Specific Nuclear Magnetic Resonance Lipoprotein Subclass Profiles and Central Arterial Stiffness in Type 1 Diabetes Mellitus: A Case Control Study
Abstract
:1. Introduction
2. Methods
2.1. Study Participants
2.2. Study Design
2.2.1. Laboratory Analyses
2.2.2. Insulin Resistance
2.2.3. Assessment of Microvascular Complications
2.2.4. Lipoprotein Analysis by NMR Spectroscopy
2.2.5. Measurement of Central Arterial Stiffness
2.3. Statistical Analyses
3. Results
3.1. Study Population
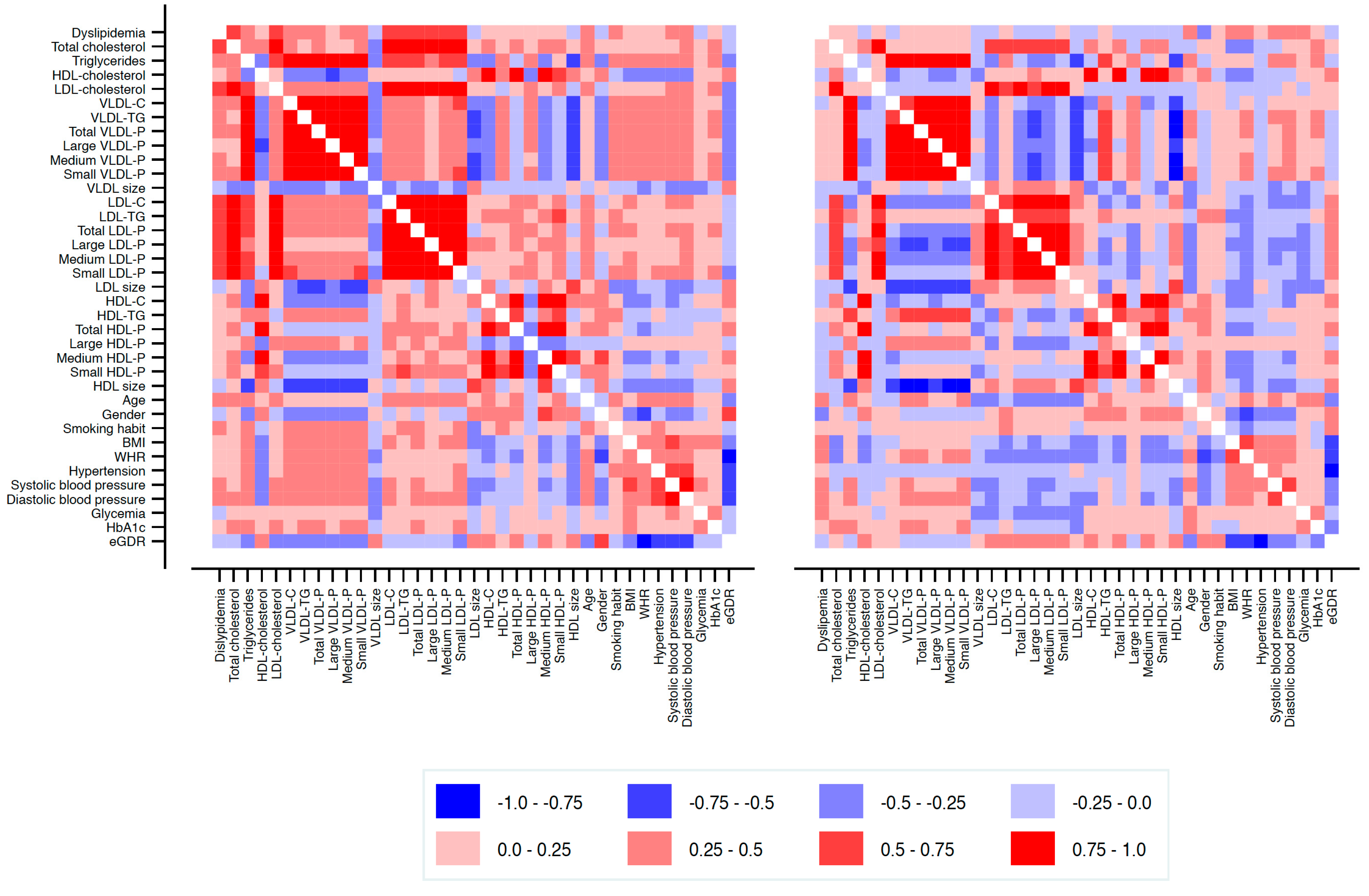
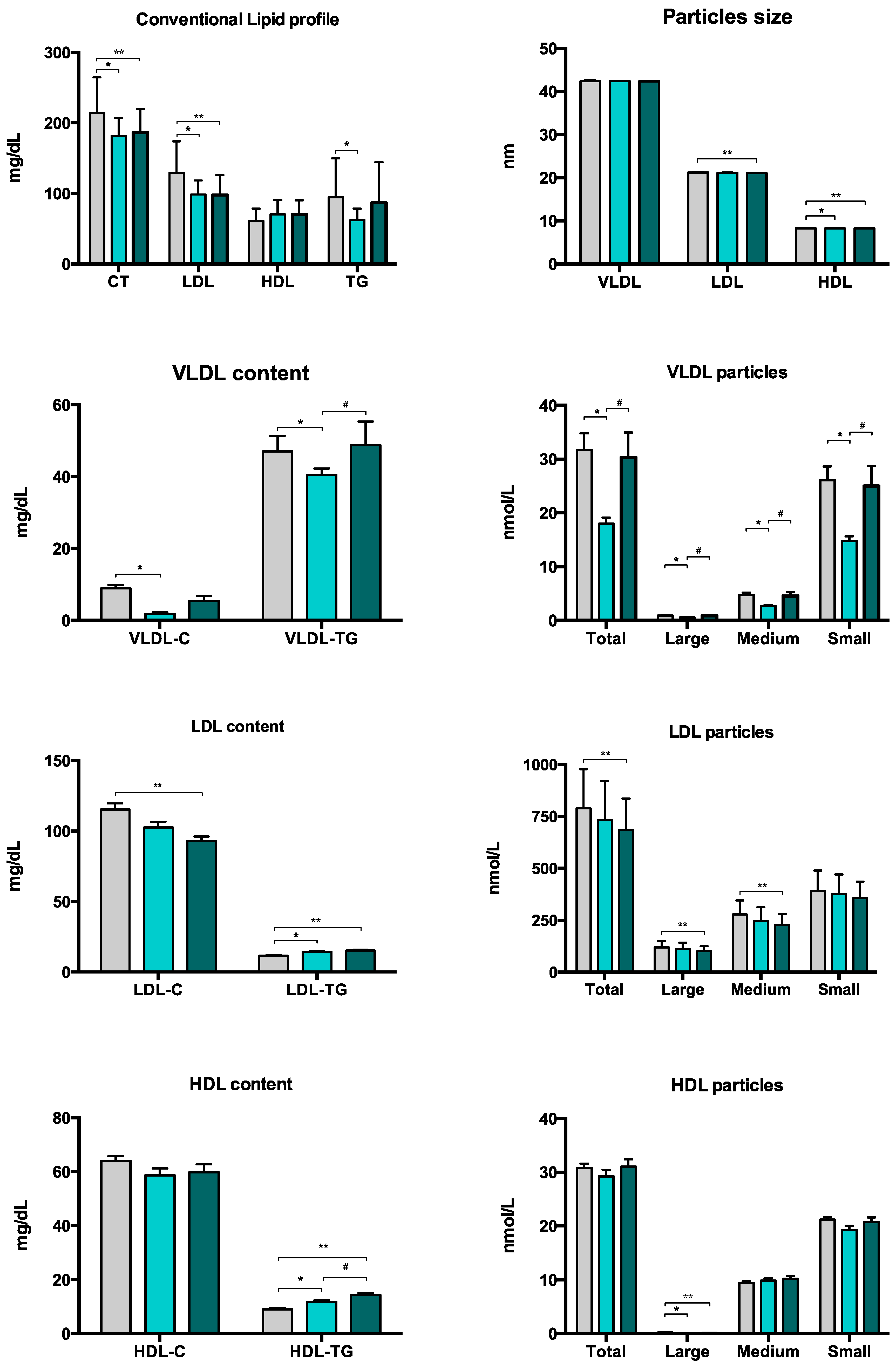
3.2. Lipoprotein Subclass Profiles
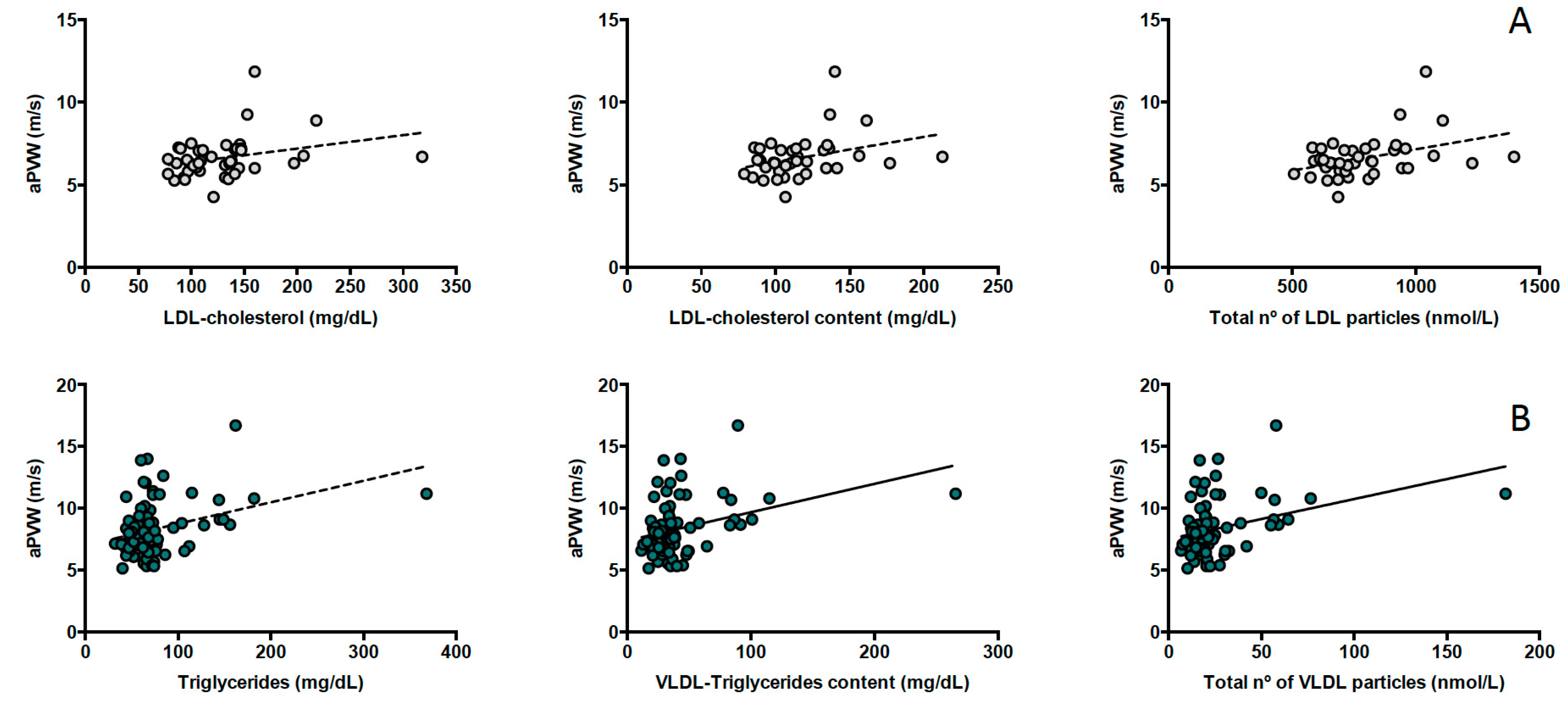
3.3. Lipoproteins and Central Arterial Stiffness
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- de Ferranti, S.D.; de Boer, I.H.; Fonseca, V.; Fox, C.S.; Golden, S.H.; Lavie, C.J.; Magge, S.N.; Marx, N.; McGuire, D.K.; Orchard, T.J.; et al. Type 1 diabetes mellitus and cardiovascular disease: A scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care 2014, 37, 2843–2863. [Google Scholar] [CrossRef] [PubMed]
- Lind, M.; Svensson, A.M.; Kosiborod, M.; Gudbjornsdottir, S.; Pivodic, A.; Wedel, H.; Dahlqvist, S.; Clements, M.; Rosengren, A. Glycemic control and excess mortality in type 1 diabetes. N. Engl. J. Med. 2014, 371, 1972–1982. [Google Scholar] [CrossRef] [PubMed]
- Rawshani, A.; Rawshani, A.; Franzen, S.; Eliasson, B.; Svensson, A.M.; Miftaraj, M.; McGuire, D.K.; Sattar, N.; Rosengren, A.; Gudbjornsdottir, S. Mortality and Cardiovascular Disease in Type 1 and Type 2 Diabetes. N. Engl. J. Med. 2017, 376, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.R.; Peters, S.A.; Mishra, G.D.; Woodward, M. Risk of all–cause mortality and vascular events in women versus men with type 1 diabetes: A systematic review and meta–analysis. Lancet Diabetes Endocrinol. 2015, 3, 198–206. [Google Scholar] [CrossRef]
- Rawshani, A.; Sattar, N.; Franzen, S.; Rawshani, A.; Hattersley, A.T.; Svensson, A.M.; Eliasson, B.; Gudbjornsdottir, S. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: A nationwide, register–based cohort study. Lancet 2018, 392, 477–486. [Google Scholar] [CrossRef]
- Bebu, I.; Braffett, B.H.; Orchard, T.J.; Lorenzi, G.M.; Lachin, J.M.; Group, D.E.R. Mediation of the Effect of Glycemia on the Risk of CVD Outcomes in Type 1 Diabetes: The DCCT/EDIC Study. Diabetes Care 2019, 42, 1284–1289. [Google Scholar] [CrossRef] [Green Version]
- Maahs, D.M.; Daniels, S.R.; de Ferranti, S.D.; Dichek, H.L.; Flynn, J.; Goldstein, B.I.; Kelly, A.S.; Nadeau, K.J.; Martyn–Nemeth, P.; Osganian, S.K.; et al. Cardiovascular disease risk factors in youth with diabetes mellitus: A scientific statement from the American Heart Association. Circulation 2014, 130, 1532–1558. [Google Scholar] [CrossRef]
- Verges, B. Lipid disorders in type 1 diabetes. Diabetes Metab. 2009, 35, 353–360. [Google Scholar] [CrossRef]
- Mallol, R.; Rodriguez, M.A.; Brezmes, J.; Masana, L.; Correig, X. Human serum/plasma lipoprotein analysis by NMR: Application to the study of diabetic dyslipidemia. Prog. Nucl. Magn. Reson. Spectrosc. 2013, 70, 1–24. [Google Scholar] [CrossRef]
- Colhoun, H.M.; Otvos, J.D.; Rubens, M.B.; Taskinen, M.R.; Underwood, S.R.; Fuller, J.H. Lipoprotein subclasses and particle sizes and their relationship with coronary artery calcification in men and women with and without type 1 diabetes. Diabetes 2002, 51, 1949–1956. [Google Scholar] [CrossRef]
- Lyons, T.J.; Jenkins, A.J.; Zheng, D.; Klein, R.L.; Otvos, J.D.; Yu, Y.; Lackland, D.T.; McGee, D.; McHenry, M.B.; Lopes–Virella, M.; et al. Nuclear magnetic resonance–determined lipoprotein subclass profile in the DCCT/EDIC cohort: Associations with carotid intima–media thickness. Diabet. Med. 2006, 23, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Jenkins, A.J.; Zhang, Y.; Stoner, J.A.; Klein, R.L.; Lopes–Virella, M.F.; Garvey, W.T.; Lyons, T.J.; Group, D.E.R. Nuclear magnetic resonance–determined lipoprotein subclasses and carotid intima–media thickness in type 1 diabetes. Atherosclerosis 2016, 244, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, J.A.; Segers, P.; Hughes, T.; Townsend, R. Large–Artery Stiffness in Health and Disease: JACC State–of–the–Art Review. J. Am. Coll. Cardiol. 2019, 74, 1237–1263. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all–cause mortality with arterial stiffness: A systematic review and meta–analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Ben–Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.H.; Cruickshank, J.K.; et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta–analysis of prospective observational data from 17,635 subjects. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Hu, M.J.; Cui, Y.J.; Liang, L.; Zhou, M.M.; Yang, Y.W.; Huang, F. Carotid–Femoral Pulse Wave Velocity in the Prediction of Cardiovascular Events and Mortality: An Updated Systematic Review and Meta–Analysis. Angiology 2018, 69, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Llaurado, G.; Ceperuelo–Mallafre, V.; Vilardell, C.; Simo, R.; Freixenet, N.; Vendrell, J.; Gonzalez–Clemente, J.M. Arterial stiffness is increased in patients with type 1 diabetes without cardiovascular disease: A potential role of low–grade inflammation. Diabetes Care 2012, 35, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Hallal, P.C.; Victora, C.G. Reliability and validity of the International Physical Activity Questionnaire (IPAQ). Med. Sci. Sports Exerc. 2004, 36, 556. [Google Scholar] [CrossRef]
- Meaney, E.; Alva, F.; Moguel, R.; Meaney, A.; Alva, J.; Webel, R. Formula and nomogram for the sphygmomanometric calculation of the mean arterial pressure. Heart (Br. Card. Soc.) 2000, 84, 64. [Google Scholar] [CrossRef]
- Mancia, G.; De Backer, G.; Dominiczak, A.; Cifkova, R.; Fagard, R.; Germano, G.; Grassi, G.; Heagerty, A.M.; Kjeldsen, S.E.; Laurent, S.; et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2007, 28, 1462–1536. [Google Scholar] [CrossRef]
- National Cholesterol Education Program (US). Expert Panel on Detection; Treatment of High Blood Cholesterol in Adults. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low–density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [PubMed]
- Williams, K.V.; Erbey, J.R.; Becker, D.; Arslanian, S.; Orchard, T.J. Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes 2000, 49, 626–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilpatrick, E.S.; Rigby, A.S.; Atkin, S.L. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes: "double diabetes" in the Diabetes Control and Complications Trial. Diabetes Care 2007, 30, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.L.; Stevens, M.J.; Thomas, P.K.; Brown, M.B.; Canal, N.; Greene, D.A. A practical two–step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994, 17, 1281–1289. [Google Scholar] [CrossRef]
- Perkins, B.A.; Olaleye, D.; Zinman, B.; Bril, V. Simple screening tests for peripheral neuropathy in the diabetes clinic. Diabetes Care 2001, 24, 250–256. [Google Scholar] [CrossRef]
- American Diabetes Association. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes–2019. Diabetes Care 2019, 42, S124–S138. [Google Scholar] [CrossRef]
- Mallol, R.; Amigo, N.; Rodriguez, M.A.; Heras, M.; Vinaixa, M.; Plana, N.; Rock, E.; Ribalta, J.; Yanes, O.; Masana, L.; et al. Liposcale: A novel advanced lipoprotein test based on 2D diffusion–ordered 1H NMR spectroscopy. J. Lipid Res. 2015, 56, 737–746. [Google Scholar] [CrossRef]
- Jeyarajah, E.J.; Cromwell, W.C.; Otvos, J.D. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin. Lab. Med. 2006, 26, 847–870. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Brewer, H.B., Jr.; Chapman, M.J.; Fazio, S.; Hussain, M.M.; Kontush, A.; Krauss, R.M.; Otvos, J.D.; Remaley, A.T.; Schaefer, E.J. HDL measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin. Chem. 2011, 57, 392–410. [Google Scholar] [CrossRef]
- Mallol, R.; Rodriguez, M.A.; Heras, M.; Vinaixa, M.; Plana, N.; Masana, L.; Morris, G.A.; Correig, X. Particle size measurement of lipoprotein fractions using diffusion–ordered NMR spectroscopy. Anal. Bioanal. Chem. 2012, 402, 2407–2415. [Google Scholar] [CrossRef] [PubMed]
- Mallol, R.; Rodríguez, M.A.; Heras, M.; Vinaixa, M.; Cañellas, N.; Brezmes, J.; Plana, N.; Masana, L.; Correig, X. Surface fitting of 2D diffusion–edited 1H NMR spectroscopy data for the characterisation of human plasma lipoproteins. Metabolomics 2011, 7, 572–582. [Google Scholar] [CrossRef]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker–Boudier, H. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef] [PubMed]
- Brugnara, L.; Mallol, R.; Ribalta, J.; Vinaixa, M.; Murillo, S.; Casserras, T.; Guardiola, M.; Vallve, J.C.; Kalko, S.G.; Correig, X.; et al. Improving Assessment of Lipoprotein Profile in Type 1 Diabetes by 1H NMR Spectroscopy. PLoS ONE 2015, 10, e0136348. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2019, pehz455. [Google Scholar] [CrossRef]
- Annuzzi, G.; Iovine, C.; Mandarino, B.; Patti, L.; Di Marino, L.; Riccardi, G.; Rivellese, A.A. Effect of acute exogenous hyperinsulinaemia on very low density lipoprotein subfraction composition in normal subjects. Eur. J. Clin. Investig. 2001, 31, 118–124. [Google Scholar] [CrossRef]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [Green Version]
- Hother–Nielsen, O.; Schmitz, O.; Bak, J.; Beck–Nielsen, H. Enhanced hepatic insulin sensitivity, but peripheral insulin resistance in patients with type 1 (insulin–dependent) diabetes. Diabetologia 1987, 30, 834–840. [Google Scholar]
- Rosental, S.B.; Schreier, L.E.; Halperin, H.; Berg, G.; Paglione, A.M.; Ruiz, M.; Wikinski, R.L. [Low density lipoprotein rich in triglycerides and hepatic lipase activity in insulin–dependent diabetic patients]. Medicina (B Aires) 1995, 55, 317–323. [Google Scholar]
- Hughes, T.A.; Calderon, R.M.; Diaz, S.; Mendez, A.J.; Goldberg, R.B. Lipoprotein composition in patients with type 1 diabetes mellitus: Impact of lipases and adipokines. J. Diabetes Complicat. 2016, 30, 657–668. [Google Scholar] [CrossRef]
- Guerin, M.; Bruckert, E.; Dolphin, P.J.; Turpin, G.; Chapman, M.J. Fenofibrate reduces plasma cholesteryl ester transfer from HDL to VLDL and normalizes the atherogenic, dense LDL profile in combined hyperlipidemia. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Ikewaki, K.; Tohyama, J.; Nakata, Y.; Wakikawa, T.; Kido, T.; Mochizuki, S. Fenofibrate effectively reduces remnants, and small dense LDL, and increases HDL particle number in hypertriglyceridemic men–a nuclear magnetic resonance study. J. Atheroscler. Thromb. 2004, 11, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Soedamah–Muthu, S.S.; Colhoun, H.M.; Thomason, M.J.; Betteridge, D.J.; Durrington, P.N.; Hitman, G.A.; Fuller, J.H.; Julier, K.; Mackness, M.I.; Neil, H.A.; et al. The effect of atorvastatin on serum lipids, lipoproteins and NMR spectroscopy defined lipoprotein subclasses in type 2 diabetic patients with ischaemic heart disease. Atherosclerosis 2003, 167, 243–255. [Google Scholar] [CrossRef]
- Jenkins, A.J.; Lyons, T.J.; Zheng, D.; Otvos, J.D.; Lackland, D.T.; McGee, D.; Garvey, W.T.; Klein, R.L.; Group, D.E.R. Lipoproteins in the DCCT/EDIC cohort: Associations with diabetic nephropathy. Kidney Int. 2003, 64, 817–828. [Google Scholar] [CrossRef] [Green Version]
- Makinen, V.P.; Soininen, P.; Kangas, A.J.; Forsblom, C.; Tolonen, N.; Thorn, L.M.; Viikari, J.; Raitakari, O.T.; Savolainen, M.; Groop, P.H.; et al. Triglyceride–cholesterol imbalance across lipoprotein subclasses predicts diabetic kidney disease and mortality in type 1 diabetes: The FinnDiane Study. J. Intern. Med. 2013, 273, 383–395. [Google Scholar] [CrossRef]
- Hallman, D.M.; Brown, S.A.; Ballantyne, C.M.; Sharrett, A.R.; Boerwinkle, E. Relationship between low–density lipoprotein subclasses and asymptomatic atherosclerosis in subjects from the Atherosclerosis Risk in Communities (ARIC) Study. Biomarkers 2004, 9, 190–202. [Google Scholar] [CrossRef]
- Mora, S.; Szklo, M.; Otvos, J.D.; Greenland, P.; Psaty, B.M.; Goff, D.C., Jr.; O’Leary, D.H.; Saad, M.F.; Tsai, M.Y.; Sharrett, A.R. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi–Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2007, 192, 211–217. [Google Scholar] [CrossRef]
- Gallo, L.M.; Silverstein, J.H.; Shuster, J.J.; Haller, M.J. Arterial stiffness, lipoprotein particle size, and lipoprotein particle concentration in children with type 1 diabetes. J. Pediatric Endocrinol. Metab. JPEM 2010, 23, 661–667. [Google Scholar] [CrossRef]
- Cote, A.T.; Harris, K.C.; Panagiotopoulos, C.; Sandor, G.G.; Devlin, A.M. Childhood obesity and cardiovascular dysfunction. J. Am. Coll. Cardiol. 2013, 62, 1309–1319. [Google Scholar] [CrossRef]
- Soedamah–Muthu, S.S.; Chang, Y.F.; Otvos, J.; Evans, R.W.; Orchard, T.J.; Pittsburgh Epidemiology of Diabetes Complications Study. Lipoprotein subclass measurements by nuclear magnetic resonance spectroscopy improve the prediction of coronary artery disease in Type 1 diabetes. A prospective report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia 2003, 46, 674–682. [Google Scholar] [CrossRef]
- Llaurado, G.; Simo, R.; Villaplana, M.; Berlanga, E.; Vendrell, J.; Gonzalez–Clemente, J.M. Can augmentation index substitute aortic pulse wave velocity in the assessment of central arterial stiffness in type 1 diabetes? Acta Diabetol. 2012, 49 (Suppl. 1), S253–S257. [Google Scholar] [CrossRef]
- Austin, M.A. Small, dense low–density lipoprotein as a risk factor for coronary heart disease. Int. J. Clin. Lab. Res. 1994, 24, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Ganjali, S.; Dallinga–Thie, G.M.; Simental–Mendia, L.E.; Banach, M.; Pirro, M.; Sahebkar, A. HDL functionality in type 1 diabetes. Atherosclerosis 2017, 267, 99–109. [Google Scholar] [CrossRef] [PubMed]
- van der Steeg, W.A.; Holme, I.; Boekholdt, S.M.; Larsen, M.L.; Lindahl, C.; Stroes, E.S.; Tikkanen, M.J.; Wareham, N.J.; Faergeman, O.; Olsson, A.G.; et al. High–density lipoprotein cholesterol, high–density lipoprotein particle size, and apolipoprotein A–I: Significance for cardiovascular risk: The IDEAL and EPIC–Norfolk studies. J. Am. Coll. Cardiol. 2008, 51, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G. Triglyceride–Rich Lipoproteins and Atherosclerotic Cardiovascular Disease: New Insights From Epidemiology, Genetics, and Biology. Circ. Res. 2016, 118, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.V.; Millwood, I.Y.; Kartsonaki, C.; Hill, M.R.; Bennett, D.A.; Boxall, R.; Guo, Y.; Xu, X.; Bian, Z.; Hu, R.; et al. Lipids, Lipoproteins, and Metabolites and Risk of Myocardial Infarction and Stroke. J. Am. Coll. Cardiol. 2018, 71, 620–632. [Google Scholar] [CrossRef]
- Girona, J.; Amigo, N.; Ibarretxe, D.; Plana, N.; Rodriguez–Borjabad, C.; Heras, M.; Ferre, R.; Gil, M.; Correig, X.; Masana, L. HDL Triglycerides: A New Marker of Metabolic and Cardiovascular Risk. Int. J. Mol. Sci. 2019, 20, 3151. [Google Scholar] [CrossRef]
- Allaire, J.; Vors, C.; Couture, P.; Lamarche, B. LDL particle number and size and cardiovascular risk: Anything new under the sun? Curr. Opin. Lipidol. 2017, 28, 261–266. [Google Scholar] [CrossRef]
- Contois, J.H.; McConnell, J.P.; Sethi, A.A.; Csako, G.; Devaraj, S.; Hoefner, D.M.; Warnick, G.R.; Lipoproteins, A.; Vascular Diseases Division Working Group on Best Practices. Apolipoprotein B and cardiovascular disease risk: Position statement from the AACC Lipoproteins and Vascular Diseases Division Working Group on Best Practices. Clin. Chem. 2009, 55, 407–419. [Google Scholar] [CrossRef]
- Cole, T.G.; Contois, J.H.; Csako, G.; McConnell, J.P.; Remaley, A.T.; Devaraj, S.; Hoefner, D.M.; Mallory, T.; Sethi, A.A.; Warnick, G.R.; et al. Association of apolipoprotein B and nuclear magnetic resonance spectroscopy–derived LDL particle number with outcomes in 25 clinical studies: Assessment by the AACC Lipoprotein and Vascular Diseases Division Working Group on Best Practices. Clin. Chem. 2013, 59, 752–770. [Google Scholar] [CrossRef]
- Cleland, S.J. Cardiovascular risk in double diabetes mellitus––when two worlds collide. Nat. Rev. Endocrinol. 2012, 8, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Chillaron, J.J.; Flores Le–Roux, J.A.; Benaiges, D.; Pedro–Botet, J. Type 1 diabetes, metabolic syndrome and cardiovascular risk. Metab. Clin. Exp. 2014, 63, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Orchard, T.J.; Olson, J.C.; Erbey, J.R.; Williams, K.; Forrest, K.Y.; Smithline Kinder, L.; Ellis, D.; Becker, D.J. Insulin resistance–related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10–year follow–up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care 2003, 26, 1374–1379. [Google Scholar] [CrossRef] [PubMed]
- Pambianco, G.; Costacou, T.; Orchard, T.J. The prediction of major outcomes of type 1 diabetes: A 12–year prospective evaluation of three separate definitions of the metabolic syndrome and their components and estimated glucose disposal rate: The Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes Care 2007, 30, 1248–1254. [Google Scholar] [PubMed]
- The Diabetes Control; Complications Trial/Epidemiology of Diabetes Interventions; Complications (DCCT/EDIC) Research Group. Risk Factors for Cardiovascular Disease in Type 1 Diabetes. Diabetes 2016, 65, 1370–1379. [Google Scholar] [CrossRef] [Green Version]
- Llaurado, G.; Cano, A.; Hernandez, C.; Gonzalez–Sastre, M.; Rodriguez, A.A.; Punti, J.; Berlanga, E.; Albert, L.; Simo, R.; Vendrell, J.; et al. Type 1 diabetes: Developing the first risk–estimation model for predicting silent myocardial ischemia. The potential role of insulin resistance. PLoS ONE 2017, 12, e0174640. [Google Scholar] [CrossRef]
- Maahs, D.M.; Hokanson, J.E.; Wang, H.; Kinney, G.L.; Snell–Bergeon, J.K.; East, A.; Bergman, B.C.; Schauer, I.E.; Rewers, M.; Eckel, R.H. Lipoprotein subfraction cholesterol distribution is proatherogenic in women with type 1 diabetes and insulin resistance. Diabetes 2010, 59, 1771–1779. [Google Scholar] [CrossRef]
| T1DM (n = 84) | Controls (n = 42) | p-Value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age (years) | 50.1 (9.3) | 41.7 (7.5) | <0.001 |
| Gender (male/female), n (%) | 42 (50.0)/42 (50.0) | 23 (54.8)/19 (45.2) | 0.614 |
| Current smokers, n (%) | 31 (36.9) | 12 (28.6) | 0.648 |
| Physical activity (MET–min/w) | 1386 (693–2286) | 1530 (873–2079) | 0.202 |
| Family history of premature CVD, n (%) | 14 (16.7) | 3 (7.1) | 0.174 |
| Family history of T2DM, n (%) | 23 (27.4) | 12 (28.6) | 0.888 |
| Blood pressure | |||
| Hypertension, n (%) | 34 (40.5) | 3 (7.1) | <0.001 |
| Systolic BP (mmHg) | 126.4 (12.4) | 121.5 (11.3) | 0.031 |
| Diastolic BP (mmHg) | 71.9 (9.1) | 72.2 (9.3) | 0.897 |
| MAP (mmHg) | 90.1 (9.3) | 88.6 (9.5) | 0.395 |
| Anthropometric parameters | |||
| Weight (kg) | 71.8 (13.5) | 73.3 (12.7) | 0.549 |
| Body mass index (kg/m2) | 26.0 (4.2) | 24.7 (2.8) | 0.075 |
| Waist-to-hip ratio | 0.91 (0.85–0.96) | 0.89 (0.81–0.94) | 0.082 |
| Diabetes | |||
| Disease duration (years) | 19.0 (15.0–27.5) | – | – |
| Total insulin doses (IU/kg/day) | 0.60 (0.53–0.72) | – | – |
| Microvascular complications, n (%) | 43 (51.2) | – | – |
| Retinopathy, n (%) | |||
| Non-proliferative, n (%) | 13 (15.5) | – | – |
| Proliferative, n (%) | 12 (14.3) | – | – |
| Nephropathy, n (%) | 27 (32.1) | – | |
| Peripheral neuropathy, n (%) | 5 (6.0) | – | |
| Laboratory parameters | |||
| Fasting plasma glucose (mg/dL) | 133 (91–192) | 86 (79–93) | <0.001 |
| HbA1c (%) | 7.9 (7.1–8.7) | 5.4 (5.3–5.5) | <0.001 |
| Urinary ACR (mg/g) | 5.1 (3.2–12.5) | 3.7 (2.7–5.6) | 0.069 |
| Insulin resistance | |||
| eGDR (mg·kg–1·min–1) | 7.8 (5.5–9.4) | 10.6 (9.8–11.4) | <0.001 |
| Arterial stiffness | |||
| aPWV (m/s) | 7.9 (6.9–9.1) | 6.4 (6.0–7.2) | <0.001 |
| T1DM (n = 84) | Controls (n = 42) | p-Value | Adjusted p-Value * | |
|---|---|---|---|---|
| Dyslipidemia, n (%) | 59 (70.2) | 26 (61.9) | 0.347 | – |
| Dyslipidemia treatment, n (%) | 45 (53.6) | 1 (2.4) | <0.001 | – |
| Conventional lipid profile | ||||
| Total cholesterol (mg/dL) | 180 (162–201) | 205 (175–238) | <0.001 | <0.001 |
| HDL cholesterol (mg/dL) | 68 (55–86) | 58 (50–72) | 0.012 | 0.049 |
| LDL cholesterol (mg/dL) | 95 (82–111) | 127 (98–145) | <0.001 | <0.001 |
| Triglycerides (mg/dL) | 65 (52–74) | 76 (60–117) | 0.009 | 0.005 |
| NMR subclasses | ||||
| VLDL-P number (nmol/L) | ||||
| Total | 18.1 (13.7–24.5) | 24.7 (19.0–40.0) | <0.001 | 0.010 |
| Large | 0.53 (0.40–0.74) | 0.84 (0.63–1.16) | <0.001 | 0.005 |
| Medium | 2.72 (2.09–3.66) | 3.53 (2.79–6.18) | <0.001 | 0.011 |
| Small | 15.1 (11.2–20.0) | 19.5 (14.9–32.0) | <0.001 | 0.010 |
| VLDL-P composition (mg/dL) | ||||
| VLDL-C | 3.56 (7.41) | 8.93 (6.49) | <0.001 | <0.001 |
| VLDL-TG | 39.7 (32.3) | 47.0 (27.9) | 0.036 | 0.034 |
| Ratio VLDL-C/VLDL-TG | 0.05 (0.07) | 0.18 (0.05) | <0.001 | <0.001 |
| VLDL size (nm) | 42.2 (42.2–42.6) | 42.4 (42.1–42.6) | 0.987 | 0.649 |
| LDL-P number (nmol/L) | ||||
| Total | 688.4 (584.1–801.4) | 726.2 (661.3–912.3) | 0.020 | 0.171 |
| Large | 101.5 (86.3–122.4) | 116.3 (100.0–133.9) | 0.012 | 0.159 |
| Medium | 232.6 (190.7–276.9) | 263.4 (232.6–313.6) | <0.001 | 0.039 |
| Small | 356.7 (306.3–415.6) | 362.8 (327.4–436.1) | 0.252 | 0.440 |
| LDL-P composition (mg/dL) | ||||
| LDL-C | 94.0 (79.9–111.3) | 107.7 (98.8–132.5) | <0.001 | 0.024 |
| LDL-TG | 14.4 (12.0–16.9) | 11.1 (8.9–13.5) | <0.001 | 0.002 |
| Ratio LDL-C/LDL-TG | 6.7 (5.9–7.6) | 10.0 (9.3–11.6) | <0.001 | <0.001 |
| LDL size (nm) | 21.1 (0.1) | 21.2 (0.1) | 0.010 | 0.152 |
| HDL-P number (nmol/L) | ||||
| Total | 29.5 (24.0–34.5) | 29.6 (27.1–33.8) | 0.374 | 0.103 |
| Large | 0.10 (0.08–0.14) | 0.19 (0.13–0.24) | <0.001 | <0.001 |
| Medium | 9.9 (7.9–11.5) | 9.1 (8.0–11.2) | 0.310 | 0.779 |
| Small | 19.2 (15.9–24.0) | 20.7 (19.1–23.0) | 0.062 | 0.013 |
| HDL-P composition (mg/dL) | ||||
| HDL-C | 58.2 (46.0–69.0) | 62.7 (56.6–73.0) | 0.063 | 0.030 |
| HDL-TG | 12.2 (9.9–15.7) | 8.3 (6.8–10.2) | <0.001 | 0.008 |
| Ratio HDL-C/HDL-TG | 4.9 (3.7–5.7) | 7.8 (6.2–9.3) | <0.001 | <0.001 |
| HDL size (nm) | 8.2 (0.02) | 8.2 (0.03) | <0.001 | <0.001 |
| T1DM | Controls | |||
|---|---|---|---|---|
| Beta (95% CI) | p-Value | Beta (95% CI) | p-Value | |
| Conventional lipid profile | ||||
| Total cholesterol (mg/dL) | 0.093 (−0.062–0.248) | 0.234 | 0.120 (−0.114–0.355) | 0.305 |
| HDL cholesterol (mg/dL) | −0.002 (−0.156–0.151) | 0.979 | 0.016 (−0.235–0.266) | 0.899 |
| LDL cholesterol (mg/dL) | 0.023 (−0.130–0.176) | 0.765 | 0.074 (−0.162–0.310) | 0.531 |
| Triglycerides (mg/dL) | 0.244 (0.091–0.397) | 0.002 | 0.164 (−0.085–0.413) | 0.191 |
| NMR subclasses | ||||
| VLDL-P number (nmol/L) | ||||
| Total | 0.225 (0.084–0.366) | 0.002 | 0.109 (−0.147–0.366) | 0.393 |
| Large | 0.199 (0.057–0.341) | 0.007 | 0.113 (−0.133–0.359) | 0.360 |
| Medium | 0.213 (0.071–0.354) | 0.004 | 0.150 (−0.104–0.404) | 0.238 |
| Small | 0.228 (0.088–0.369) | 0.002 | 0.102 (−0.155–0.358) | 0.428 |
| VLDL-P composition (mg/dL) | ||||
| VLDL-C | 0.268 (0.076–0.460) | 0.007 | 0.086 (−0.161–0.335) | 0.482 |
| VLDL-TG | 0.224 (0.082–0.366) | 0.002 | 0.118 (−0. 139–0.374) | 0.358 |
| VLDL size (nm) | −0.040 (–0.182–0.102) | 0.574 | 0.072 (−0.162–0.307) | 0.537 |
| LDL-P number (nmol/L) | ||||
| Total | −0.112 (–0.255– 0.032) | 0.123 | 0.095 (−0.160–0.350) | 0.455 |
| Large | −0.139 (–0.281– 0.004) | 0.057 | −0.032 (–0.273–0.209) | 0.787 |
| Medium | −0.111 (–0.259–0.038) | 0.141 | 0.112 (−0.136–0.361) | 0.365 |
| Small | −0.088 (–0.233–0.056) | 0.227 | 0.118 (−0.143–0.379) | 0.365 |
| LDL-P composition (mg/dL) | ||||
| LDL-C | −0.128 (−0.271–0.014) | 0.077 | 0.059 (−0.193–0.311) | 0.637 |
| LDL-TG | −0.066 (−0.214–0.083) | 0.383 | 0.124 (−0.120–0.368) | 0.308 |
| LDL size (nm) | −0.125 (−0.268– 0.019) | 0.087 | −0.202 (−0.441–0.036) | 0.094 |
| HDL-P number (nmol/L) | ||||
| Total | 0.029 (−0.131–0.189) | 0.720 | 0.076 (−0.187–0.340) | 0.561 |
| Large | 0.131 (−0.018–0.281) | 0.085 | 0.033 (−0.197–0.264) | 0.771 |
| Medium | 0.003 (−0.162–0.167) | 0.975 | 0.051 (−0.217–0.318) | 0.703 |
| Small | 0.039 (−0.118–0.197) | 0.621 | 0.080 (−0.174–0.335) | 0.527 |
| HDL-P composition (mg/dL) | ||||
| HDL-C | 0.010 (−0.150–0.170) | 0.900 | –0.007 (−0.264–0.279) | 0.954 |
| HDL-TG | 0.038 (−0.120–0.196) | 0.633 | 0.042 (−0.192–0.276) | 0.719 |
| HDL size (nm) | −0.170 (−0.311– –0.030) | 0.018 | 0.006 (−0.243–0.255) | 0.963 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llauradó, G.; Amigó, N.; Cano, A.; Ballesta, S.; Albert, L.; Mazarico, I.; Fernández-Veledo, S.; Pedro-Botet, J.; Vendrell, J.; González-Clemente, J.-M. Specific Nuclear Magnetic Resonance Lipoprotein Subclass Profiles and Central Arterial Stiffness in Type 1 Diabetes Mellitus: A Case Control Study. J. Clin. Med. 2019, 8, 1875. https://doi.org/10.3390/jcm8111875
Llauradó G, Amigó N, Cano A, Ballesta S, Albert L, Mazarico I, Fernández-Veledo S, Pedro-Botet J, Vendrell J, González-Clemente J-M. Specific Nuclear Magnetic Resonance Lipoprotein Subclass Profiles and Central Arterial Stiffness in Type 1 Diabetes Mellitus: A Case Control Study. Journal of Clinical Medicine. 2019; 8(11):1875. https://doi.org/10.3390/jcm8111875
Chicago/Turabian StyleLlauradó, Gemma, Núria Amigó, Albert Cano, Silvia Ballesta, Lara Albert, Isabel Mazarico, Sonia Fernández-Veledo, Juan Pedro-Botet, Joan Vendrell, and José-Miguel González-Clemente. 2019. "Specific Nuclear Magnetic Resonance Lipoprotein Subclass Profiles and Central Arterial Stiffness in Type 1 Diabetes Mellitus: A Case Control Study" Journal of Clinical Medicine 8, no. 11: 1875. https://doi.org/10.3390/jcm8111875






