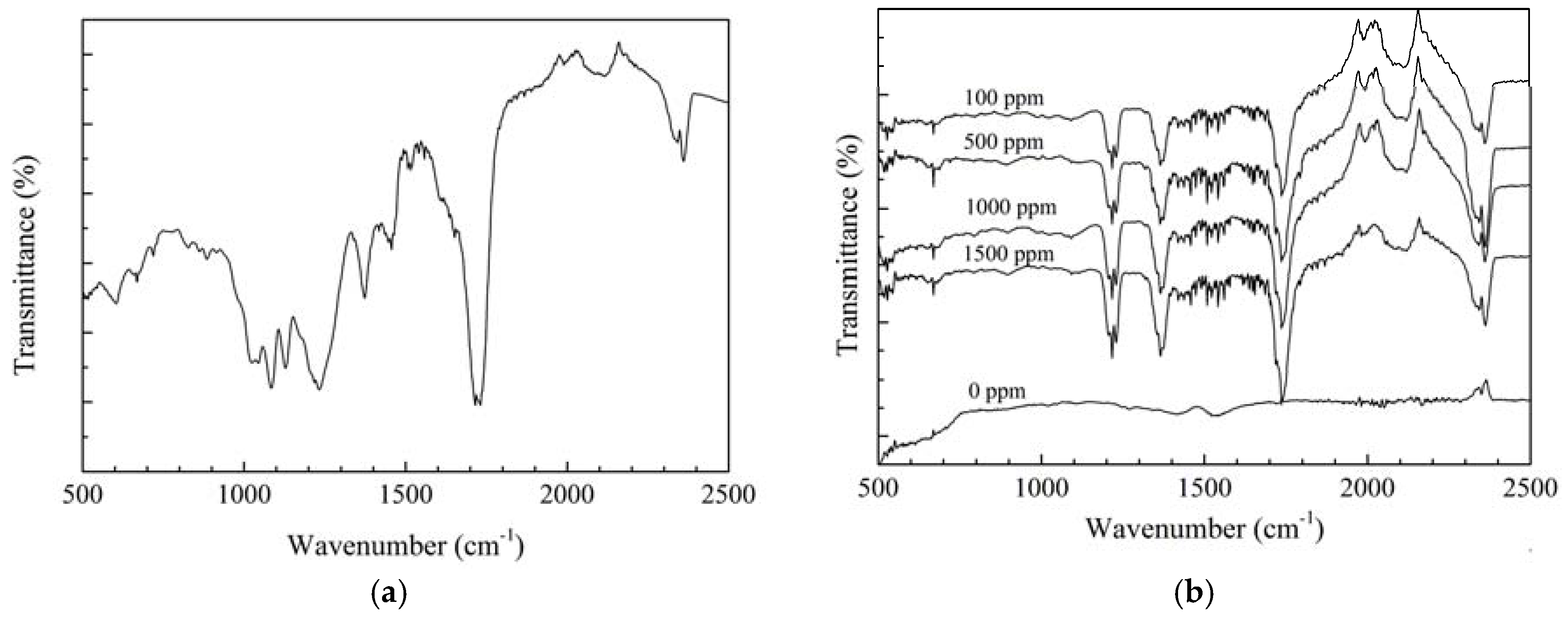
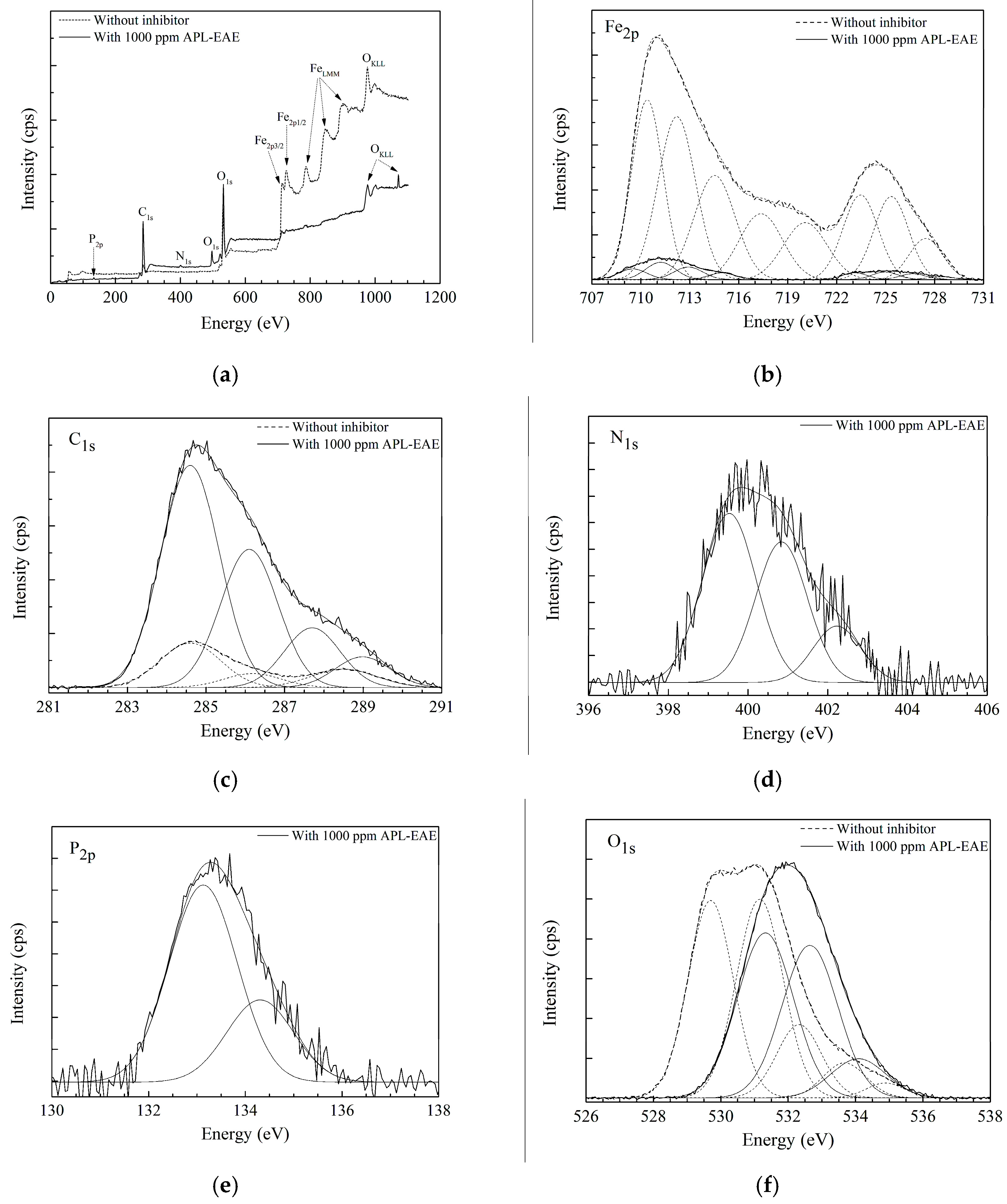
2.4. Surface Analysis
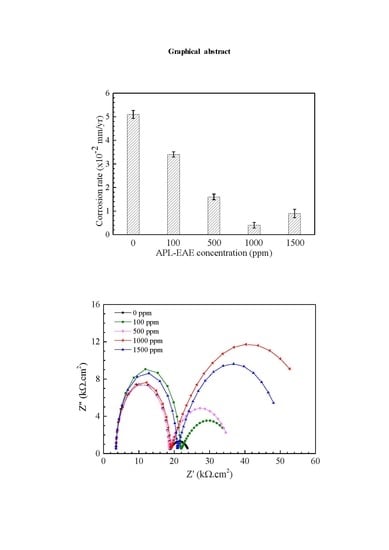
3. Results and Discussion
3.1. Surface Analysis
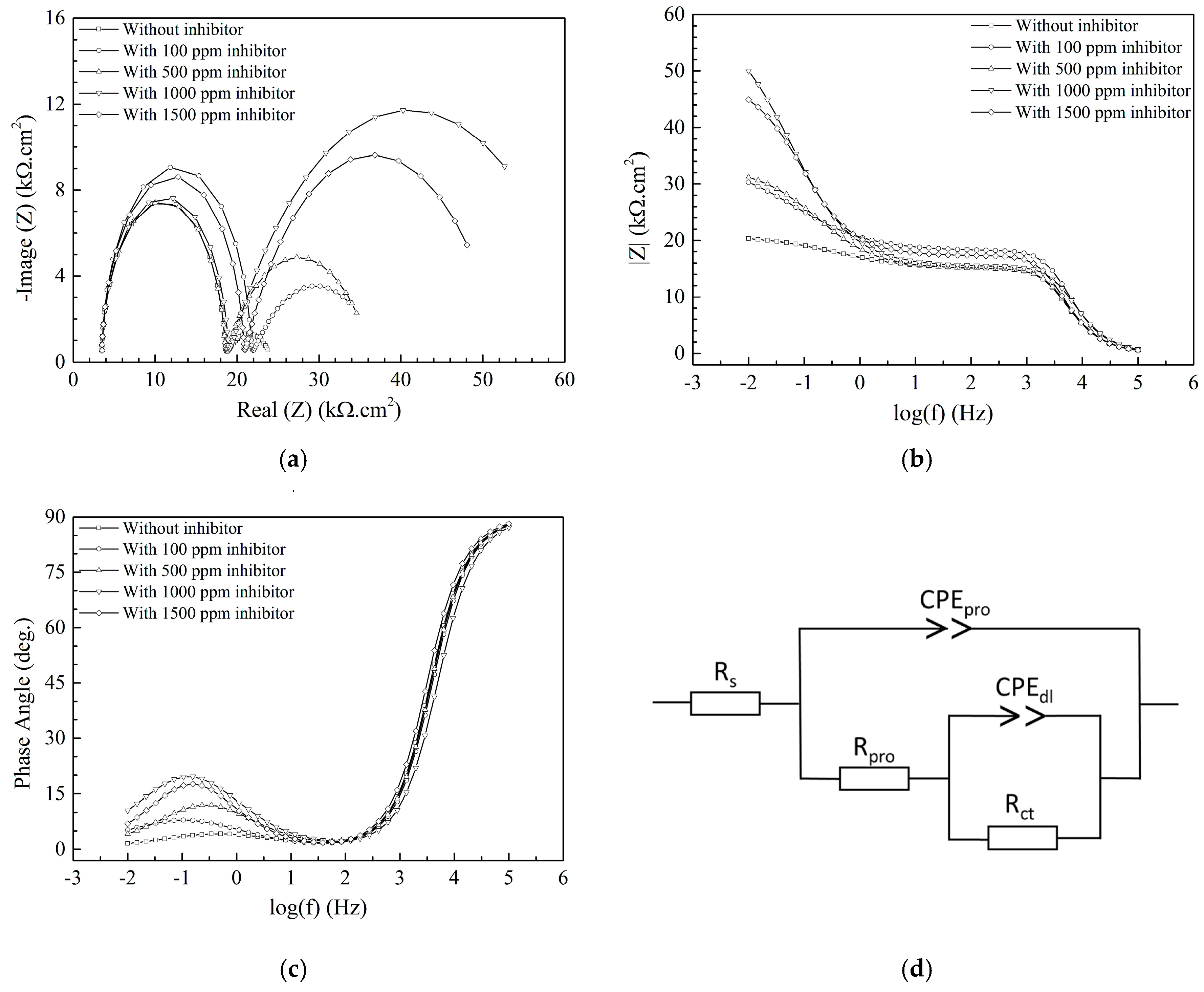
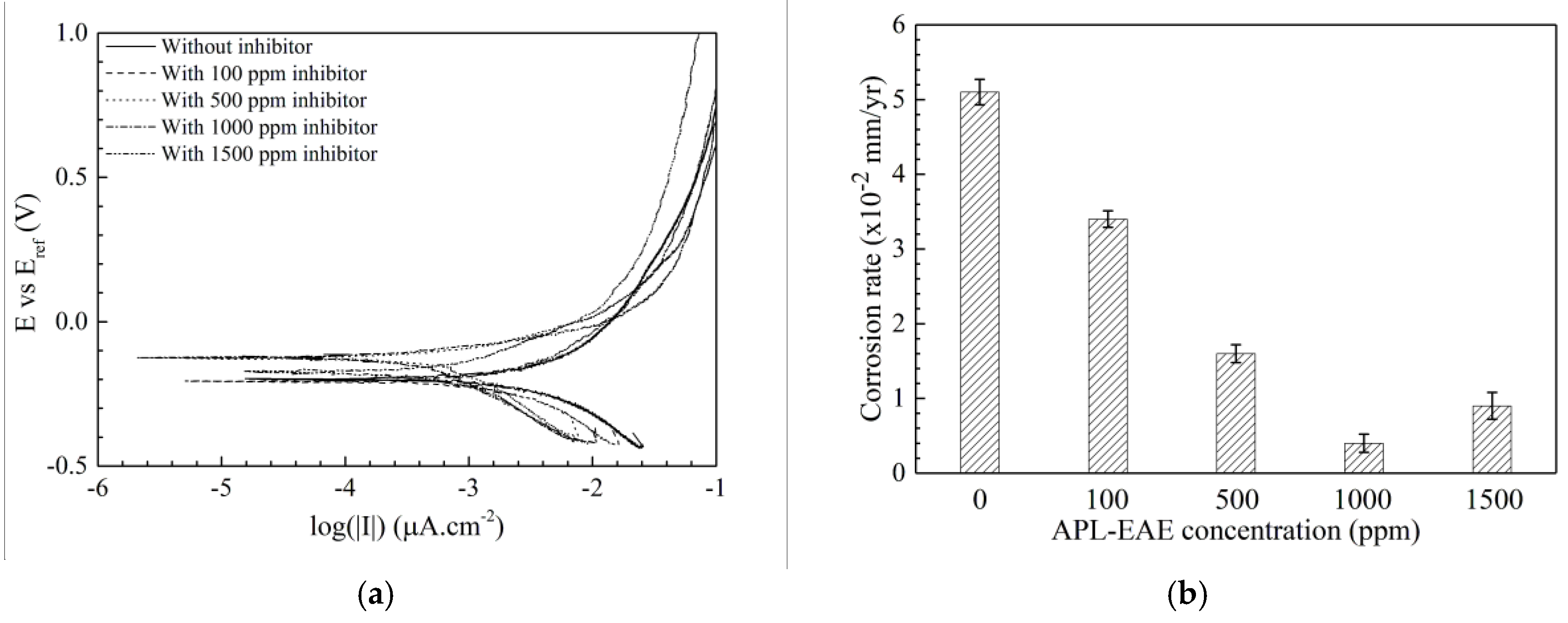
3.2. Electrochemical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P. Climate Change 2014: Synthesis Report; Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Solomon, S.; Plattner, G.K.; Knutti, R.; Friedlingstein, P. Irreversible climate change due to carbon dioxide emissions. Proc. Natl. Acad. Sci. USA 2009, 106, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Petrou, E.C.; Pappis, C.P. Biofuels: A survey on pros and cons. Energy Fuels 2009, 23, 1055–1066. [Google Scholar] [CrossRef]
- Niven, R.K. Ethanol in gasoline: Environmental impacts and sustainability review article. Renew. Sustain. Energy Rev. 2005, 9, 535–555. [Google Scholar] [CrossRef]
- Monteiro, M.R.; Ambrozin, A.R.P.; Santos, A.O.; Contri, P.P.; Kuri, S.E. Evaluation of metallic corrosion caused by alcohol fuel and some contaminants. Mater. Sci. Forum 2010, 636–637, 1024–1029. [Google Scholar] [CrossRef]
- Thangavelu, S.K.; Ahmed, A.S.; Ani, F.N. Corrosive characteristics of bioethanol and gasoline blends for metals. Int. J. Energy Res. 2016, 40, 1704–1711. [Google Scholar] [CrossRef]
- Jafari, H.; Idris, M.H.; Ourdjini, A.; Rahimi, H.; Ghobadian, B. Effect of ethanol as gasoline additive on vehicle fuel delivery system corrosion. Mater. Corros. 2010, 61, 432–440. [Google Scholar] [CrossRef]
- Little, B.; Wagner, P.; Mansfeld, F. An overview of microbiologically influenced corrosion. Electrochim. Acta 1992, 37, 2185–2194. [Google Scholar] [CrossRef]
- Jafari, H.; Idris, M.H.; Ourdjini, A.; Rahimi, H.; Ghobadian, B. EIS study of corrosion behavior of metallic materials in ethanol blended gasoline containing water as a contaminant. Fuel 2011, 90, 1181–1187. [Google Scholar] [CrossRef]
- Nam, N.D.; Kim, M.J.; Kim, J.G. Corrosion behavior of low alloy steels containing manganese in mixed chloride sulfate solution. Metall. Mater. Trans. A 2014, 45, 893–905. [Google Scholar] [CrossRef]
- Khaksar, L.; Shirokoff, J. Effect of elemental sulfur and sulfide on the corrosion behavior of Cr-Mo low alloy steel for tubing and tubular components in oil and gas industry. Materials 2017, 10, 430. [Google Scholar] [CrossRef] [PubMed]
- Nam, N.D.; Lee, S.H.; Kim, J.G.; Yi, J.W.; Lee, K.R. Effect of stress on the passivation of Si-DLC coating as stent materials in simulated body environment. Diam. Relat. Mater. 2009, 18, 1145–1151. [Google Scholar] [CrossRef]
- Sazou, D.; Deshpande, P.P. Conducting polyaniline nanocomposite-based paints for corrosion protection of steel. Chem. Pap. 2017, 71, 459–487. [Google Scholar] [CrossRef]
- Lee, H.S.; Ryu, H.S.; Park, W.J.; Ismail, M.A. Comparative study on corrosion protection of reinforcing steel by using amino alcohol and lithium nitrite inhibitors. Materials 2015, 8, 251–269. [Google Scholar] [CrossRef] [PubMed]
- Hien, P.V.; Vu, N.S.H.; Thu, V.T.H.; Somers, A.; Nam, N.D. Study of yttrium 4-nitrocinnamate to promote surface interactions with AS1020 steel. Appl. Surf. Sci. 2017, 711, 215–221. [Google Scholar] [CrossRef]
- Tijing, L.D.; Ruelo, M.T.G.; Park, C.H.; Amarjargal, A.; Kim, H.J.; Pant, H.R.; Lee, D.H.; Kim, C.S. Efficacy of zinc and tourmaline in mitigating corrosion of carbon steel in non-flow mode. Chem. Pap. 2013, 67, 1304–1310. [Google Scholar] [CrossRef]
- Nam, N.D.; Lee, D.Y.; Kim, J.G.; Park, N.J. Effect of cold rolling on the corrosion properties of low-alloy steel in an acid-chloride solution. Met. Mater. Int. 2014, 20, 469–474. [Google Scholar] [CrossRef]
- Greim, H.; Bury, D.; Klimisch, H.J.; Oeben-Negele, M.; Ziegler-Skylakakis, K. Toxicity of aliphatic amines: Structure-activity relationship. Chemosphere 1998, 36, 271–295. [Google Scholar] [CrossRef]
- Benigni, R.; Passerini, L. Carcinogenicity of the aromatic amines: from structure–activity relationships to mechanisms of action and risk assessment. Mutat. Res., Rev. Mutat. Res. 2002, 511, 191–206. [Google Scholar] [CrossRef]
- Raja, P.B.; Sethuraman, M.G. Natural products as corrosion inhibitor for metals in corrosive media—A review. Mater. Lett. 2008, 62, 113–116. [Google Scholar] [CrossRef]
- Kesavan, D.; Gopiraman, M.; Sulochana, N. Green inhibitors for corrosion of metals: A review. Chem. Sci. Rev. Lett. 2012, 1, 1–8. [Google Scholar]
- Al-Otaibi, M.S.; Al-Mayouf, A.M.; Khan, M.; Mousa, A.A.; Al-Mazroa, S.A.; Alkhathlan, H.Z. Corrosion inhibitory action of some plant extracts on the corrosion of mild steel in acidic media. Arabian J. Chem. 2014, 7, 340–346. [Google Scholar] [CrossRef]
- Nam, N.D.; Hung, T.V.; Ngan, D.T.; Hung, N.L.T.; Hoi, T.K.N. Film formation in Y(4NO2Cin)3 compound on 6061 aluminum alloy to protect against corrosion in chloride ion media. J. Taiwan Inst. Chem. Eng. 2016, 67, 495–504. [Google Scholar] [CrossRef]
- Nam, N.D.; Thang, V.Q.; Hoai, N.T.; Hien, P.V. Yttrium 3-(4-nitrophenyl)-2-propenoate used as inhibitor against copper alloy corrosion in 0.1 M NaCl solution. Corros. Sci. 2016, 112, 451–461. [Google Scholar] [CrossRef]
- Cao, L.; Frankel, G.S.; Sridhar, N. Supporting electrolyte for corrosion and cracking studies in deaerated simulated fuel grade ethanol. J. Electrochem. Soc. 2013, 160, C19–C27. [Google Scholar] [CrossRef]
- ASTM D7577-12. Standard Test Method for Determining the Accelerated Iron Corrosion Rating of Denatured Fuel Ethanol and Ethanol Fuel Blends; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- ASTM G1-03. Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- ASTM G31-12a. Standard Guide for Laboratory Immersion Corrosion Testing of Metals; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- ASTM G5-14. Standard Reference Test Method for Making Potentiodynamic Anodic Polarization Measurements; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Li, L.; Zhang, X.; Lei, J.; He, J.; Zhang, S.; Pan, F. Adsorption and corrosion inhibition of Osmanthus fragran leaves extract on carbon steel. Corros. Sci. 2012, 63, 82–90. [Google Scholar] [CrossRef]
- Deng, S.; Li, X. Inhibition by Ginkgo leaves extract of the corrosion of steel in HCl and H2SO4 solutions. Corros. Sci. 2012, 55, 407–415. [Google Scholar] [CrossRef]
- Loganayagi, C.; Kamal, C.; Sethuraman, M.G. Opuntiol: An active principle of Opuntia elatior as an eco-friendly inhibitor of corrosion of mild steel in acid medium. ACS Sustain. Chem. Eng. 2014, 2, 606–613. [Google Scholar] [CrossRef]
- Graat, P.C.J.; Somers, M.A.J. Simultaneous determination of composition and thickness of thin iron-oxide films from XPS Fe 2p spectra. Appl. Surf. Sci. 1996, 100, 36–40. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, H.; Wang, F. [BMIM]BF4 ionic liquids as effective inhibitor for carbon steel in alkaline chloride solution. Electrochim. Acta 2011, 56, 4268–4275. [Google Scholar] [CrossRef]
- Jones, D.A. Principles and Prevention of Corrosion, 2nd ed.; Printice Hall: Upper Saddle River, NJ, USA, 1996; pp. 366–367. [Google Scholar]
- ASTM G102-89e1. Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]

| Chemical | Origin | Minimum Purity (%) | Proportion |
|---|---|---|---|
| Ethanol | Merck | 99.8 | 75.6% v/v |
| Methanol | Merck | 99.8 | 4.2% v/v |
| Iso-Propanol | Merck | 99.5 | 4.2% v/v |
| RON92 | Commercial, unleaded | - | 15% v/v |
| Deionized water | ELGA Purelab Ultra | ASTM D1193 | 1% v/v |
| Sodium chloride | Merck | 99.5 | 15 ppm |
| Formic acid | Merck | 95.0 | 10 ppm |
| Acetic acid | Merck | 99.7 | 20 ppm |
| Chemical Elements (%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Mn | Si | S | P | Ni | Cr | Mo | Cu | V | Nb | Ti | Al | B | Fe |
| 0.16 | 0.73 | 0.21 | 0.01 | 0.02 | <0.01 | 0.03 | <0.01 | <0.01 | 0.01 | <0.01 | <0.01 | <0.005 | <0.005 | Bal. |
| Concentration (ppm) | Rs (kΩ.cm2) | CPEpro (µF/cm2) | Α (0~1) | Rpro (kΩ.cm2) | CPEdl (nF/cm2) | Α (0~1) | Rct (kΩ.cm2) | χ2 (%) |
|---|---|---|---|---|---|---|---|---|
| 0 | 3.52 | 140.0 | 0.442 | 14.95 | 21.4 | 0.899 | 5.86 | 0.136 |
| 100 | 3.56 | 106.0 | 0.569 | 18.14 | 15.2 | 0.939 | 15.14 | 0.161 |
| 500 | 3.55 | 68.1 | 0.604 | 21.74 | 4.9 | 0.982 | 15.80 | 0.182 |
| 1000 | 3.58 | 50.1 | 0.743 | 44.18 | 3.0 | 0.998 | 18.14 | 0.116 |
| 1500 | 3.68 | 58.4 | 0.617 | 26.51 | 3.9 | 0.994 | 17.48 | 0.170 |
| Concentration (ppm) | Ecorr (mVAg/AgCl) | icorr (µA/cm2) | βa (mV/decade) | βc (mV/decade) | Rp (kΩ.cm2) |
|---|---|---|---|---|---|
| 0 | −198 | 2.17 | 377 | 295 | 33 |
| 100 | −206 | 1.46 | 296 | 304 | 45 |
| 500 | −127 | 0.69 | 133 | 275 | 56 |
| 1000 | −125 | 0.17 | 80 | 211 | 148 |
| 1500 | −173 | 0.39 | 172 | 255 | 114 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, N.S.H.; Hien, P.V.; Man, T.V.; Hanh Thu, V.T.; Tri, M.D.; Nam, N.D. A Study on Corrosion Inhibitor for Mild Steel in Ethanol Fuel Blend. Materials 2018, 11, 59. https://doi.org/10.3390/ma11010059
Vu NSH, Hien PV, Man TV, Hanh Thu VT, Tri MD, Nam ND. A Study on Corrosion Inhibitor for Mild Steel in Ethanol Fuel Blend. Materials. 2018; 11(1):59. https://doi.org/10.3390/ma11010059
Chicago/Turabian StyleVu, Nguyen Si Hoai, Pham Van Hien, Tran Van Man, Vu Thi Hanh Thu, Mai Dinh Tri, and Nguyen Dang Nam. 2018. "A Study on Corrosion Inhibitor for Mild Steel in Ethanol Fuel Blend" Materials 11, no. 1: 59. https://doi.org/10.3390/ma11010059