Biosorbents for Removing Hazardous Metals and Metalloids †
Abstract
:1. Introduction
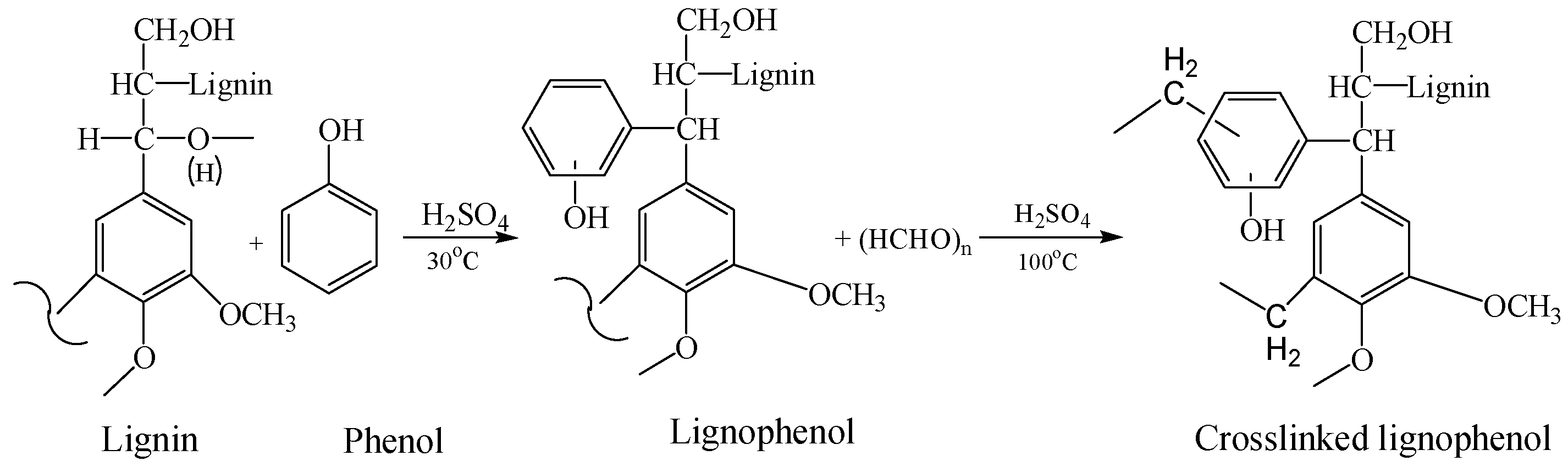
2. Lignophenols
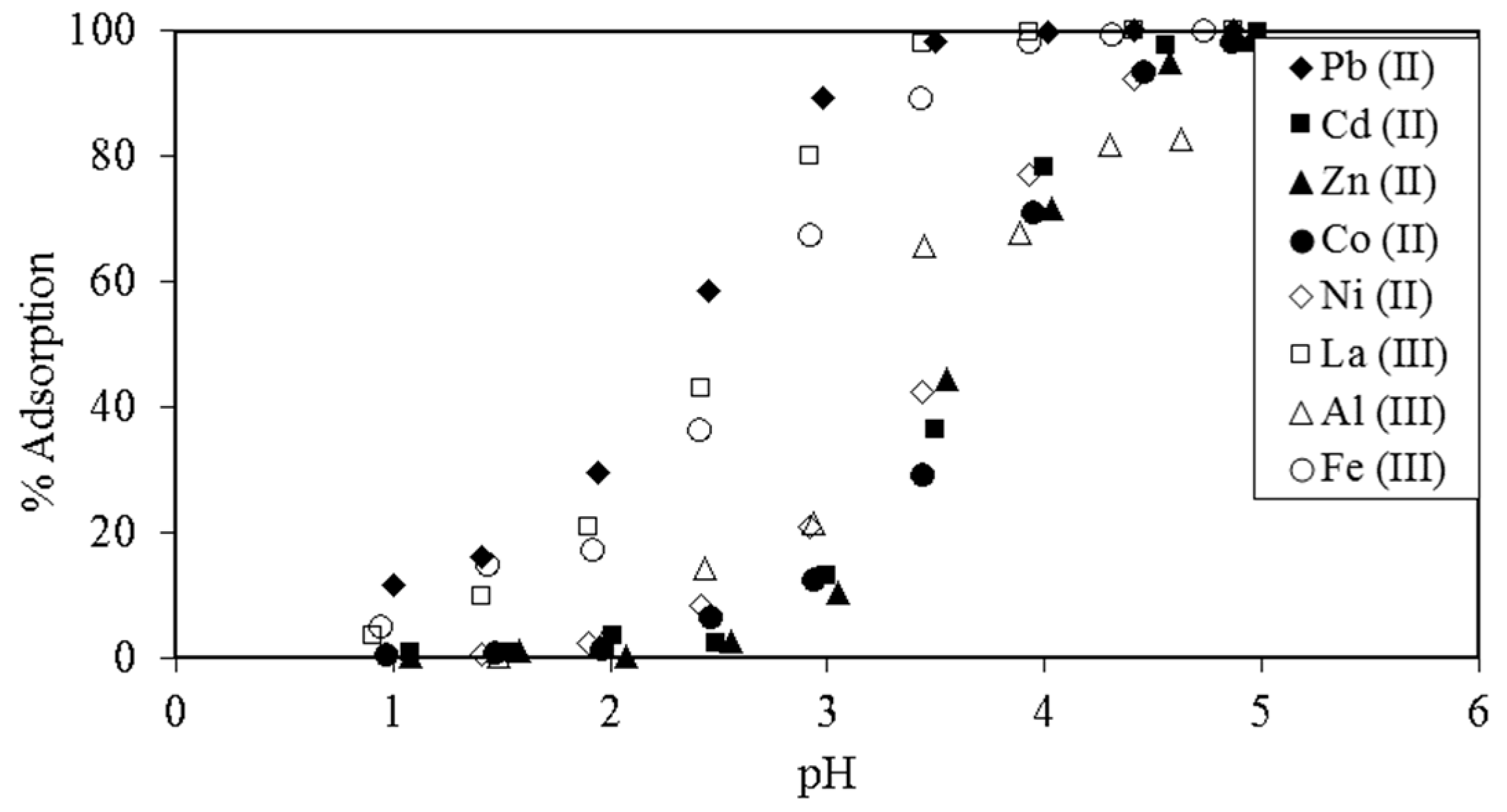
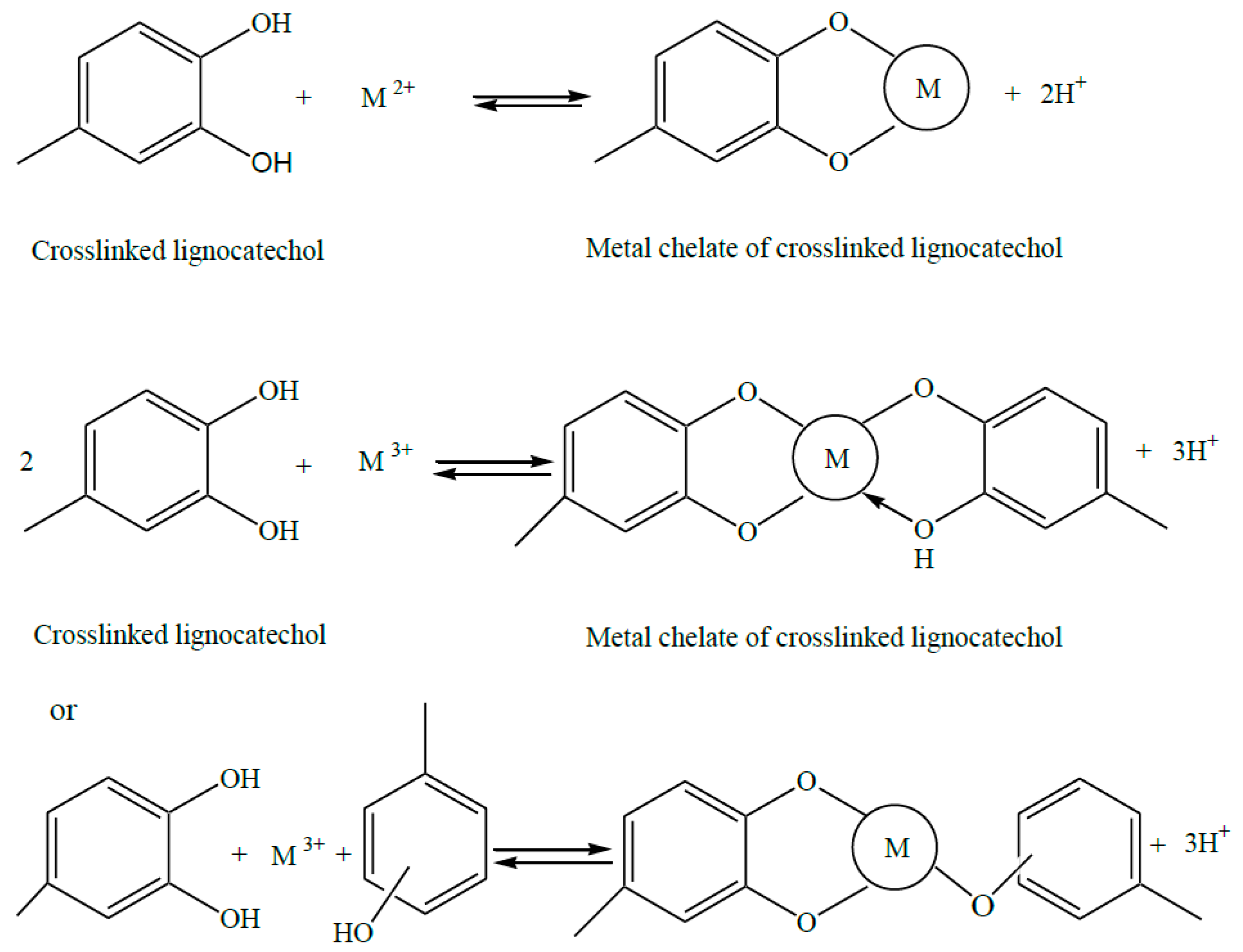
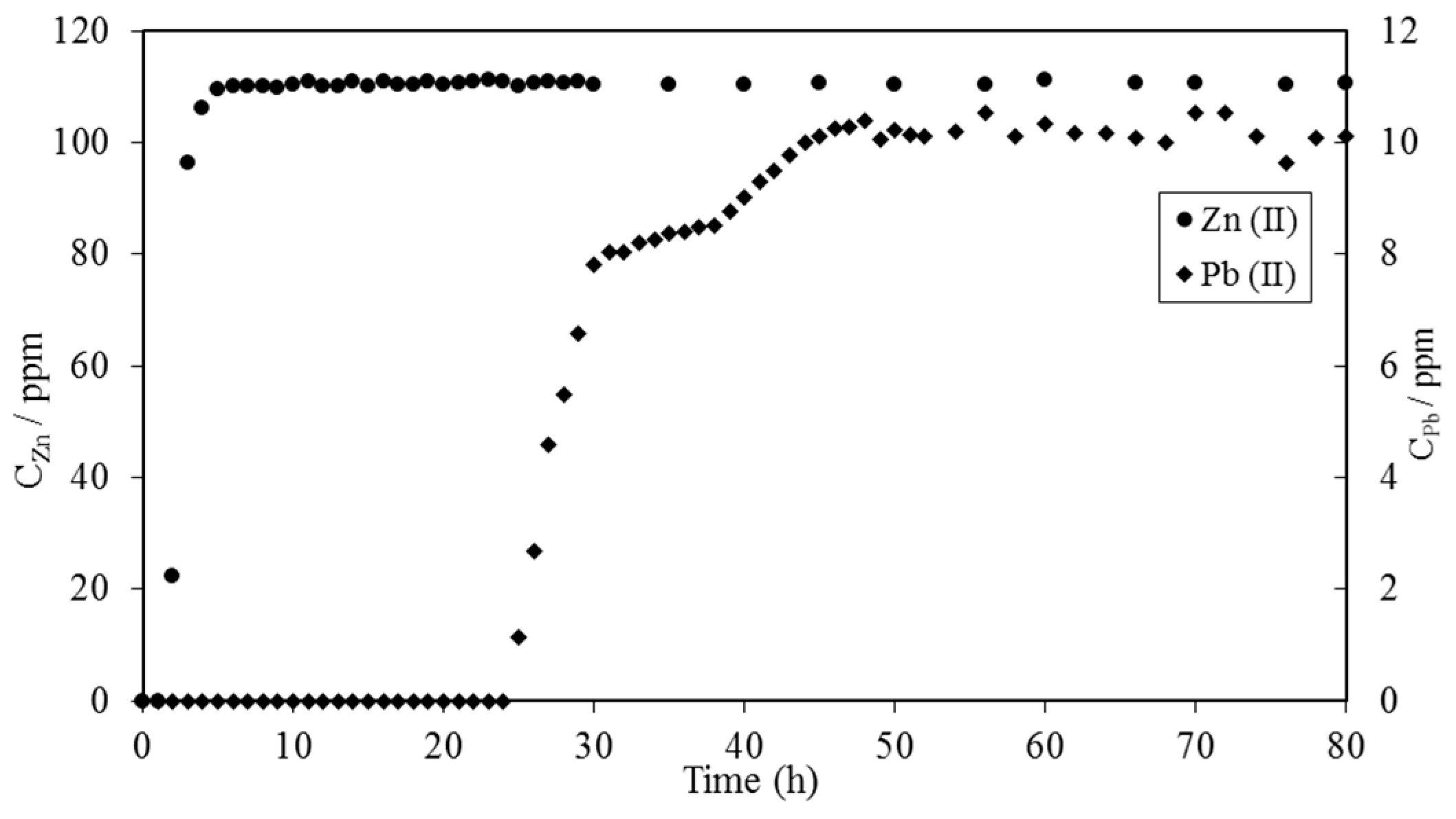
3. Crosslinked Lignosulfonate
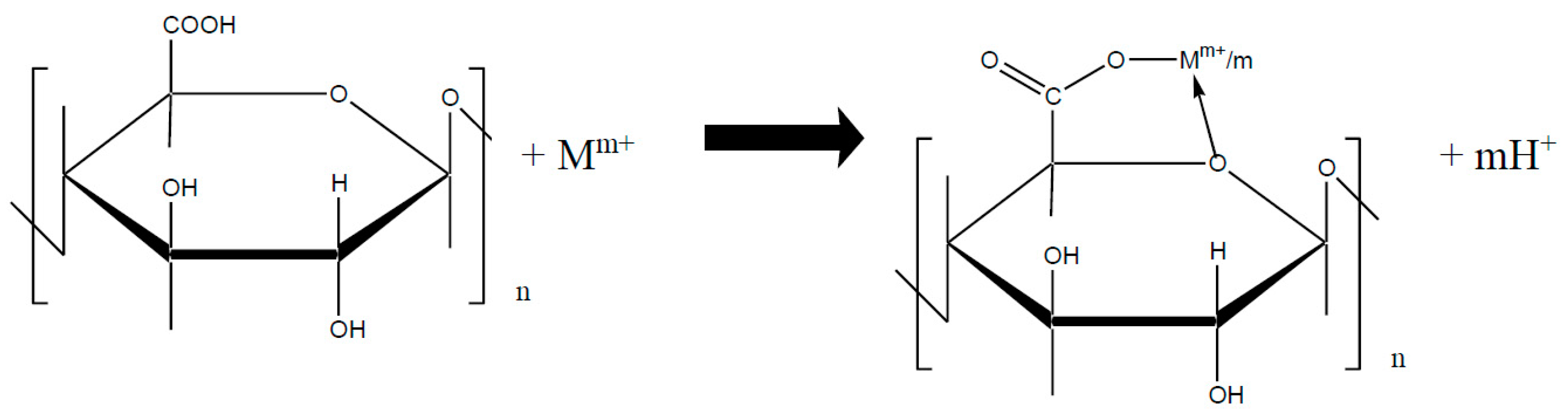
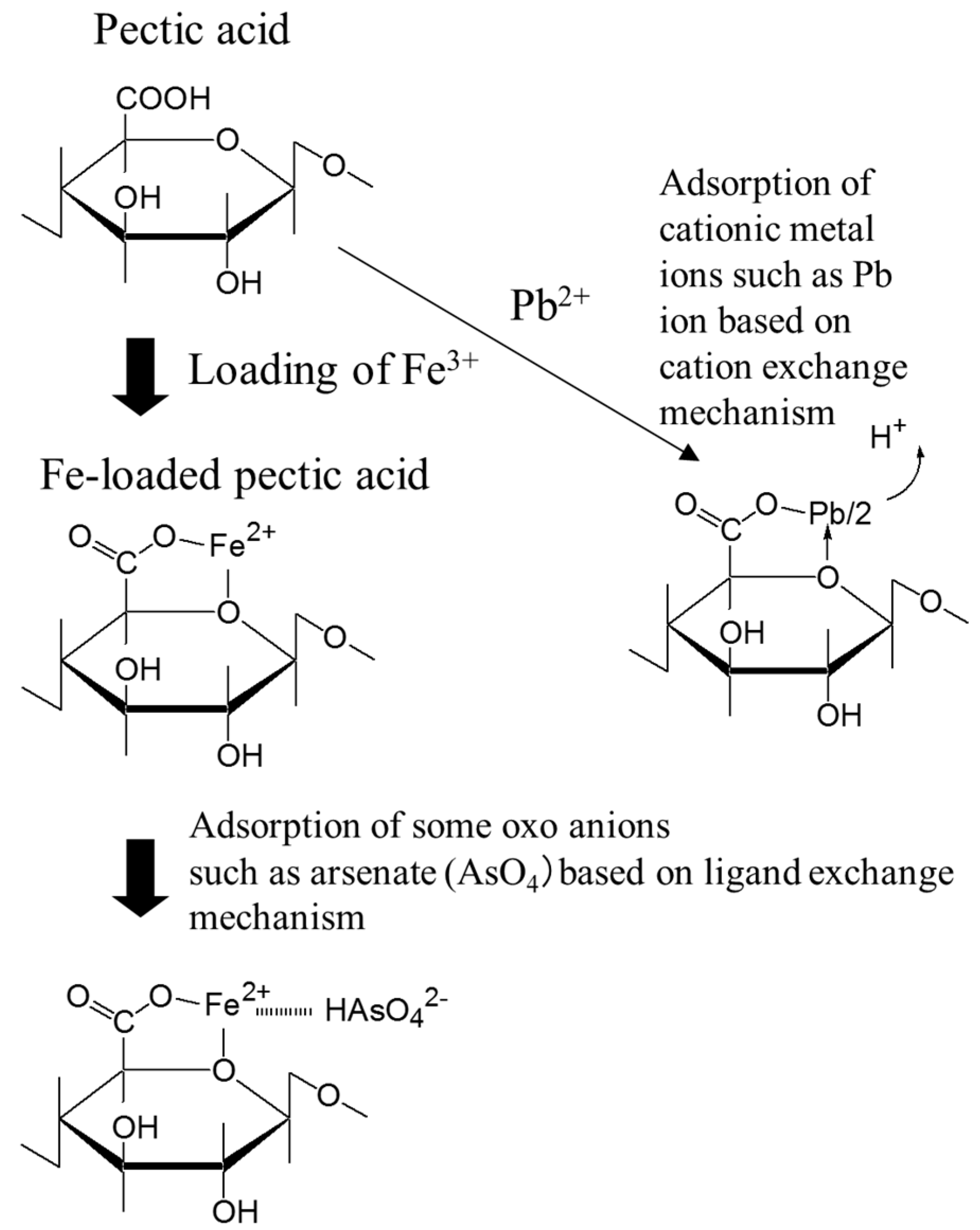
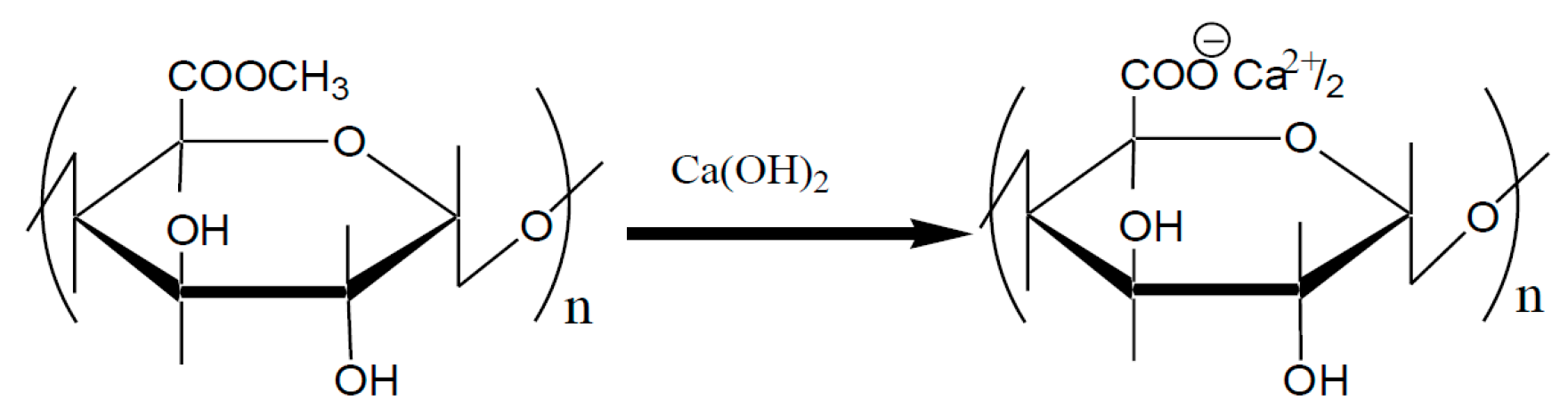
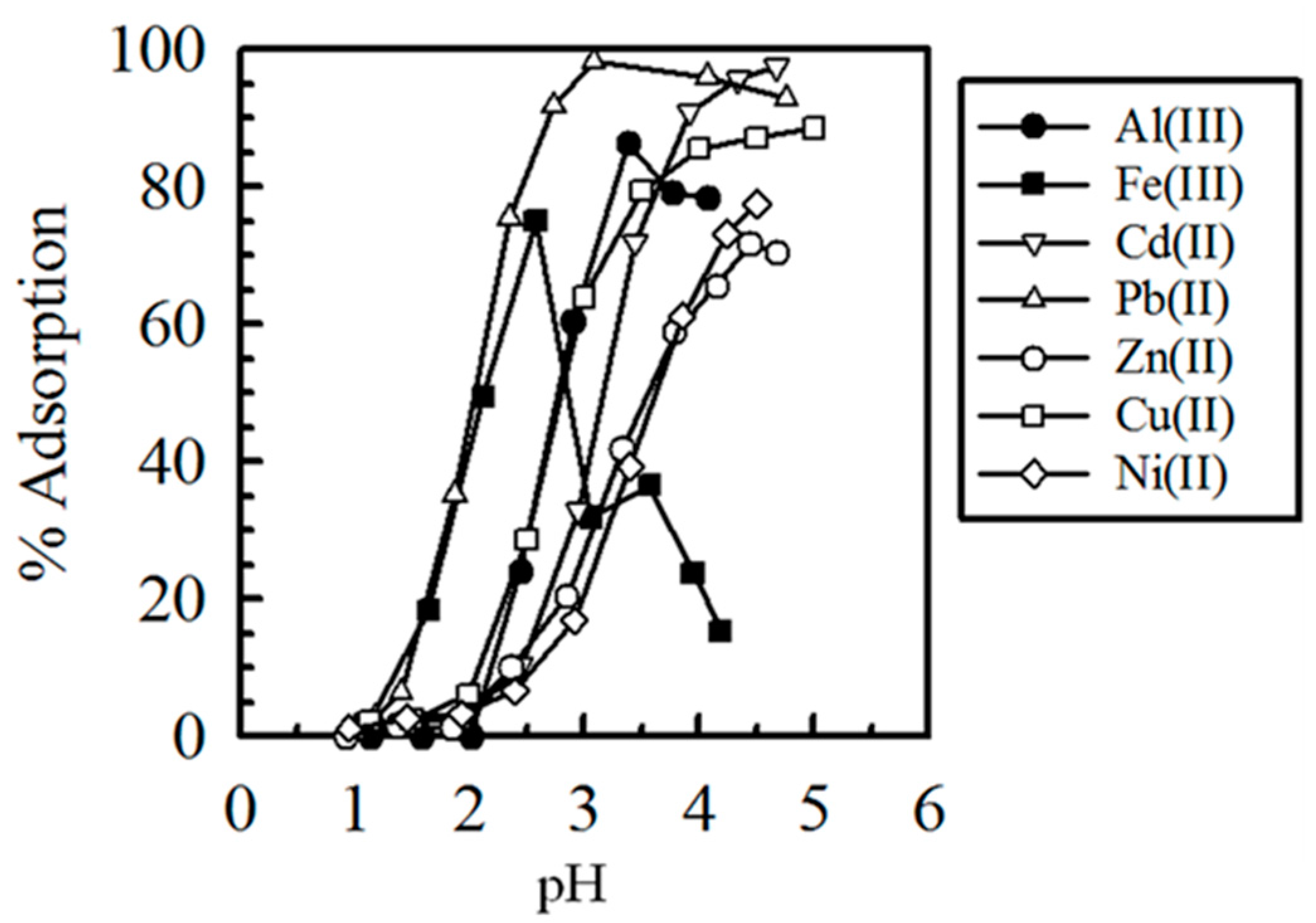
4. Crosslinked Pectic Acid and Alginic Acid
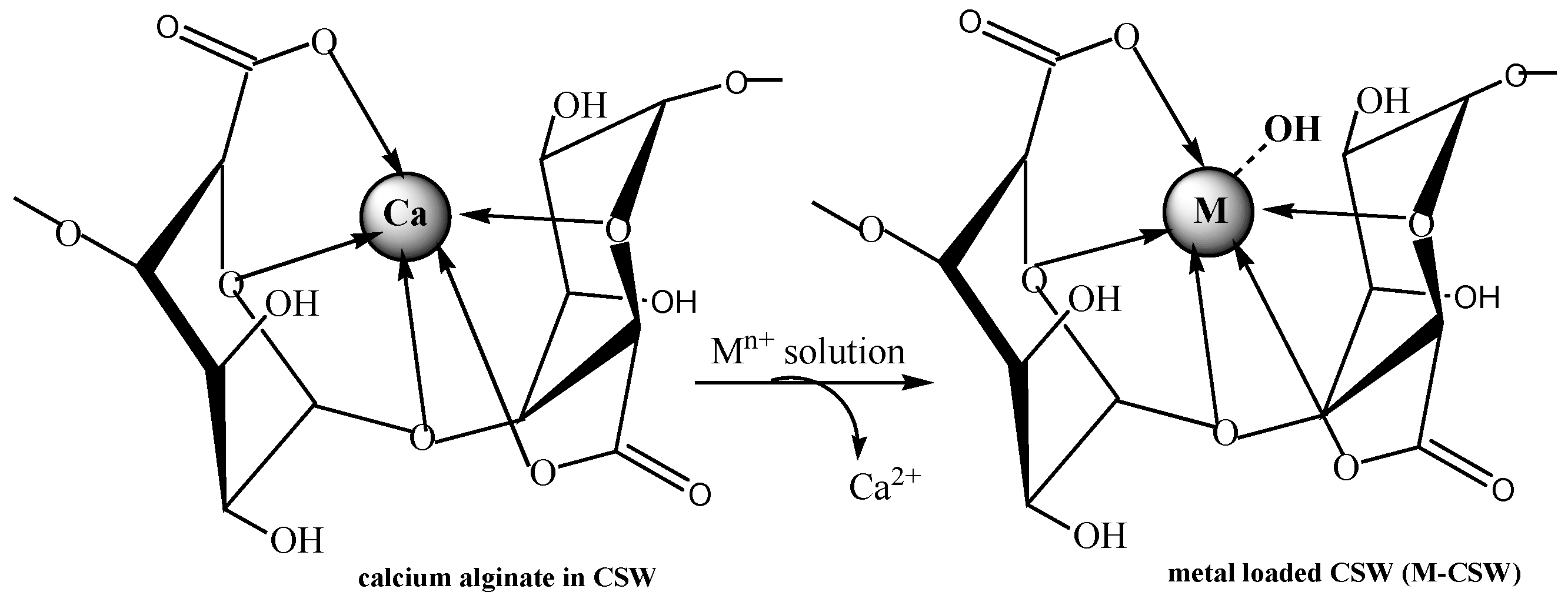
5. Biosorbents Prepared from Orange and Apple Juice Residues
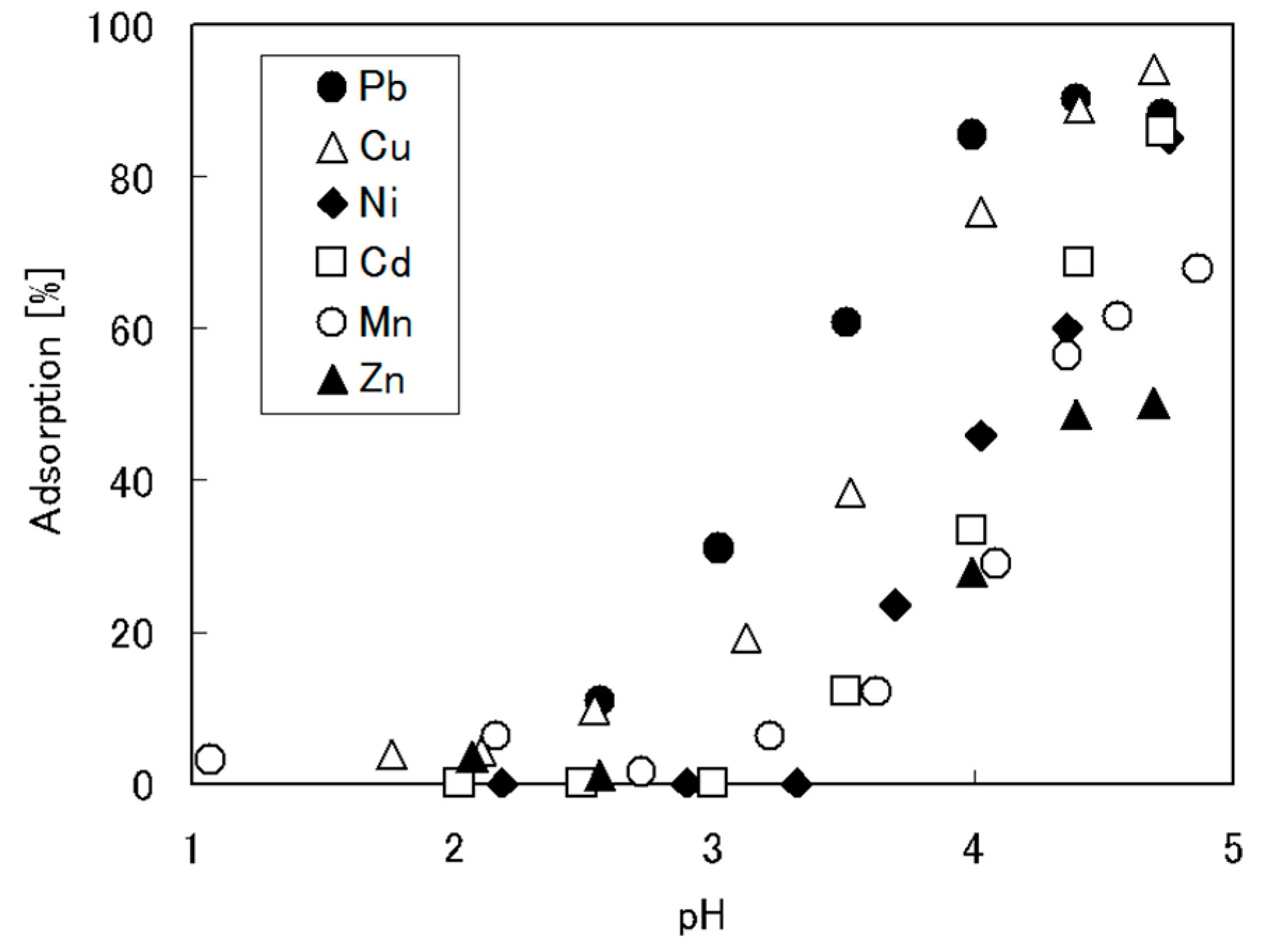
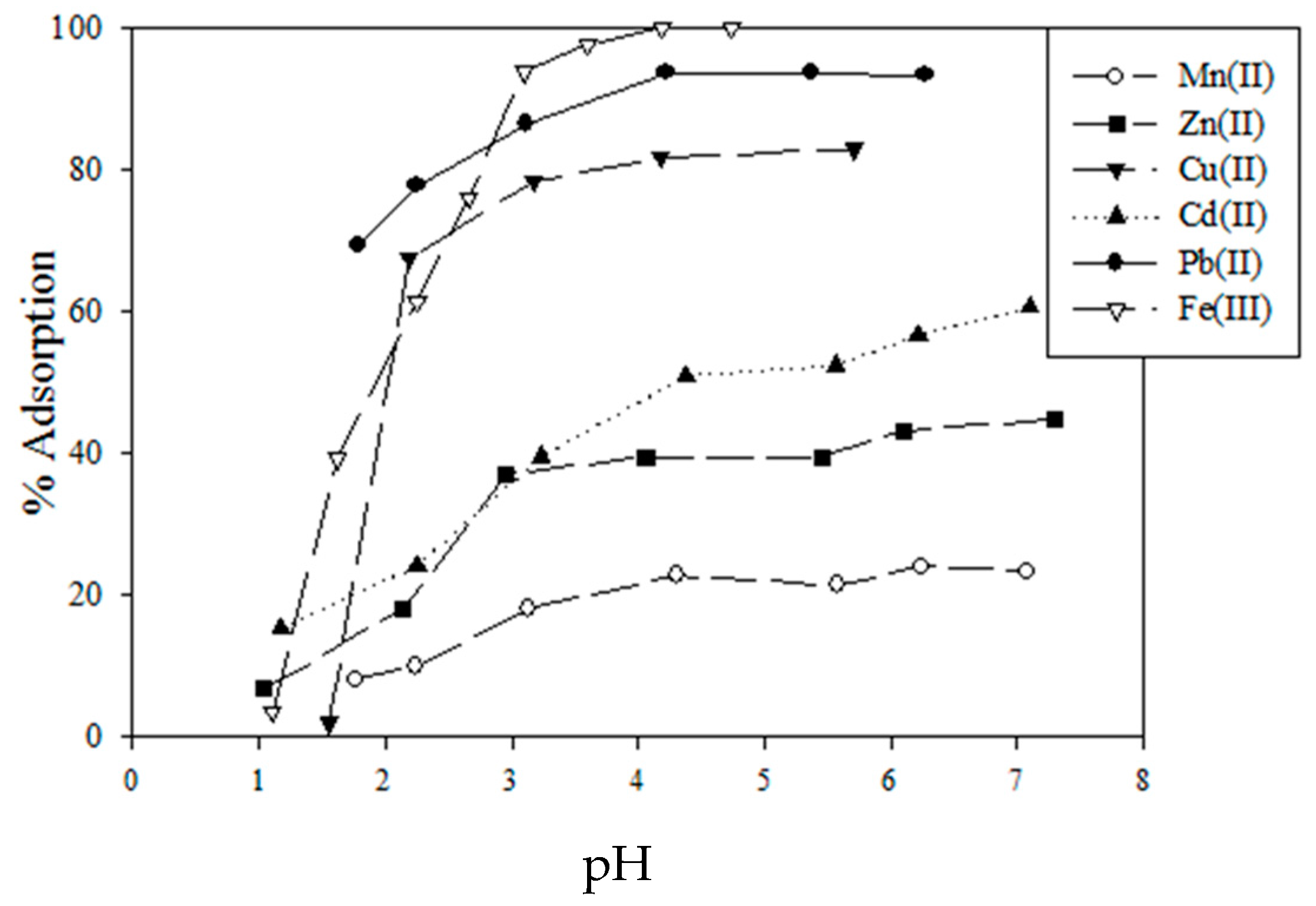
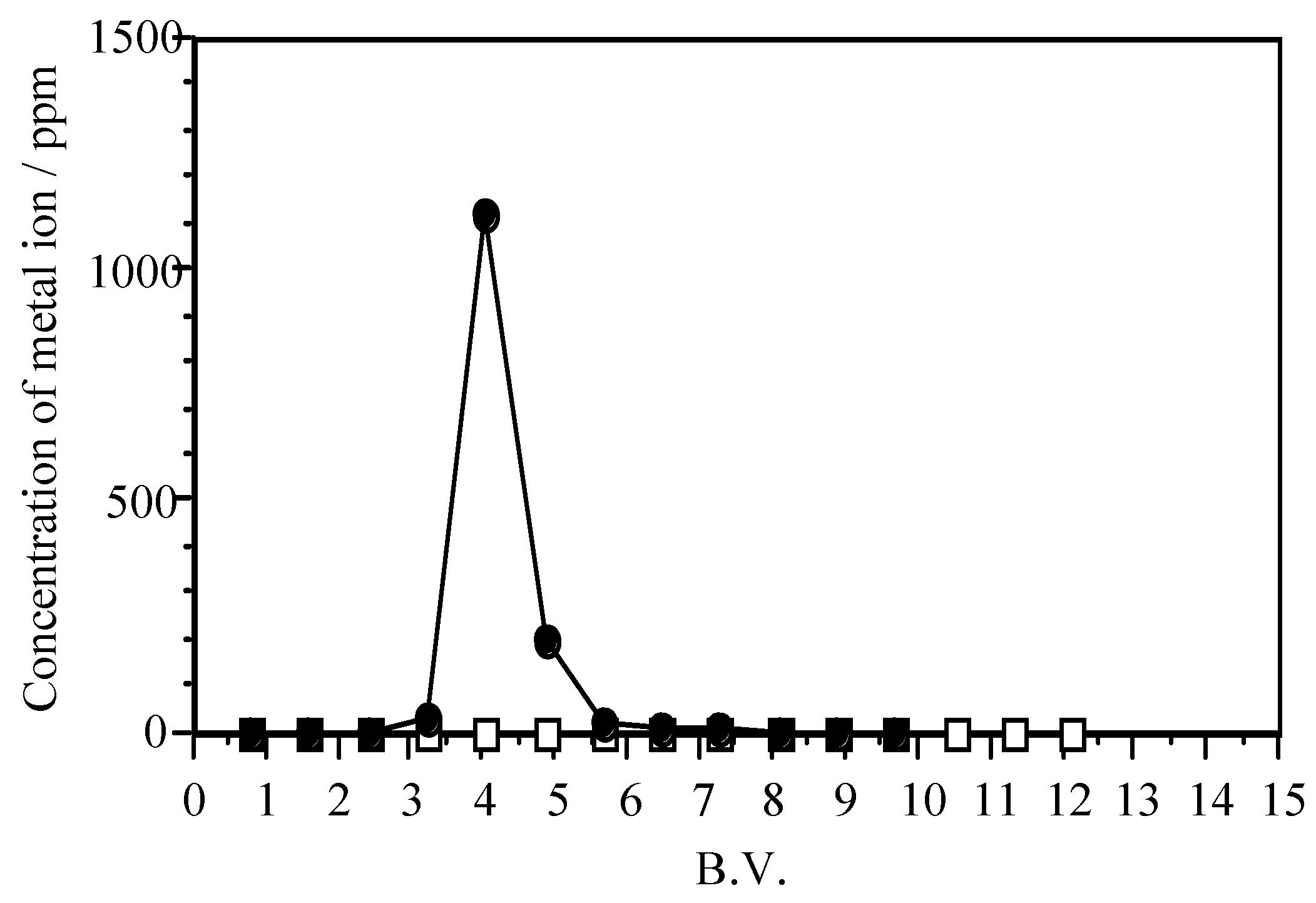
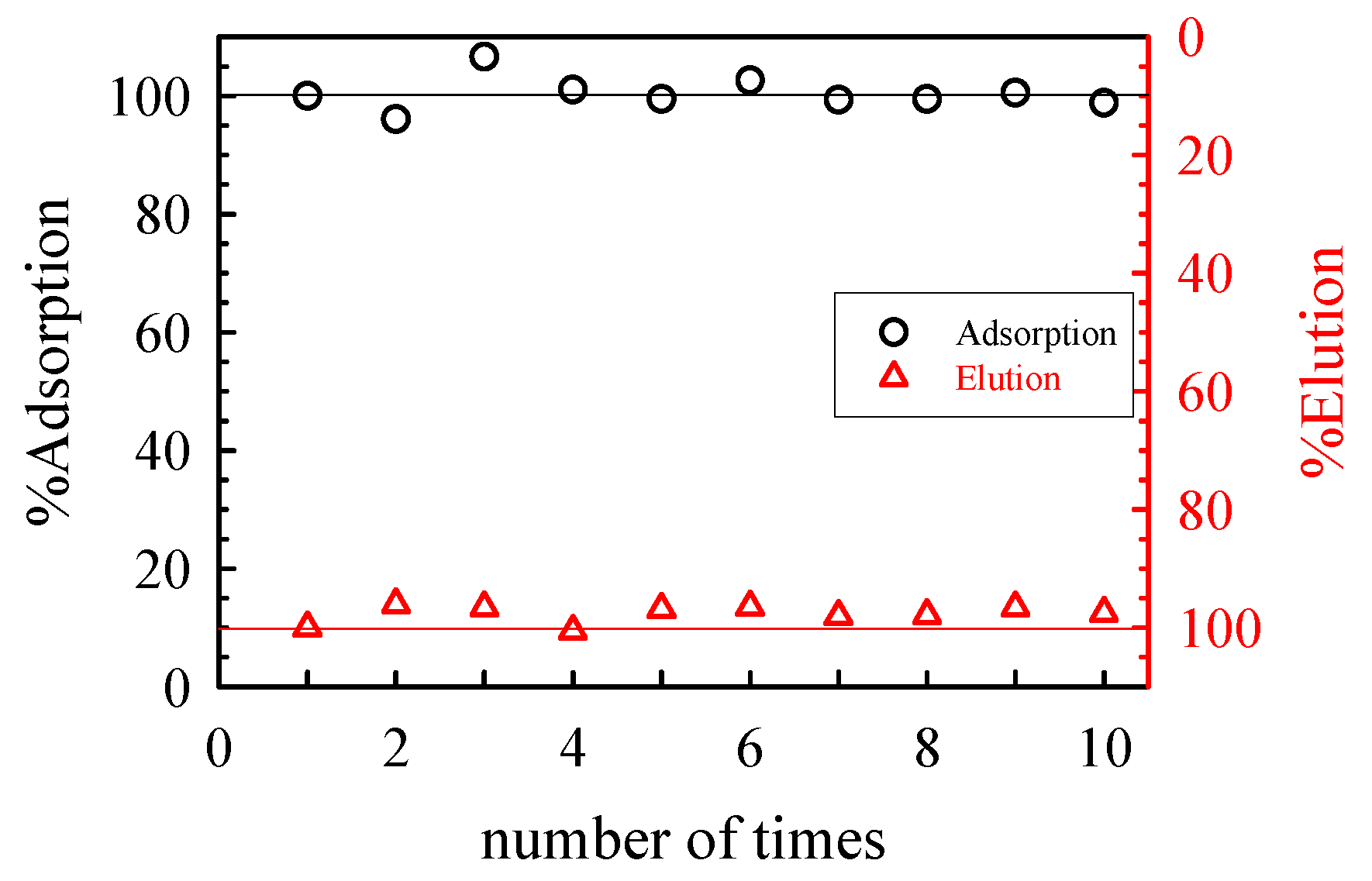
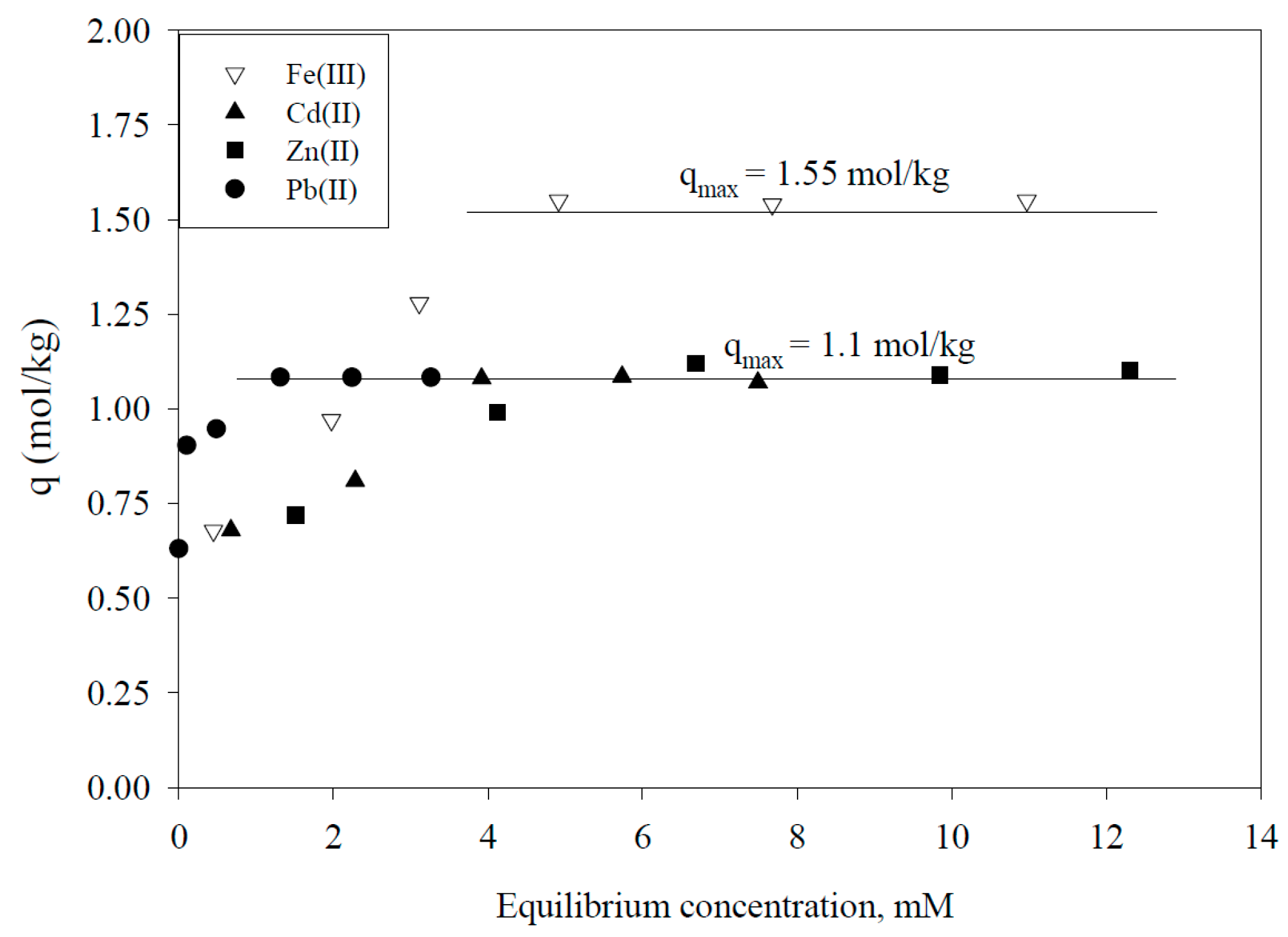
5.1. Removal of Hazardous Metal Cations Using Adsorption Gels Prepared from Orange and Apple Juice Residues
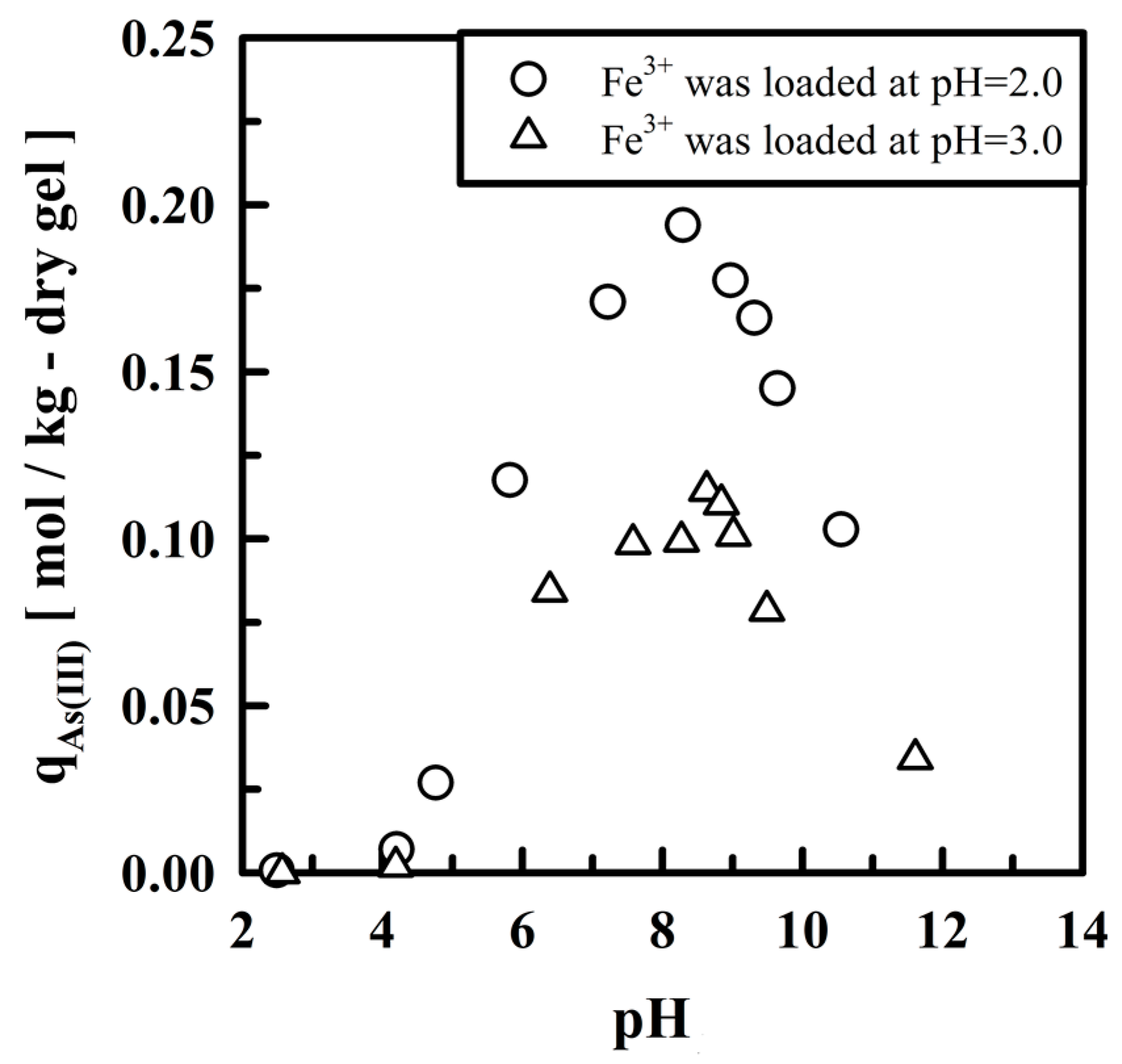
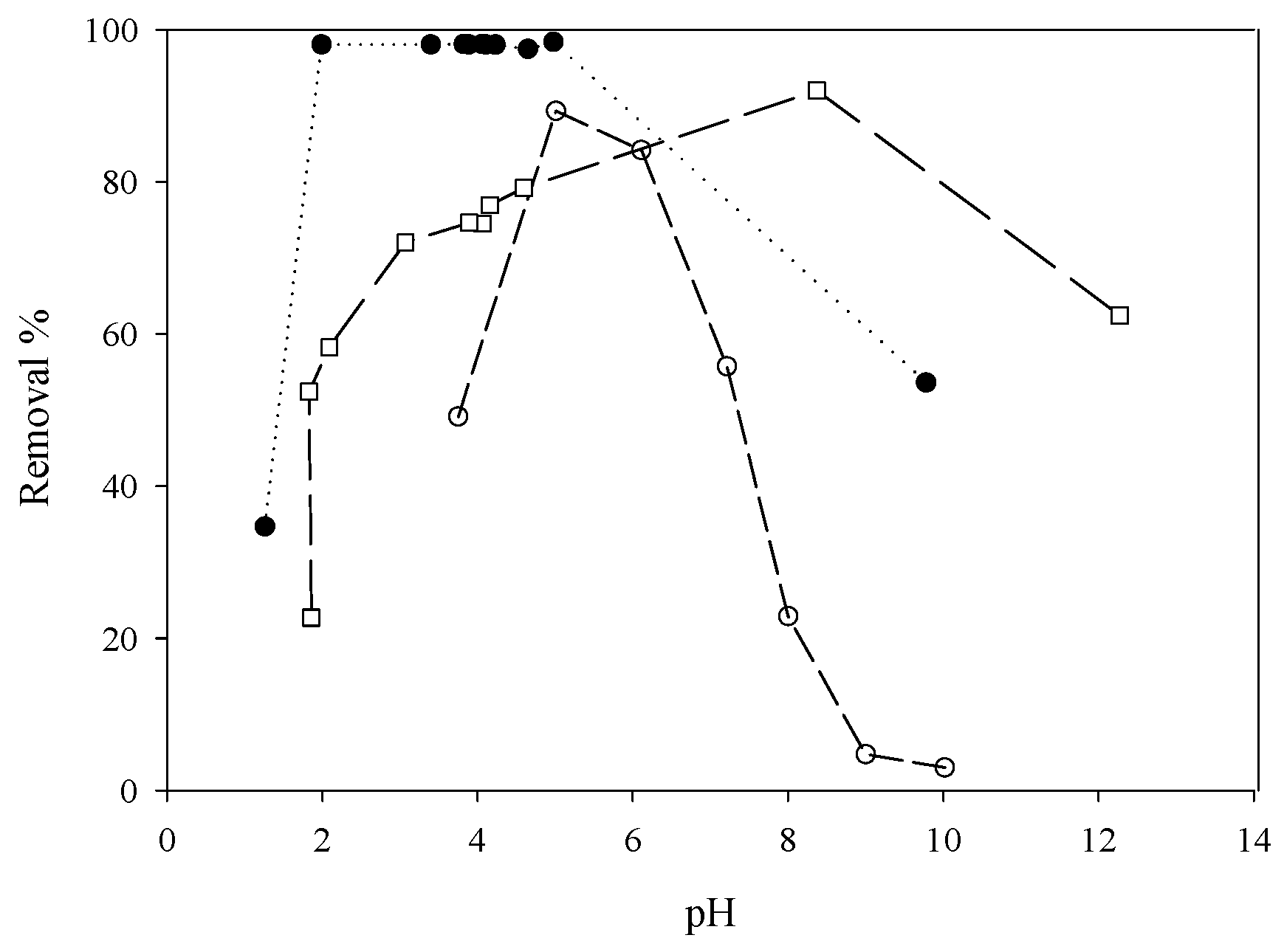
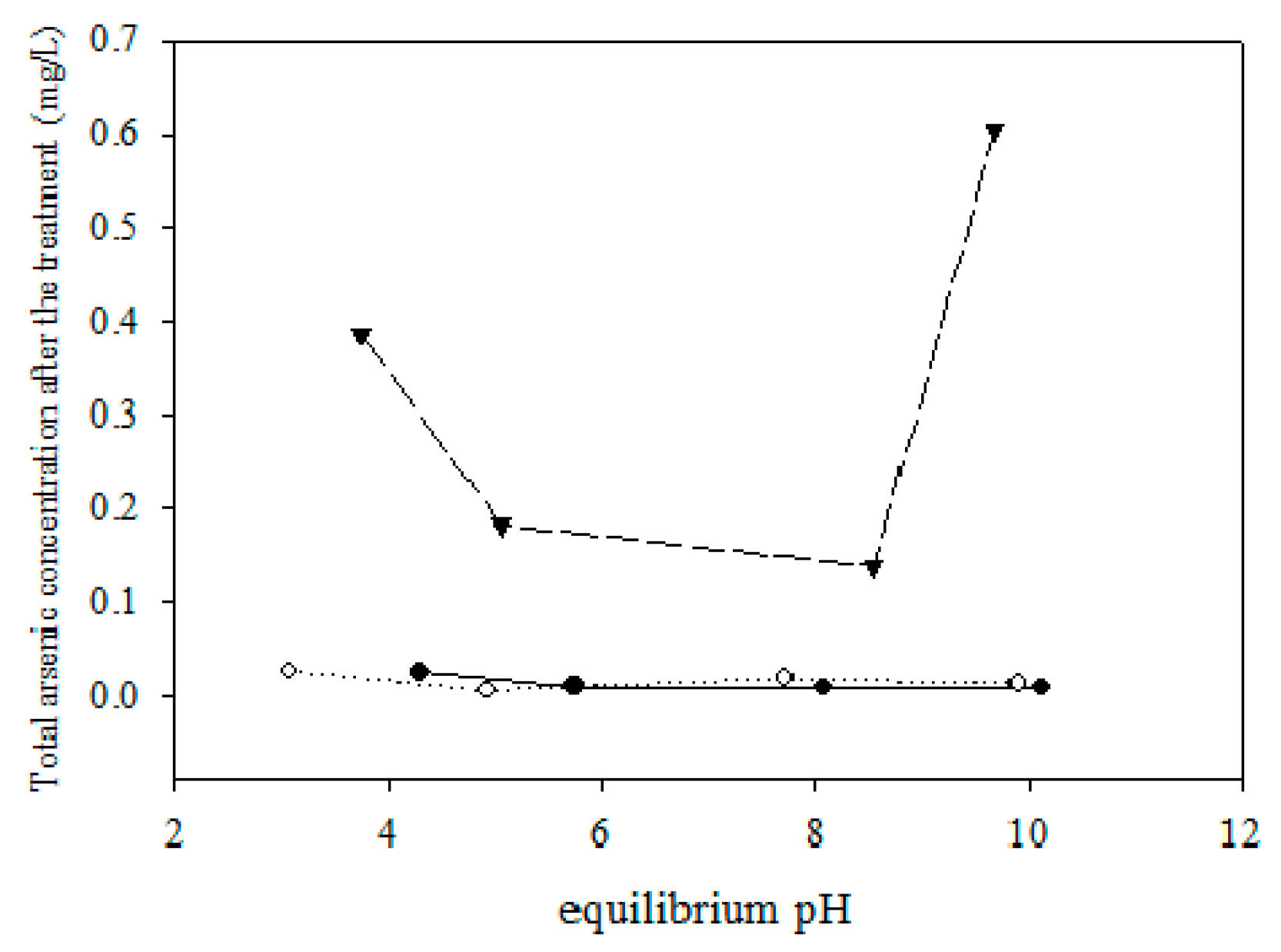
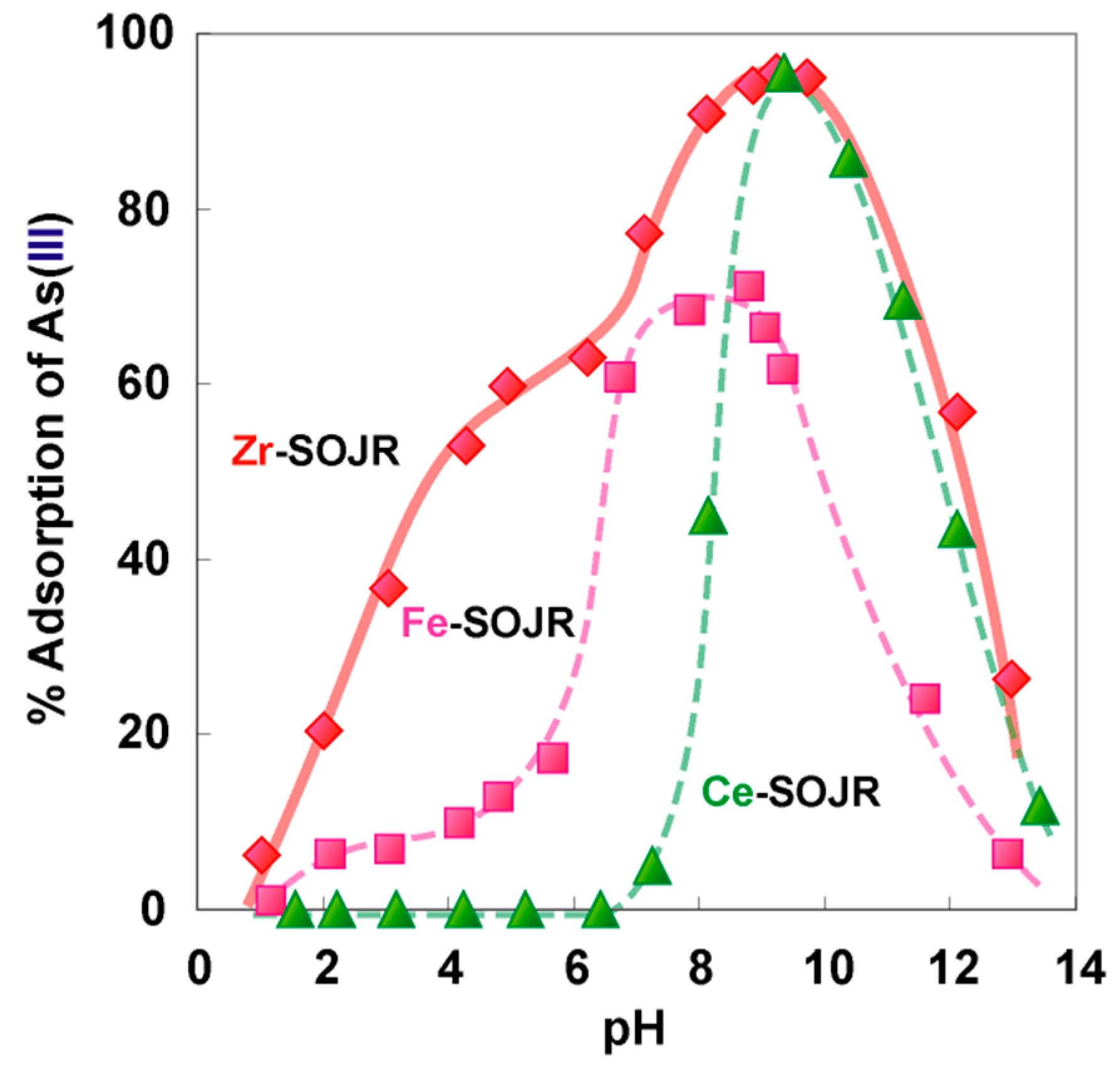
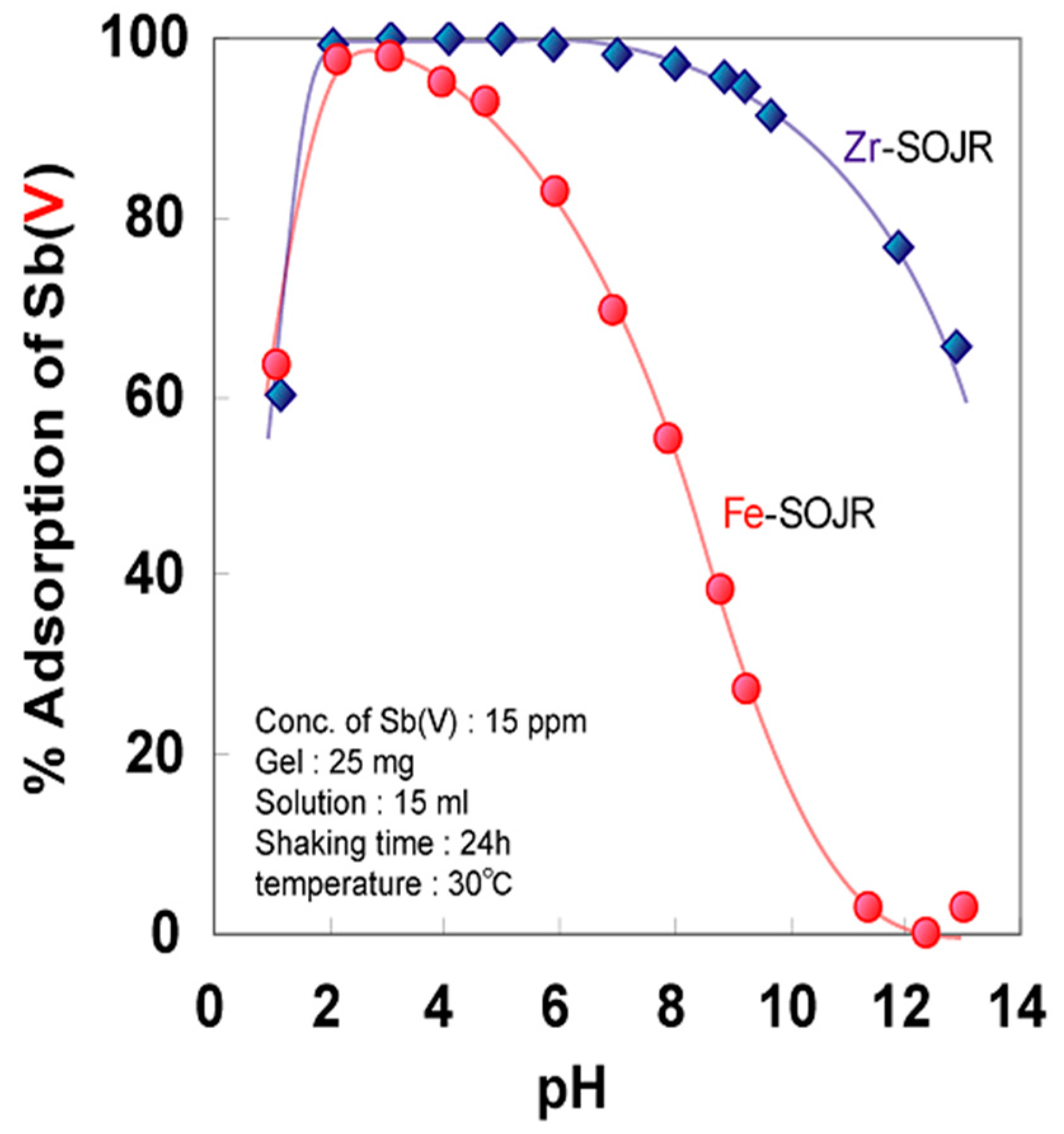
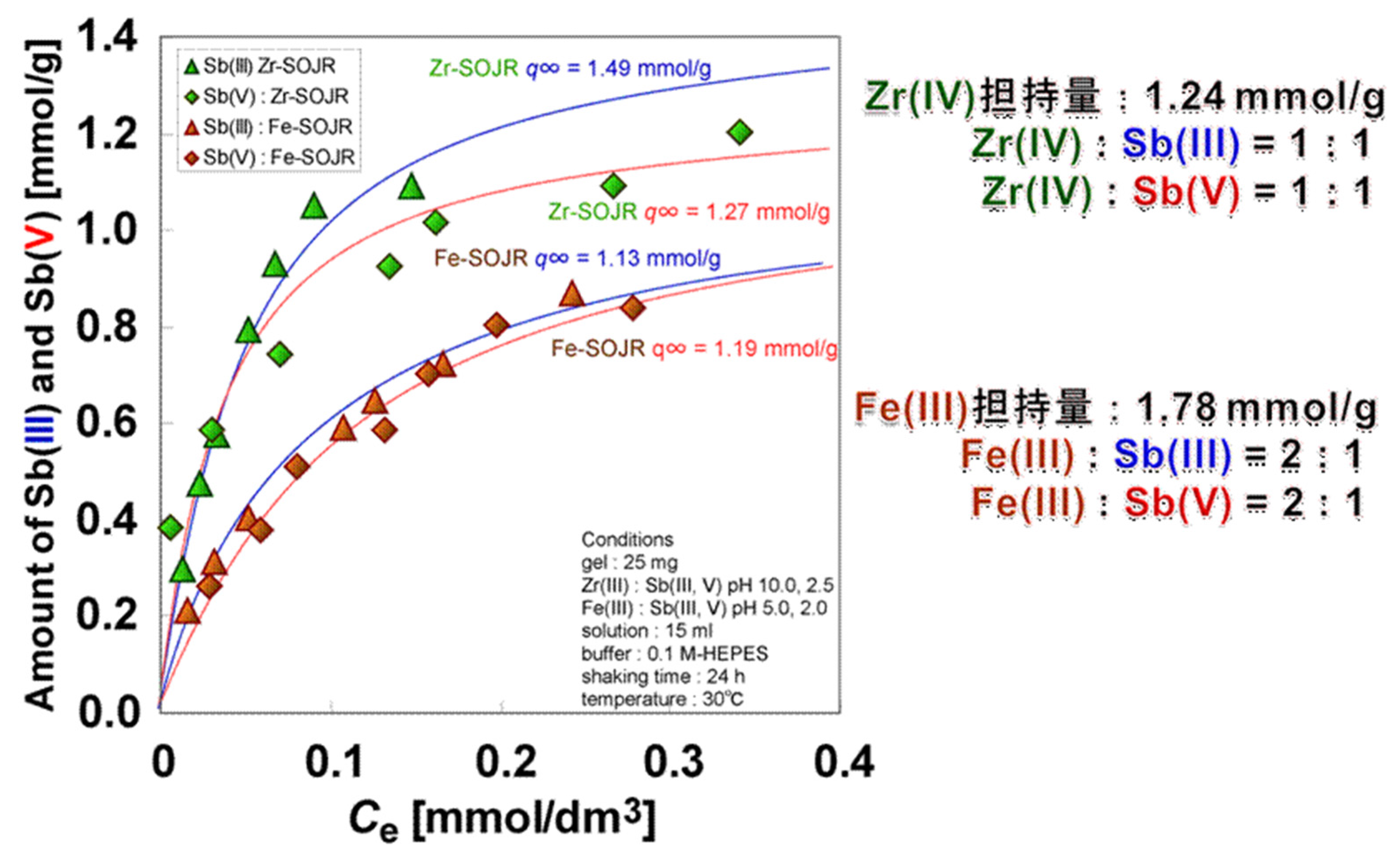
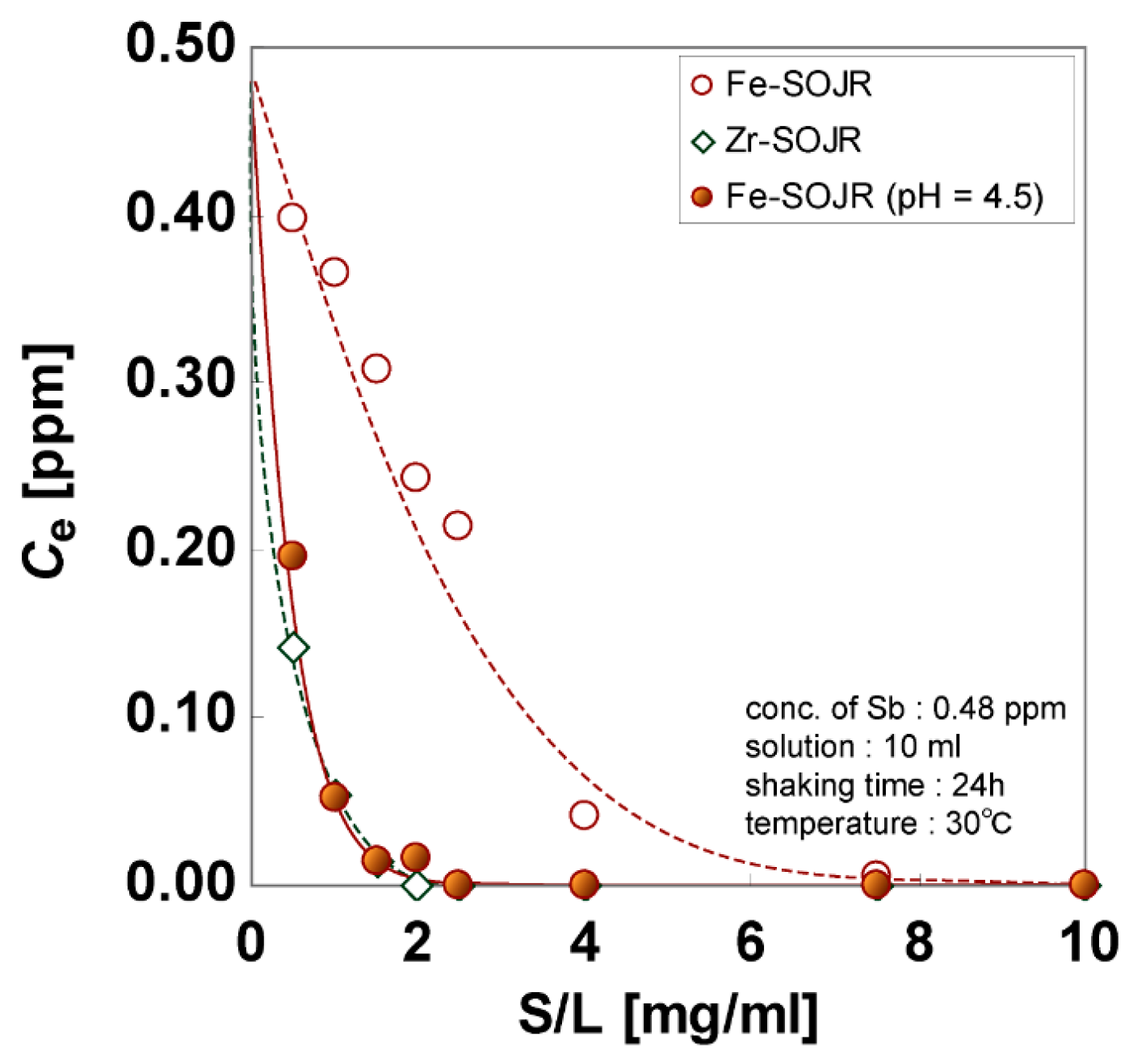
5.2. Removing As and Sb Using Adsorption Gels Prepared from Orange Juice Residue
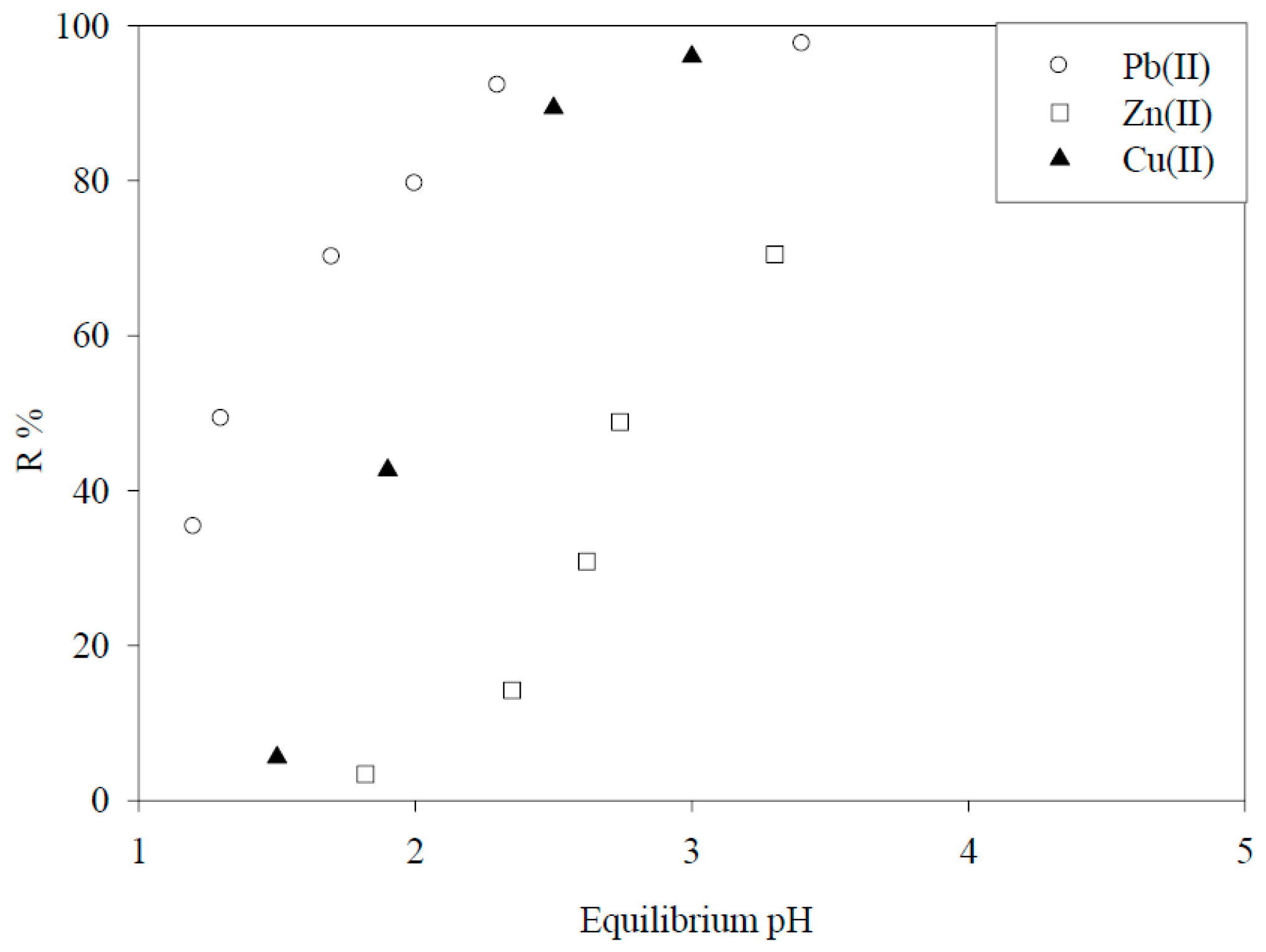
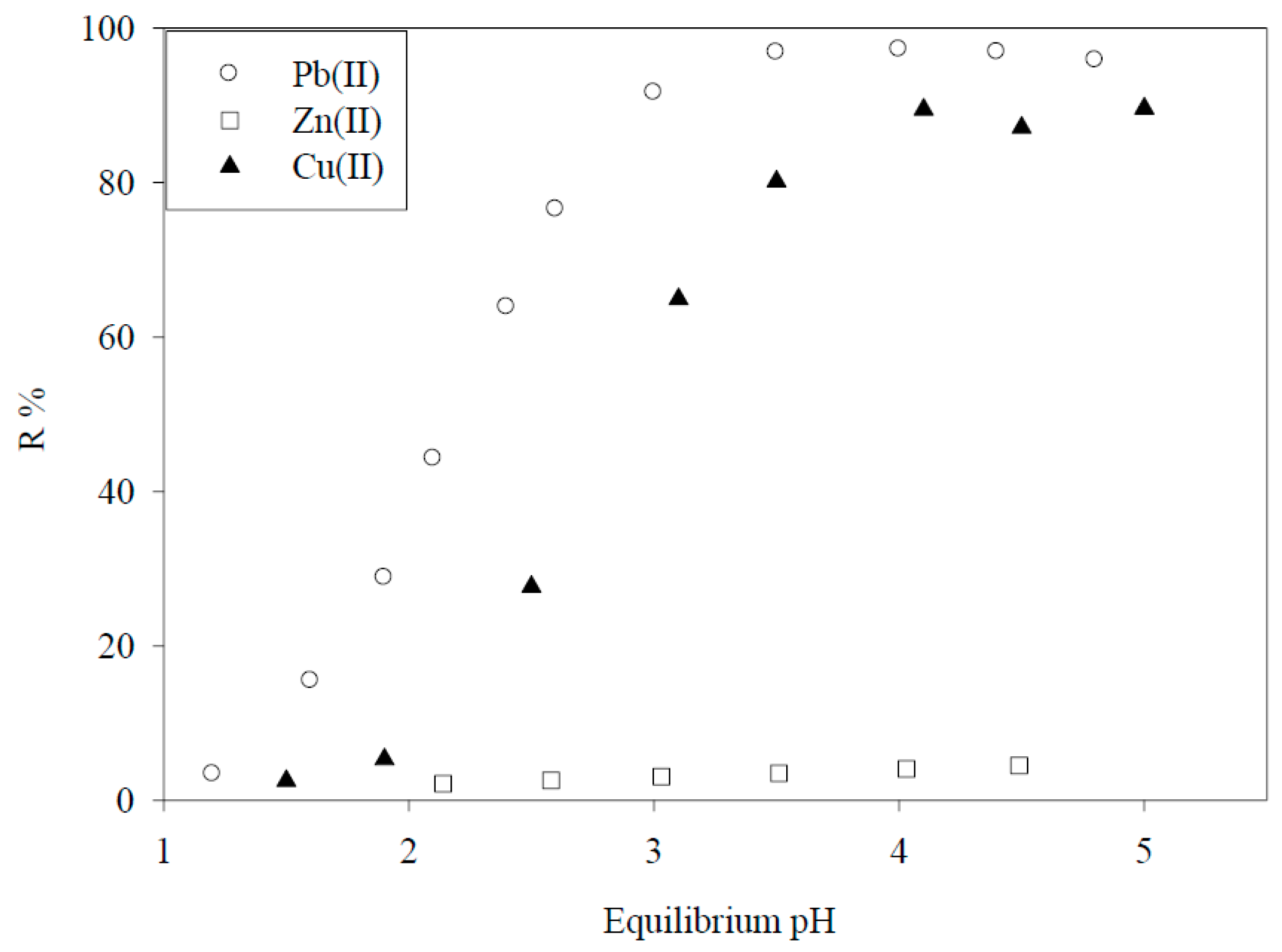
6. Biosorbents Prepared from Seaweed
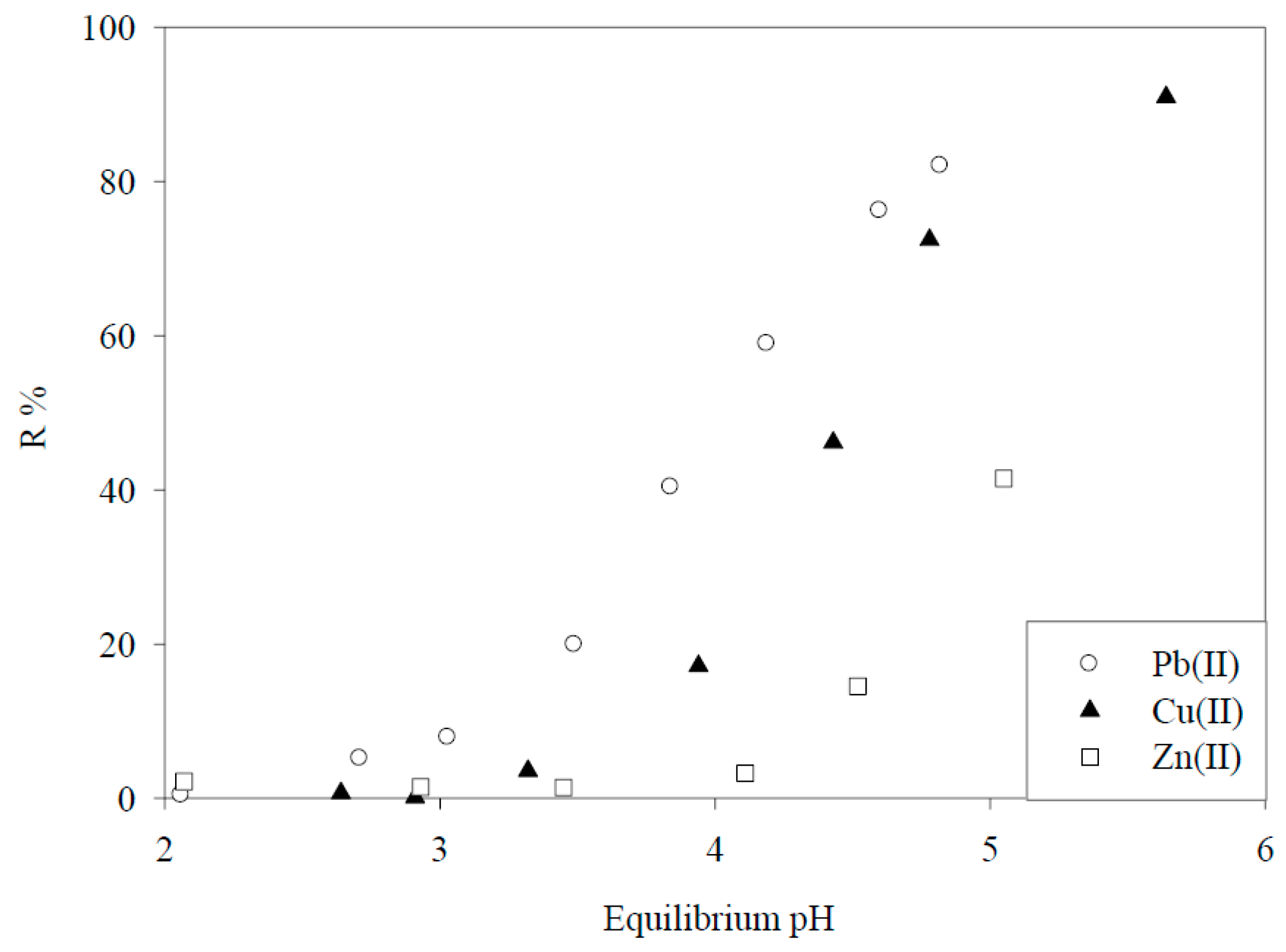
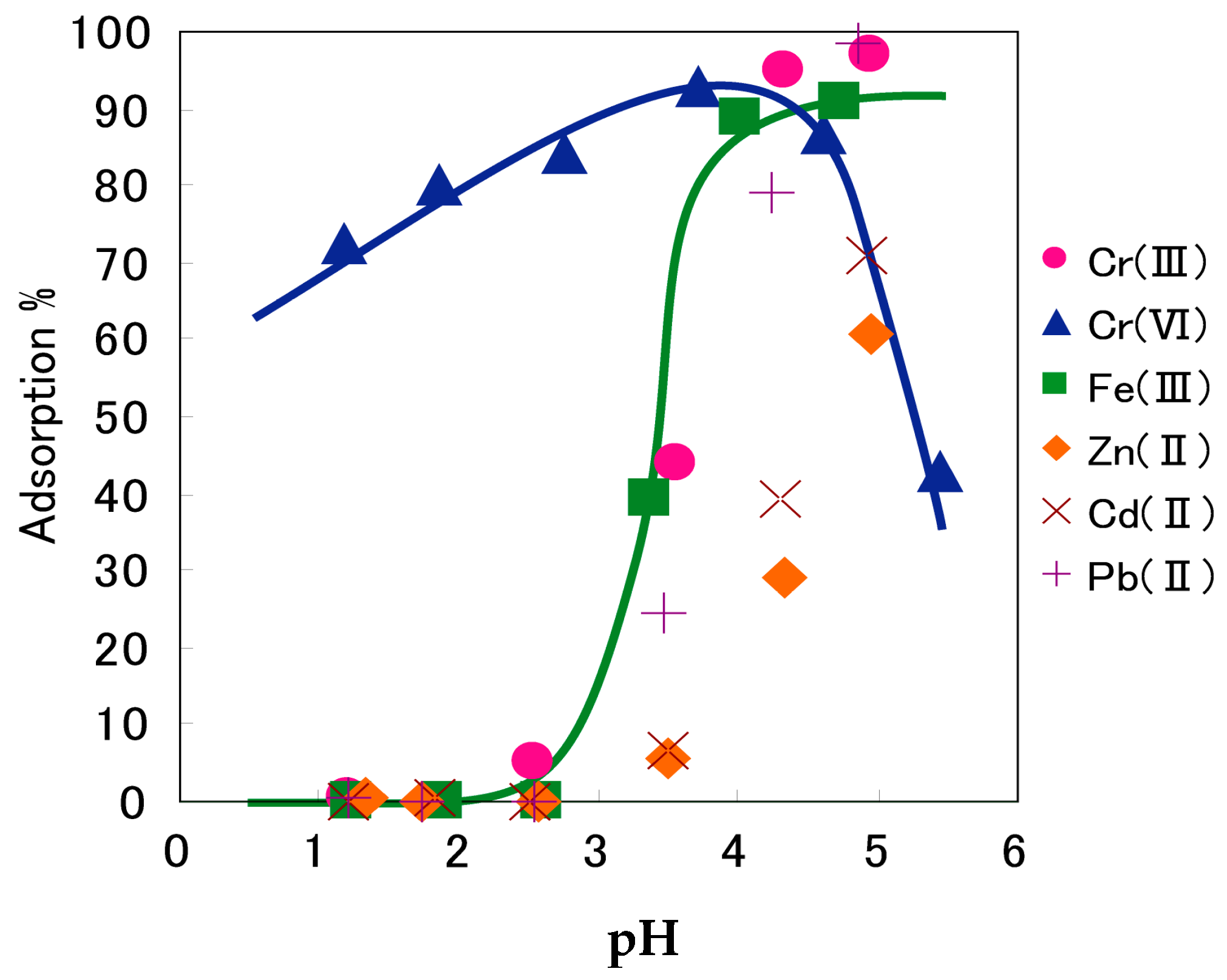
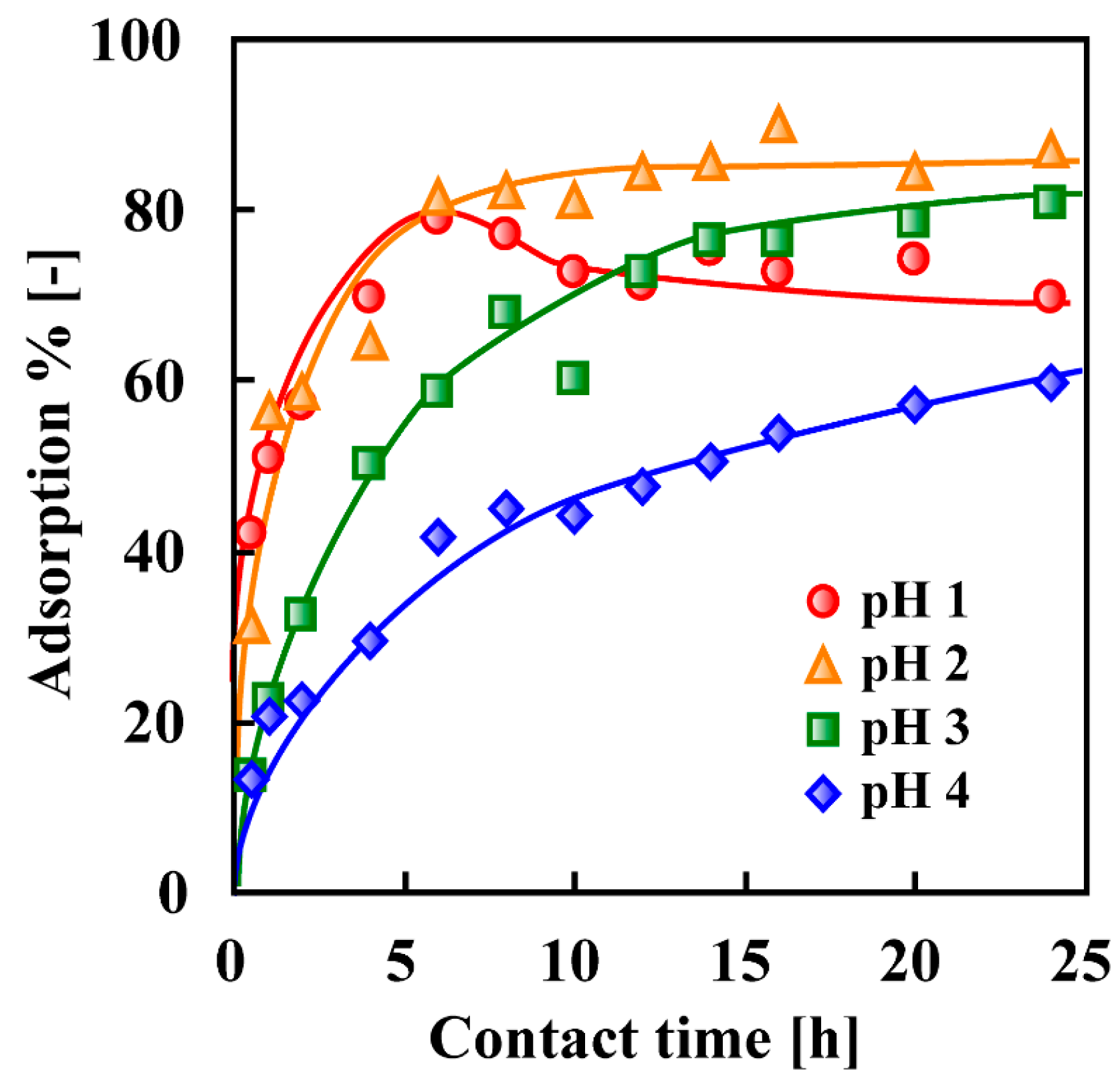
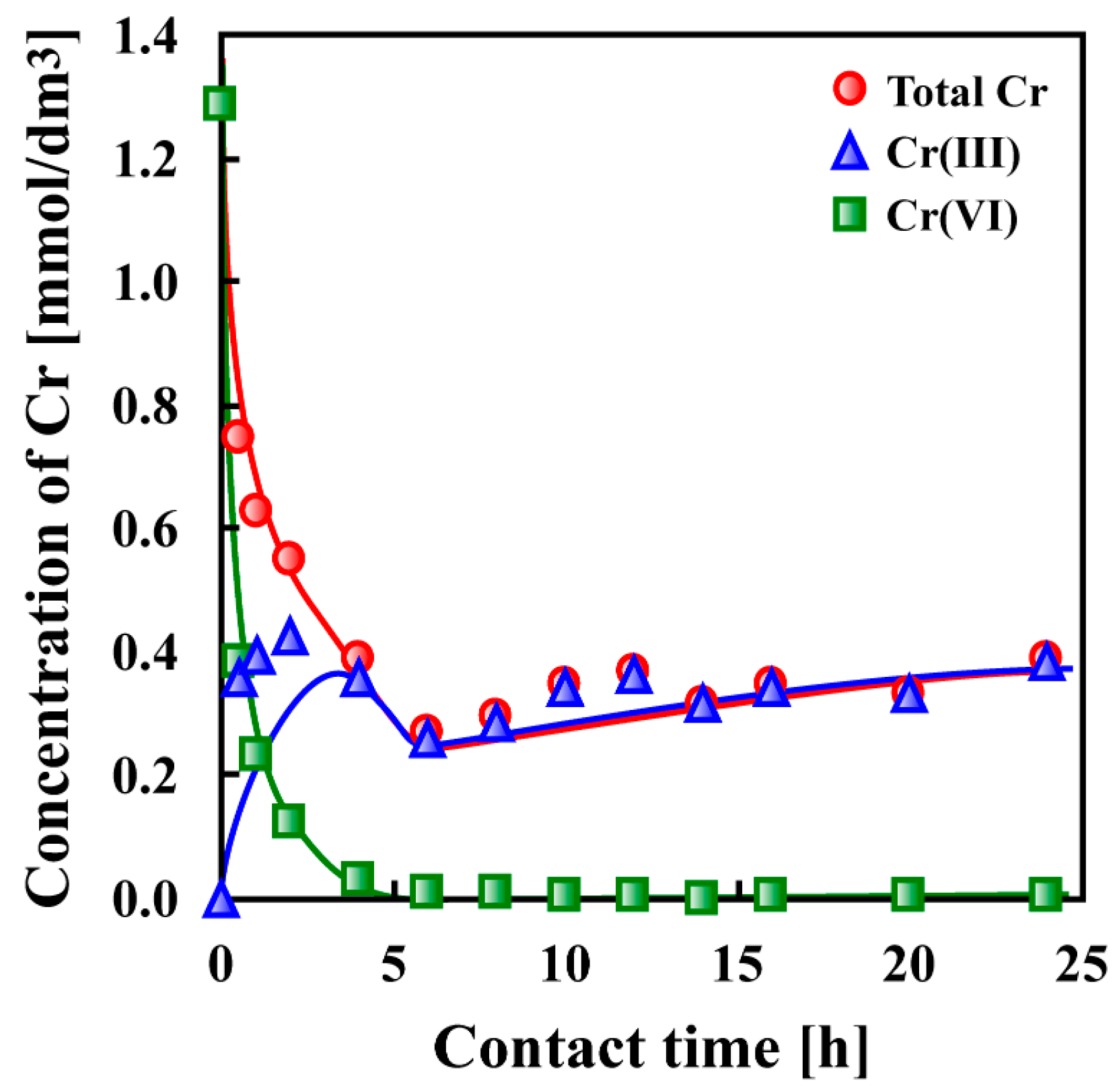
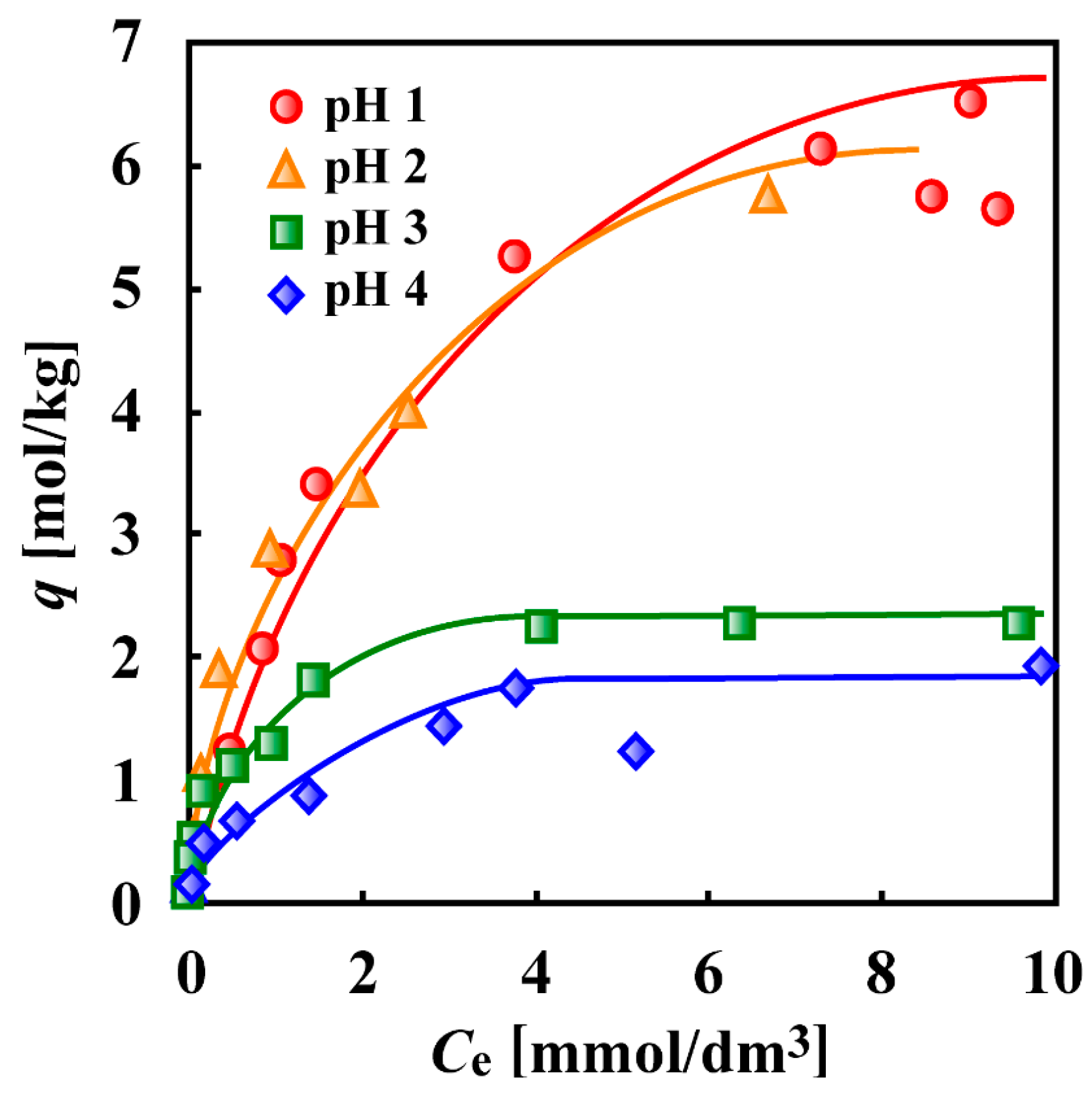
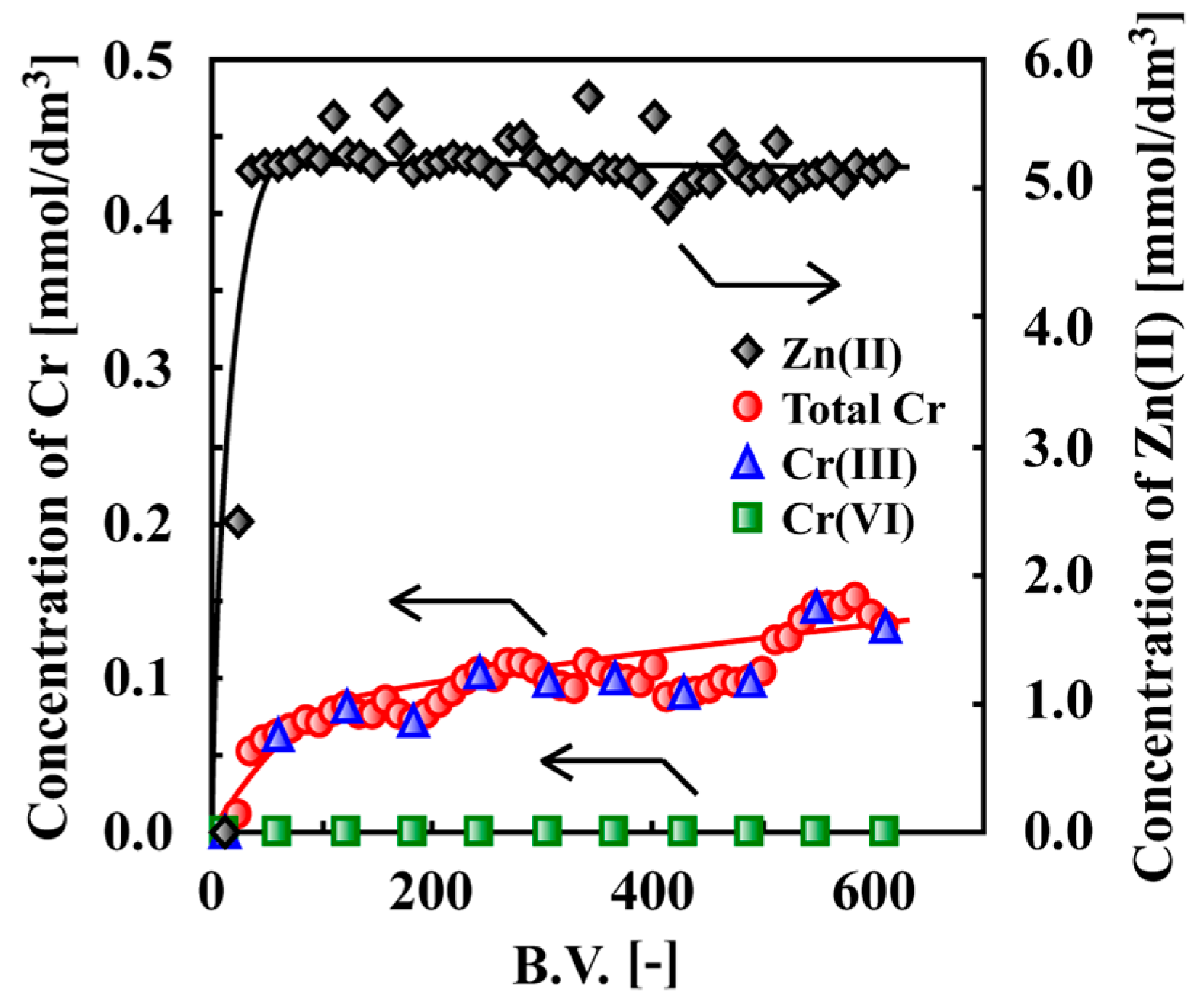
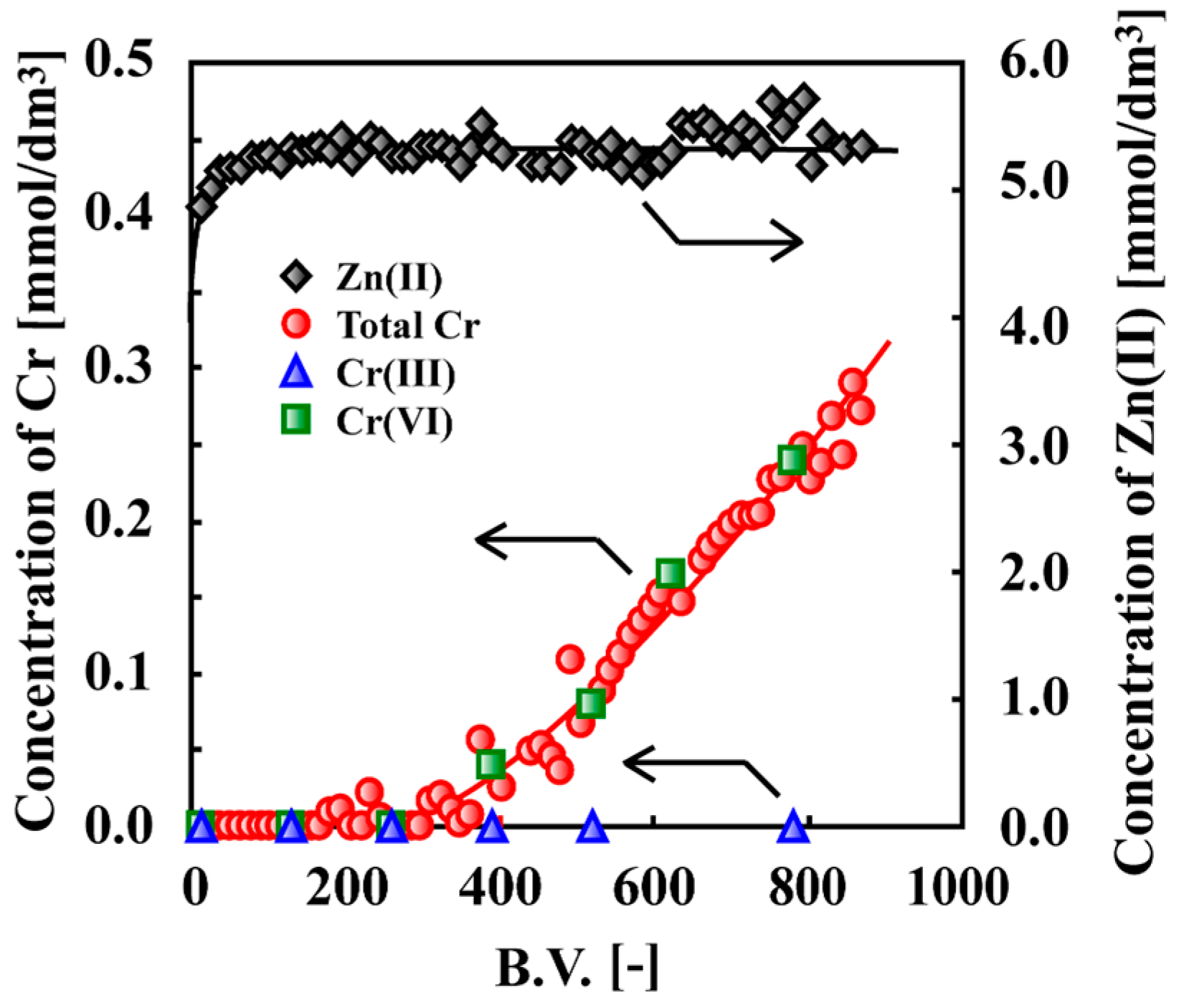
7. Adsorptive Removal of Hexa-Valent Cr Using Biosorbents Prepared from Persimmon Extract Residue and Grape Juice Residues


8. Conclusions
Author Contributions
Conflicts of Interest
References
- Volesky, B.; Holan, Z.R. Biosorption of heavy metals. Biotechnol. Prog. 1995, 11, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Baba, Y.; Yoshizuka, K. Adsorption of metal ions on chitosan and crosslinked copper(II)-complexed chitosan. Bull. Chem. Soc. Jpn. 1993, 66, 2915–2921. [Google Scholar] [CrossRef]
- Inoue, K.; Baba, Y. Chitosan: A Versatile biopolymer for separation, purification, and concentration of metal ions. In Ion Exchange and Solvent Extraction; Sengupta, A.K., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2007; Volume 18, pp. 339–374. [Google Scholar]
- Monties, B.; Fukushima, K. Occurrence, function and biosynthesis of lignins. In Biopolymers, Lignin, Humic Substances and Coal; Hoflichter, M., Steinbuchel, A., Eds.; WILEY-VCH: New York, NY, USA, 2001; pp. 1–64. [Google Scholar]
- Funaoka, M. A new type of phenolic lignin-based network polymer with the structure-variable function composed of 1,1-diarylpropane units. Polym. Int. 1998, 47, 277–290. [Google Scholar] [CrossRef]
- Parajuli, D.; Inoue, K.; Ohto, K.; Oshima, T.; Murota, A.; Funaoka, M.; Makino, K. Adsorption of heavy metals on crosslinked lignocatechol: A modified lignin gel. React. Funct. Polym. 2005, 62, 129–139. [Google Scholar] [CrossRef]
- Ametani, T. Additives of lignin compounds for cement and concrete. Gekkan Namakonkurito 2000, 19, 37–40. (In Japanese) [Google Scholar]
- Inoue, K.; Ohto, K.; Oshima, T.; Onishi, S.; Makino, K. Adsorption of metal ions on crosslinked lignosulphonic acid. Kagaku Kogaku Ronbunshu 2004, 30, 822–825. (In Japanese) [Google Scholar] [CrossRef]
- Dhakal, R.P.; Ghimire, K.N.; Inoue, K.; Yano, M.; Makino, K. Acidic polysaccharide gels for selective adsorption of lead(II) ion. Sep. Purif. Technol. 2005, 42, 219–225. [Google Scholar] [CrossRef]
- Helfferich, F. Ion Exchange; McGraw-Hill: New York, NY, USA, 1962. [Google Scholar]
- Inoue, K. Development of the technology for removing toxic metals and recovering valuable metals by the effective use of biomass wastes. J. MMIJ 2007, 123, 59–67. (In Japanese) [Google Scholar] [CrossRef]
- Inoue, K.; Shioya, A.; Zhu, Y.; Sedlackova, I.; Makino, K.; Makino, K.; Baba, Y. Adsorptive removal of arsenic by ferric ion-loaded crosslinked pectic acid gel. Kagaku Kogaku Ronbunshu 2003, 29, 389–394. (In Japanese) [Google Scholar] [CrossRef]
- Dhakal, R.P.; Ghimire, K.N.; Inoue, K. Adsorptive separation of heavy metals from an aquatic environment using orange waste. Hydrometallurgy 2005, 79, 182–190. [Google Scholar] [CrossRef]
- Inoue, K.; Ghimire, K.N.; Makino, K. Removal of impurity metal ions from waste plating solutions by using orange and apple juice residues. In EPD Congress 2003; Schlesinger, M.E., Ed.; TMS: Warrendale, PA, USA, 2003. [Google Scholar]
- Inoue, K.; Ghimire, K.N.; Hayashida, H.; Oshima, T.; Ohto, K.; Makino, K.; Kuboki, E.; Hashimoto, K. Removal of arsenic from mine water by the effective use of biomass. J. MMIJ 2003, 119, 767–771. (In Japanese) [Google Scholar] [CrossRef]
- Inoue, K. Adsorptive removal of hazardous inorganic elements from water by using orange waste. Indones. J. Chem. 2008, 8, 293–299. [Google Scholar]
- Biswas, B.K.; Inoue, K.; Ghimire, K.N.; Kawakita, H.; Ohto, K.; Harada, H. Effective removal of arsenic with lanthanum(III)- and cerium(III)-loaded orange waste gels. Sep. Sci. Technol. 2008, 43, 2144–2165. [Google Scholar] [CrossRef]
- Biswas, B.K.; Inoue, J.; Inoue, K.; Ghimire, K.N.; Harada, H.; Ohto, K.; Kawakita, H. Adsorptive removal of As(V) and As(III) from water by Zr(IV)-loaded orange waste gel. J. Hazard. Mater. 2008, 154, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, K.N.; Inoue, K.; Yamaguchi, H.; Makino, K.; Miyajima, T. Adsorptive separation of arsenate and arsenite anions from aqueous medium by using orange waste. Water Res. 2003, 37, 4945–4953. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Chen, K.Y. Arsenic removal by adsorption. J. Water Pollut. Control Fed. 1978, 50, 493–506. [Google Scholar]
- Wasay, S.A.; Tokunaga, S.; Park, S.W. Removal of hazardous anions from aqueous solutions by La(III)- and Y(III)-impregnated alumina. Sep. Sci. Technol. 1996, 31, 1501–1514. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, M.; Huang, X. Arsenic(V) removal with a Ce(IV)-doped iron oxide adsorbent. Chemosphere 2003, 51, 945–952. [Google Scholar] [CrossRef]
- Balaji, T.; Yokoyama, T.; Matsunaga, H. Adsorption and removal of As(V) and As(III) using Zr-loaded lysine diacetic acid chelating resin. Chemosphere 2005, 59, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.K.; Ribeiro, A.; Mateus, E. Biosorption of arsenic(V) with Lessonia nigrescens. Miner. Eng. 2006, 19, 486–490. [Google Scholar] [CrossRef]
- Matsunaga, H.; Yokoyama, T.; Eldridge, R.J.; Bolto, B.A. Adsorption characteristics of arsenic(III) and arsenic(V) on iron(III)-loaded chelating resin having lysine-N,N-diacetic acid moiety. React. Funct. Polym. 1996, 29, 167–174. [Google Scholar] [CrossRef]
- Haque, M.N.; Morrison, G.M.; Perrusquia, G.; Gutierrez, M.; Aguilera, A.F.; C-Aguilera, I.; G-Torresdey, J.L. Characteristics of arsenic adsorption to sorghum biomass. J. Hazard. Mater. 2007, 145, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Biswas, B.K.; Inoue, J.; Kawakita, H.; Ohto, K.; Inoue, K. Effective removal and recovery of antimony using metal-loaded saponified orange waste. J. Hazard. Mater. 2009, 172, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Deorkar, N.V.; Tavlarides, L.L. A chemically bonded adsorbent for separation of antimony, copper and lead. Hydrometallurgy 1997, 46, 121–135. [Google Scholar] [CrossRef]
- Thanabalasingam, P.; Pickering, W.F. Specific sorption of antimony(III) by the hydrous oxides of Mn, Fe and Al. Water Air Soil Pollut. 1990, 49, 175–185. [Google Scholar] [CrossRef]
- Xu, Y.H.; Ohki, A.; Maeda, S. Adsorption and removal of antimony from aqueous solution by an activated alumina 1. Adsorption capacity of adsorbent and effect of process variable. Toxicol. Environ. Chem. 2001, 80, 133–144. [Google Scholar] [CrossRef]
- Saeed, M.M.; Ahmed, M.; Ghaffar, A. Adsorption modeling of antimony(V) on diphenylthiocarbazone loaded polyurethane form. J. Radioanal. Nucl. Chem. 2003, 256, 121–126. [Google Scholar] [CrossRef]
- Ghimire, K.N.; Inoue, K.; Ohto, K.; Hayashida, T. Adsorption study of metal ions onto crosslinked seaweed Laminaria japonica. Biores. Technol. 2008, 99, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, K.N.; Inoue, K.; Ohto, K.; Hayashida, T. Adsorptive separation of metallic pollutants onto seaweeds, Porphyra Yezoensis and Ulva Japonica. Sep. Sci. Technol. 2007, 42, 2003–2018. [Google Scholar] [CrossRef]
- Matsuo, T.; Ito, S. The chemical structure of Kali-tannin from immature fruit of the persimmon (Diospyros kaki L.). Agric. Biol. Chem. 1978, 42, 1637–1643. [Google Scholar]
- Inoue, K.; Paudyal, H.; Nakagawa, H.; Kawakita, H.; Ohto, K. Selective adsorption of chromium(VI) from zinc and other metal ions using persimmon waste gel. Hydrometallurgy 2010, 104, 123–128. [Google Scholar] [CrossRef]
- Chand, R.; Narimura, K.; Kawakita, H.; Ohto, K.; Watari, T.; Inoue, K. Grape waste as a biosorbent for removing Cr(VI) from aqueous solution. J. Hazard. Mater. 2009, 163, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Baba, Y. Mechanism of hexavalent chromium adsorption by persimmon tannin gel. Water Res. 2004, 38, 2859–2864. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Takeshita, K.; Tsutsumi, T. Adsorption mechanism of hexavalent chromium by redox within condensed-tannin gel. Water Res. 2001, 35, 496–500. [Google Scholar] [CrossRef]
- Spinelli, V.A.; Lalanjeira, M.C.M.; Favere, V.T. Preparation and characterization of quaternary chitosan salt: Adsorption equilibrium of chromium(VI) ion. React. Funct. Polym. 2004, 61, 347–352. [Google Scholar] [CrossRef]
- Wartelle, L.H.; Marshall, W.E. Chromate ion adsorption by agricultural by-product modified with dimethyloldihydroxylethylene urea and choline chloride. Water Res. 2005, 39, 2869–2871. [Google Scholar] [CrossRef] [PubMed]
- Levankumar, L.; Muthukumaran, V.; Gobinath, M.B. Batch adsorption and kinetics of chromium(VI) removal from aqueous solutions by Ocimun americanum L. seed pods. J. Hazard. Mater. 2009, 161, 709–713. [Google Scholar] [CrossRef] [PubMed]














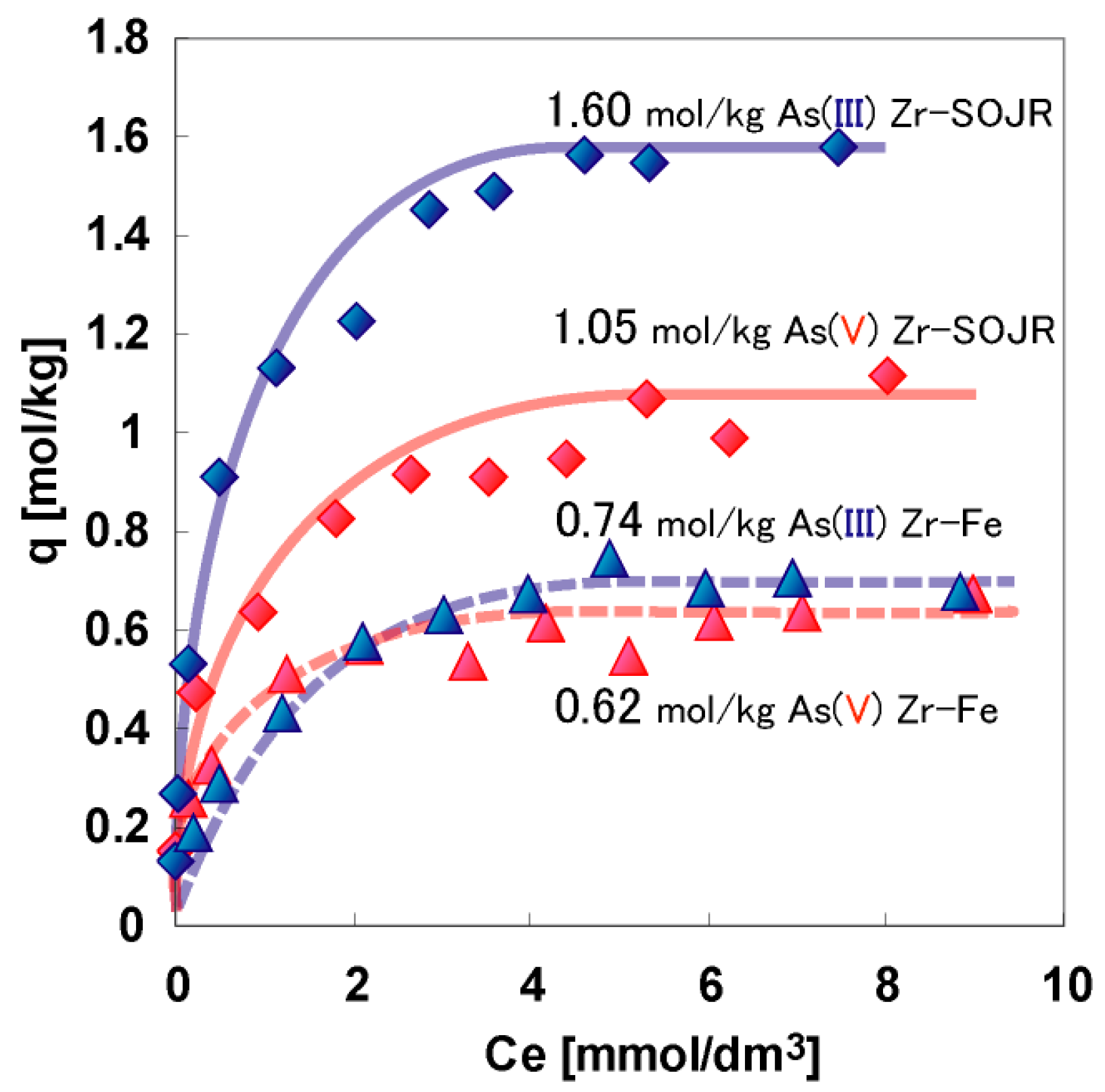







| Adsorbent | Maximum Adsorption Capacity (mol/kg) | As Species | Authors and Reference |
|---|---|---|---|
| Zr(IV)-loaded SOJR | 1.05 | As(V) | Biswas et al. [18] |
| Ce(III)-loaded SOJR | 0.56 | As(V) | Biswas et al. [17] |
| La(III)-loaded SOJR | 0.56 | As(V) | Biswas et al. [17] |
| Fe(III)-loaded POJR | 0.91 | As(V) | Ghimire et al. [19] |
| zirconium ferrite | 0.62 | As(V) | Inoue [16] |
| activated carbon | 0.014 | As(V) | Gupta and Chen [20] |
| La(III)-impregnated alumina | 0.172 | As(V) | Wasay et al. [21] |
| Ce(IV)-doped iron oxide | 0.213 | As(V) | Zhang et al. [22] |
| Zr(IV)-loaded LDA chelating resin | 0.655 | As(V) | Balaji et al. [23] |
| Lessinia nigrescens, an algae | 0.376 | As(V) | Hansen et al. [24] |
| Zr(IV)-loaded SOJR | 1.6 | As(III) | Biswas et al. [18] |
| Ce(III)-loaded SOJR | 0.57 | As(III) | Biswas et al. [17] |
| La(III)-loaded SOJR | 0.57 | As(III) | Biswas et al. [17] |
| Fe(III)-loaded LDA chelating resin | 0.83 | As(III) | Matsunaga et al. [25] |
| Fe(III)-loaded POJR | 0.91 | As(III) | Ghimire et al. [19] |
| Sorghum biomass | 0.05 | As(III) | Haque et al. [26] |
| Species | Adsorbent | Maximum Adsorption Capacity (mmol/g) | Authors and Reference |
|---|---|---|---|
| Sb(III) | Zr(IV)-loaded SOJR | 0.94 | Biswas et al. [27] |
| Sb(III) | Fe(III)-loaded SOJR | 1.12 | Biswas et al. [27] |
| Sb(V) | Zr(IV)-loaded SOJR | 1.19 | Biswas et al. [27] |
| Sb(V) | Fe(III)-loaded SOJR | 1.19 | Biswas et al. [27] |
| Sb(III) | silica gel immobilized with pyrogallol functional groups | 0.18 | Deorkar et al. [28] |
| Sb(III) | hydrous oxide of Mn | 0.14 | Thanabalasingam et al. [29] |
| Sb(III) | hydrous oxide of Fe | 0.10 | Thanabalasingam et al. [29] |
| Sb(V) | activated alumina of different mesh | 0.051–0.774 | Xu et al. [30] |
| Sb(V) | aluminum-loaded Shirasu zeolite | 0.020 | Xu et al. [30] |
| Sb(V) | diphenylthiocarbazone-loaded polyurethane foam | 0.22 | Saeed et al. [31] |
| Seaweeds Metal Ions | Laminaria Japonica | Ulva Japonica | Porphyra Yezoensis |
|---|---|---|---|
| Pb(II) | 1.35 | 0.77 | 0.27 |
| Cd(II) | 1.10 | 0.77 | 0.32 |
| La(III) | 0.87 | 0.51 | 0.23 |
| Ce(III) | 0.87 | 0.50 | 0.23 |
| Feed Material of the Biosorbent | pH | Maximum Adsorption Capacity (mol/kg) | Reference |
|---|---|---|---|
| Mimosa tannin | 2 | 5.52 | Nakano et al. [38] |
| Persimmon extract | 3 | 5.27 | Nakajima and Baba [37] |
| Quaternary chitosan | 4.5 | 0.58 | Spinelli et al. [39] |
| Sugarcane bagasse | 3 | 1.97 | Wartell and Marshal [40] |
| Ocimun americanum | 1.5 | 1.6 | Levankumar et al. [41] |
| Grape juice residue | 4 | 1.91 | Chand et al. [36] |
| Persimmon extract residue | 1 | 7.18 | Inoue et al. [35] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, K.; Parajuli, D.; Ghimire, K.N.; Biswas, B.K.; Kawakita, H.; Oshima, T.; Ohto, K. Biosorbents for Removing Hazardous Metals and Metalloids. Materials 2017, 10, 857. https://doi.org/10.3390/ma10080857
Inoue K, Parajuli D, Ghimire KN, Biswas BK, Kawakita H, Oshima T, Ohto K. Biosorbents for Removing Hazardous Metals and Metalloids. Materials. 2017; 10(8):857. https://doi.org/10.3390/ma10080857
Chicago/Turabian StyleInoue, Katsutoshi, Durga Parajuli, Kedar Nath Ghimire, Biplob Kumar Biswas, Hidetaka Kawakita, Tatsuya Oshima, and Keisuke Ohto. 2017. "Biosorbents for Removing Hazardous Metals and Metalloids" Materials 10, no. 8: 857. https://doi.org/10.3390/ma10080857





