Dry Eye Disease: Emerging Approaches to Disease Analysis and Therapy
Abstract
:1. Introduction
2. Emerging Insights into DED Pathogenesis
2.1. DED-Associated Metabolic Dysregulation
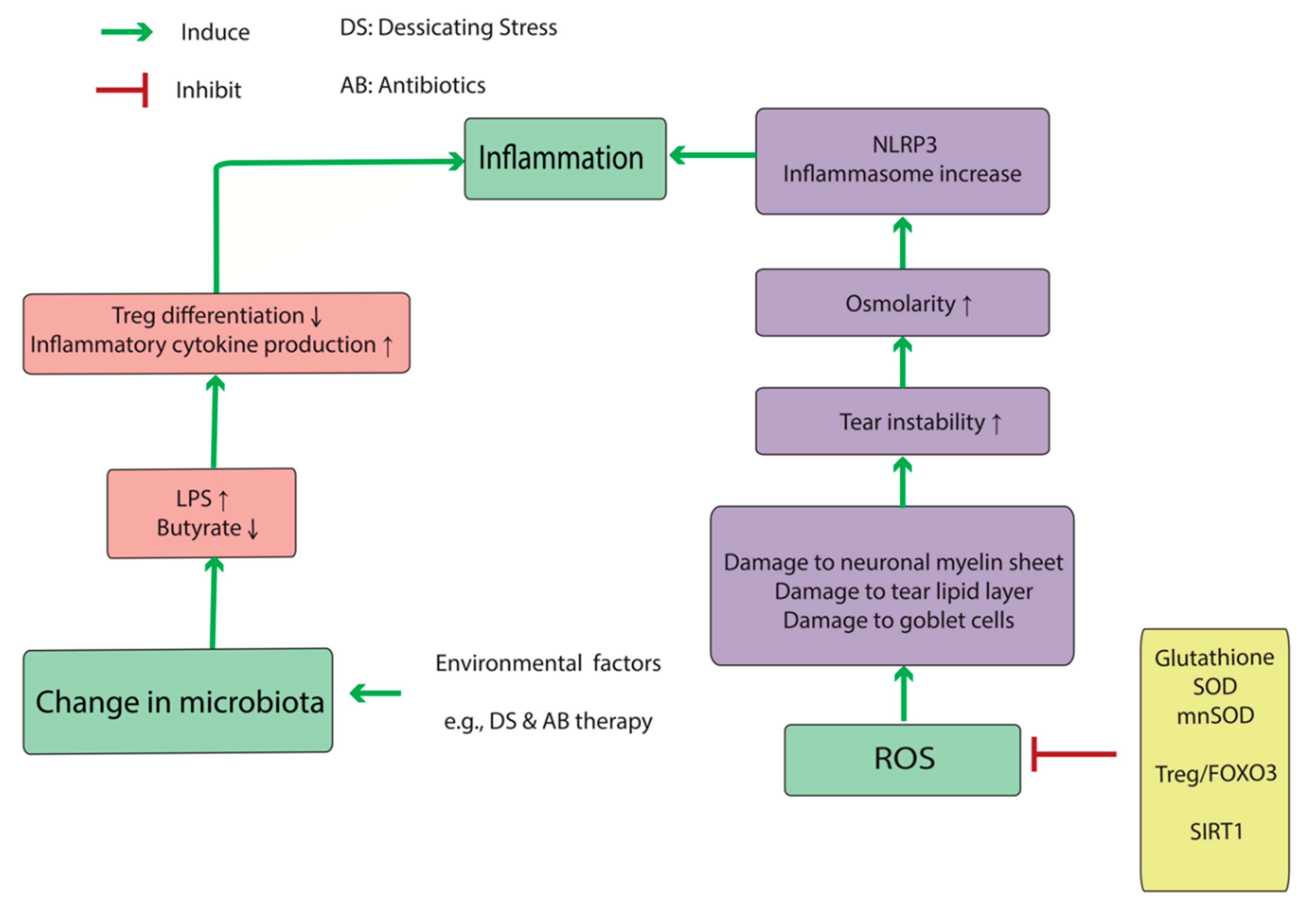
2.2. DED Immunity and Immunometabolism
2.3. DED-Associated Changes in Normal Microbiota
3. Emerging Measurement Techniques and Disease Models
3.1. Clinical Approaches
3.2. Molecular Profiling and Omics Approaches
3.3. Bioengineering Approaches
4. Emerging DED-Treatment Strategies
5. Future Directions
Funding
Acknowledgments
Conflicts of Interest
References
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.; Zhou, X.; Ge, L.; Wu, L.; Hong, J.; Xu, J. Impact of Dry Eye Syndrome on Vision-Related Quality of Life in a Non-Clinic-Based General Population. BMC Ophthalmol. 2012, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Inomata, T.; Shiang, T.; Iwagami, M.; Sakemi, F.; Fujimoto, K.; Okumura, Y.; Ohno, M.; Murakami, A. Changes in Distribution of Dry Eye Disease by the New 2016 Diagnostic Criteria from the Asia Dry Eye Society. Sci. Rep. 2018, 8, 1918. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, A.J.; Cruickshanks, K.J.; Fischer, M.E.; Huang, G.-H.; Klein, B.E.K.; Klein, R.; Dalton, D.S. Dry Eye in the Beaver Dam Offspring Study: Prevalence, Risk Factors, and Health-Related Quality of Life. Am. J. Ophthalmol. 2014, 157, 799–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pflugfelder, S.C.; Stern, M.E. Mucosal environmental sensors in the pathogenesis of dry eye. Expert Rev. Clin. Immunol. 2014, 10, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.D.; Craig, J.P.; Akpek, E.K.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Clayton, J.A.; Dogru, M.; Dua, H.S.; Foulks, G.N. TFOS DEWS II Introduction. Ocul. Surf. 2017, 15, 269–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, L.A.J.; Kishton, R.J.; Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016, 16, 553–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhoads, J.P.; Major, A.S.; Rathmell, J.C. Fine tuning of immunometabolism for the treatment of rheumatic diseases. Nat. Rev. Rheumatol. 2017, 13, 313–320. [Google Scholar] [CrossRef]
- Morel, L. Immunometabolism in systemic lupus erythematosus. Nat. Rev. Rheumatol. 2017, 13, 280–290. [Google Scholar] [CrossRef]
- Huang, N.; Perl, A. Metabolism as a Target for Modulation in Autoimmune Diseases. Trends Immunol. 2018, 39, 562–576. [Google Scholar] [CrossRef] [PubMed]
- Gaber, T.; Strehl, C.; Buttgereit, F. Metabolic regulation of inflammation. Nat. Rev. Rheumatol. 2017, 13, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Foundations of Immunometabolism and Implications for Metabolic Health and Disease. Immunity 2017, 47, 406–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seen, S.; Tong, L. Dry eye disease and oxidative stress. Acta Ophthalmol. 2018, 96, e412–e420. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Hua, X.; Li, J.; Chi, W.; Zhang, Z.; Lu, F.; Zhang, L.; Pflugfelder, S.C.; Li, D.-Q. Oxidative Stress Markers Induced by Hyperosmolarity in Primary Human Corneal Epithelial Cells. PLoS ONE 2015, 10, e0126561. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Ren, Y.; Reinach, P.S.; Xiao, B.; Lu, H.; Zhu, Y.; Qu, J.; Chen, W. Reactive oxygen species activated NLRP3 inflammasomes initiate inflammation in hyperosmolarity stressed human corneal epithelial cells and environment-induced dry eye patients. Exp. Eye Res. 2015, 134, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.; Hua, X.; Chen, X.; Bian, F.; Yuan, X.; Zhang, L.; Wang, X.; Chen, D.; Deng, R.; Li, Z.; et al. Mitochondrial DNA Oxidation Induces Imbalanced Activity of NLRP3/NLRP6 Inflammasomes by Activation of Caspase-8 and BRCC36 in Dry Eye. J. Autoimmun. 2017, 80, 65–76. [Google Scholar] [CrossRef]
- Liu, H.; Sheng, M.; Liu, Y.; Wang, P.; Chen, Y.; Chen, L.; Wang, W.; Li, B. Expression of SIRT1 and oxidative stress in diabetic dry eye. Int. J. Clin. Exp. Pathol. 2015, 8, 7644–7653. [Google Scholar]
- Chen, Y.; Chauhan, S.K.; Shao, C.; Omoto, M.; Inomata, T.; Dana, R. Interferon-γ-expressing Th17 cells are required for development of severe ocular surface autoimmunity. J. Immunol. 2017, 199, 1163–1169. [Google Scholar] [CrossRef]
- Coursey, T.G.; Gandhi, N.B.; Volpe, E.A.; Pflugfelder, S.C.; de Paiva, C.S. Chemokine receptors CCR6 and CXCR3 are necessary for CD4+ T cell mediated ocular surface disease in experimental dry eye disease. PLoS ONE 2013, 8, e78508. [Google Scholar] [CrossRef]
- De Paiva, C.; Villarreal, A.; Corrales, R.; Rahman, H.; Chang, V.; Farley, W.; Stern, M.; Niederkorn, J.; Li, D.Q.; Pflugfelder, S. IFN–Promotes goblet cell loss in response to desiccating ocular stress. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5579. [Google Scholar]
- Coursey, T.G.; Henriksson, J.T.; Barbosa, F.L.; De Paiva, C.S.; Pflugfelder, S.C. Interferon-γ–Induced Unfolded Protein Response in Conjunctival Goblet Cells as a Cause of Mucin Deficiency in Sjögren Syndrome. Am. J. Pathol. 2016, 186, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Dohlman, T.H.; Chauhan, S.K.; Kodati, S.; Hua, J.; Chen, Y.; Omoto, M.; Sadrai, Z.; Dana, R. The CCR6/CCL20 Axis Mediates Th17 Cell Migration to the Ocular Surface in Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4081–4091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dohlman, T.H.; Ding, J.; Dana, R.; Chauhan, S.K. T Cell–Derived Granulocyte-Macrophage Colony-Stimulating Factor Contributes to Dry Eye Disease Pathogenesis by Promoting CD11b+ Myeloid Cell Maturation and Migration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Inomata, T.; Hua, J.; Nakao, T.; Shiang, T.; Chiang, H.; Amouzegar, A.; Dana, R. Corneal Tissue from Dry Eye Donors Leads to Enhanced Graft Rejection. Cornea 2018, 37, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; De Paiva, C.S.; Moore, Q.L.; Volpe, E.A.; Li, D.-Q.; Gumus, K.; Zaheer, M.L.; Corrales, R.M. Aqueous Tear Deficiency Increases Conjunctival Interferon-γ (IFN-γ) Expression and Goblet Cell Loss. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7545–7550. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, J.T.; Coursey, T.G.; Corry, D.B.; De Paiva, C.S.; Pflugfelder, S.C. IL-13 Stimulates Proliferation and Expression of Mucin and Immunomodulatory Genes in Cultured Conjunctival Goblet Cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4186–4197. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Posadas, L.; Hodges, R.; Li, D.; Shatos, M.; Storr-Paulsen, T.; Diebold, Y.; Dartt, D. Interaction of IFN-γ with cholinergic agonists to modulate rat and human goblet cell function. Mucosal Immunol. 2016, 9, 206. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Gao, C.; Chen, H.; Li, Y.; Jin, Y.; Qi, H. Analysis of Th17-associated cytokines and clinical correlations in patients with dry eye disease. PLoS ONE 2017, 12, e0173301. [Google Scholar] [CrossRef] [PubMed]
- Subbarayal, B.; Chauhan, S.K.; Di Zazzo, A.; Dana, R. IL-17 augments B cell activation in ocular surface autoimmunity. J. Immunol. 2016, 197, 3464–3470. [Google Scholar] [CrossRef]
- Coursey, T.G.; Bohat, R.; Barbosa, F.L.; Pflugfelder, S.C.; De Paiva, C.S. Desiccating stress-induced chemokine expression in the epithelium is dependent on upregulation of NKG2D/RAE-1 and release of IFN-γ in experimental dry eye. J. Immunol. 2014, 193, 5264–5272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Schaumburg, C.; Coursey, T.; Siemasko, K.; Volpe, E.; Gandhi, N.; Li, D.; Niederkorn, J.; Stern, M.; Pflugfelder, S. CD8+ cells regulate the T helper-17 response in an experimental murine model of Sjögren syndrome. Mucosal Immunol. 2014, 7, 417. [Google Scholar] [CrossRef] [PubMed]
- Binger, K.J.; Côrte-Real, B.F.; Kleinewietfeld, M. Immunometabolic Regulation of Interleukin-17-Producing T Helper Cells: Uncoupling New Targets for Autoimmunity. Front. Immunol. 2017, 8, 305. [Google Scholar] [CrossRef]
- Inomata, T.; Hua, J.; Di Zazzo, A.; Dana, R. Impaired Function of Peripherally Induced Regulatory T Cells in Hosts at High Risk of Graft Rejection. Sci. Rep. 2016, 6, 39924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, J.; Inomata, T.; Chen, Y.; Foulsham, W.; Stevenson, W.; Shiang, T.; Bluestone, J.A.; Dana, R. Pathological conversion of regulatory T cells is associated with loss of allotolerance. Sci. Rep. 2018, 8, 7059. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, S.; Khanolkar, V.; Namavari, A.; Chaudhary, S.; Gandhi, S.; Tibrewal, S.; Jassim, S.H.; Shaheen, B.; Hallak, J.; Horner, J.H.; et al. Ocular Surface Extracellular DNA and Nuclease Activity Imbalance: A New Paradigm for Inflammation in Dry Eye Disease. Investig. Ophtalmol. Vis. Sci. 2012, 53, 8253–8263. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; Stern, M.; Zhang, S.; Shojaei, A. LFA-1/ICAM-1 Interaction as a Therapeutic Target in Dry Eye Disease. J. Ocul. Pharmacol. Ther. 2017, 33, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 1024. [Google Scholar] [CrossRef]
- Savage, D.C. Microbial Ecology of the Gastrointestinal Tract. Annu. Rev. Microbiol. 1977, 31, 107–133. [Google Scholar] [CrossRef]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef]
- Pandiyan, P.; Bhaskaran, N.; Zou, M.; Schneider, E.; Jayaraman, S.; Huehn, J. Microbiome Dependent Regulation of Tregs and Th17 Cells in Mucosa. Front. Immunol. 2019, 10, 426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cani, P.D. Microbiota and metabolites in metabolic diseases. Nat. Rev. Endocrinol. 2019, 15, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 2019, 570, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Nichols, R.G.; Peters, J.M.; Patterson, A.D. Interplay between the Host, the Human Microbiome, and Drug Metabolism. Hum. Genom. 2019, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, L.; Kelly, L. Bringing microbiome-drug interaction research into the clinic. EBioMedicine 2019, 44, 708–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zegans, M.E.; Van Gelder, R.N. Considerations in Understanding the Ocular Surface Microbiome. Am. J. Ophthalmol. 2014, 158, 420–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; Van Der Veeken, J.; DeRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- De Paiva, C.S.; Jones, D.B.; Stern, M.E.; Bian, F.; Moore, Q.L.; Corbiere, S.; Streckfus, C.F.; Hutchinson, D.S.; Ajami, N.J.; Petrosino, J.F.; et al. Altered Mucosal Microbiome Diversity and Disease Severity in Sjögren Syndrome. Sci. Rep. 2016, 6, 23561. [Google Scholar] [CrossRef]
- Horai, R.; Zárate-Bladés, C.R.; Dillenburg-Pilla, P.; Chen, J.; Kielczewski, J.L.; Silver, P.B.; Jittayasothorn, Y.; Chan, C.-C.; Yamane, H.; Honda, K.; et al. Microbiota-Dependent Activation of an Autoreactive T Cell Receptor Provokes Autoimmunity in an Immunologically Privileged Site. Immunity 2015, 43, 343–353. [Google Scholar] [CrossRef] [Green Version]
- Honda, K.; Littman, D.R. The microbiota in adaptive immune homeostasis and disease. Nature 2016, 535, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Simmons, K.T.; Xiao, Y.; Pflugfelder, S.C.; De Paiva, C.S. Inflammatory Response to Lipopolysaccharide on the Ocular Surface in a Murine Dry Eye Model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2443–2451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omenetti, S.; Pizarro, T.T. The Treg/Th17 Axis: A Dynamic Balance Regulated by the Gut Microbiome. Front. Immunol. 2015, 6, 845. [Google Scholar] [CrossRef] [PubMed]
- Vehof, J.; Smitt-Kamminga, N.S.; Nibourg, S.A.; Hammond, C.J. Predictors of Discordance between Symptoms and Signs in Dry Eye Disease. Ophthalmology 2017, 124, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Aragona, P.; Van Setten, G.; Rolando, M.; Irkec, M.; Del Castillo, J.B.; Geerling, G.; Labetoulle, M.; Bonini, S.; ODISSEY European Consensus Group Members. Diagnosing the severity of dry eye: A clear and practical algorithm. Br. J. Ophthalmol. 2014, 98, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.D.; Keith, M.S.; Sudharshan, L.; Snedecor, S.J. Associations between signs and symptoms of dry eye disease: A systematic review. Clin. Ophthalmol. 2015, 9, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.S.; Wei, Y.; Kuklinski, E.; Asbell, P.A. The Growing Need for Validated Biomarkers and Endpoints for Dry Eye Clinical Research. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO1–BIO19. [Google Scholar] [CrossRef] [Green Version]
- Cox, S.M.; Nichols, K.K.; Nichols, J.J. Agreement between Automated and Traditional Measures of Tear Film Breakup. Optom. Vis. Sci. 2015, 92, e257–e263. [Google Scholar] [CrossRef] [Green Version]
- Wei, A.; Le, Q.; Hong, J.; Wang, W.; Wang, F.; Xu, J. Assessment of lower tear meniscus. Optom. Vis. Sci. 2016, 93, 1420–1425. [Google Scholar] [CrossRef]
- Raj, A.; Dhasmana, R.; Nagpal, R.C. Anterior Segment Optical Coherence Tomography for Tear Meniscus Evaluation and its Correlation with other Tear Variables in Healthy Individuals. J. Clin. Diagn. Res. 2016, 10, NC01–NC04. [Google Scholar] [CrossRef]
- Fukuda, R.; Usui, T.; Miyai, T.; Yamagami, S.; Amano, S. Tear Meniscus Evaluation by Anterior Segment Swept-Source Optical Coherence Tomography. Am. J. Ophthalmol. 2013, 155, 620–624.e2. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.; Doh, S.H.; Chung, S.K. Comparison of Tear Meniscus Height Measurements Obtained with the Keratograph and Fourier Domain Optical Coherence Tomography in Dry Eye. Cornea 2015, 34, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Najafi, L.; Malek, M.; Valojerdi, A.E.; Khamseh, M.E.; Aghaei, H. Dry eye disease in type 2 diabetes mellitus; comparison of the tear osmolarity test with other common diagnostic tests: A diagnostic accuracy study using STARD standard. J. Diabetes Metab. Disord. 2015, 14, 1264. [Google Scholar] [CrossRef] [PubMed]
- Stahl, U.; Willcox, M.; Stapleton, F. Osmolality and tear film dynamics. Clin. Exp. Optom. 2012, 95, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Rocha, G.; Gulliver, E.; Borovik, A.; Chan, C.C. Randomized, masked, in vitro comparison of three commercially available tear film osmometers. Clin. Ophthalmol. 2017, 11, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.; Gadaria-Rathod, N.; Oh, C.; Asbell, P.A. Precision and Accuracy of TearLab Osmometer in Measuring Osmolarity of Salt Solutions. Curr. Eye Res. 2014, 39, 1247–1250. [Google Scholar] [CrossRef] [PubMed]
- Badugu, R.; Jeng, B.H.; Reece, E.A.; Lakowicz, J.R. Contact lens to measure individual ion concentrations in tears and applications to dry eye disease. Anal. Biochem. 2018, 542, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Inomata, T.; Iwagami, M.; Hiratsuka, Y.; Fujimoto, K.; Okumura, Y.; Shiang, T.; Murakami, A. Maximum blink interval is associated with tear film breakup time: A new simple, screening test for dry eye disease. Sci. Rep. 2018, 8, 13443. [Google Scholar] [CrossRef] [PubMed]
- Johnston, P.R.; Rodriguez, J.; Lane, K.J.; Ousler, G.; Abelson, M.B. The interblink interval in normal and dry eye subjects. Clin. Ophthalmol. 2013, 7, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Palamar, M.; Degirmenci, C.; Ertam, I.; Yagci, A. Evaluation of Dry Eye and Meibomian Gland Dysfunction with Meibography in Patients with Rosacea. Cornea 2015, 34, 497–499. [Google Scholar] [CrossRef]
- Finis, D.; Ackermann, P.; Pischel, N.; König, C.; Hayajneh, J.; Borrelli, M.; Schrader, S.; Geerling, G. Evaluation of meibomian gland dysfunction and local distribution of meibomian gland atrophy by non-contact infrared meibography. Curr. Eye Res. 2015, 40, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Menzies, K.L.; Srinivasan, S.; Prokopich, C.L.; Jones, L. Infrared imaging of meibomian glands and evaluation of the lipid layer in Sjögren’s syndrome patients and nondry eye controls. Investig. Ophthalmol. Vis. Sci. 2015, 56, 836–841. [Google Scholar] [CrossRef]
- Villani, E.; Beretta, S.; De Capitani, M.; Galimberti, D.; Viola, F.; Ratiglia, R. In vivo confocal microscopy of meibomian glands in Sjögren’s syndrome. Investig. Ophthalmol. Vis. Sci. 2011, 52, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Messmer, E.M.; Von Lindenfels, V.; Garbe, A.; Kampik, A. Matrix Metalloproteinase 9 Testing in Dry Eye Disease Using a Commercially Available Point-of-Care Immunoassay. Ophthalmology 2016, 123, 2300–2308. [Google Scholar] [CrossRef]
- Chan, T.C.; Ye, C.; Chan, K.P.; Chu, K.O.; Jhanji, V. Evaluation of point-of-care test for elevated tear matrix metalloproteinase 9 in post-LASIK dry eyes. Br. J. Ophthalmol. 2016, 100, 1188–1191. [Google Scholar] [CrossRef] [PubMed]
- Sambursky, R.; Davitt, W.F., III; Friedberg, M.; Tauber, S. Prospective, multicenter, clinical evaluation of point-of-care matrix metalloproteinase-9 test for confirming dry eye disease. Cornea 2014, 33, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Lanza, N.L.; McClellan, A.L.; Batawi, H.; Felix, E.R.; Sarantopoulos, K.D.; Levitt, R.C.; Galor, A. Dry Eye Profiles in Patients with a Positive Elevated Surface Matrix Metalloproteinase 9 Point-of-Care Test Versus Negative Patients. Ocul. Surf. 2016, 14, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Sambursky, R.; Davitt, W.F.; Latkany, R.; Tauber, S.; Starr, C.; Friedberg, M.; Dirks, M.S.; McDonald, M. Sensitivity and Specificity of a Point-of-Care Matrix Metalloproteinase 9 Immunoassay for Diagnosing Inflammation Related to Dry Eye. JAMA Ophthalmol. 2013, 131, 24. [Google Scholar] [CrossRef]
- López-Miguel, A.; Gutiérrez-Gutiérrez, S.; García-Vázquez, C.; Enríquez-De-Salamanca, A. RNA Collection from Human Conjunctival Epithelial Cells Obtained with a New Device for Impression Cytology. Cornea 2017, 36, 59–63. [Google Scholar] [CrossRef]
- Inomata, T.; Nakamura, M.; Iwagami, M.; Shiang, T.; Yoshimura, Y.; Fujimoto, K.; Okumura, Y.; Eguchi, A.; Iwata, N.; Miura, M.; et al. Risk Factors for Severe Dry Eye Disease: Crowdsourced Research Using DryEyeRhythm. Ophthalmology 2019, 126, 766–768. [Google Scholar] [CrossRef]
- Basatneh, R.; Najafi, B.; Armstrong, D.G. Health Sensors, Smart Home Devices, and the Internet of Medical Things: An Opportunity for Dramatic Improvement in Care for the Lower Extremity Complications of Diabetes. J. Diabetes Sci. Technol. 2018, 12, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.M.; Tong, L.; Reux, B.; Duan, X.; Petznick, A.; Yong, S.S.; Khee, C.B.S.; Lear, M.J.; Wenk, M.R.; Shui, G. Lipidomic analysis of human tear fluid reveals structure-specific lipid alterations in dry eye syndrome. J. Lipid Res. 2014, 55, 299–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, S.M.; Tong, L.; Yong, S.S.; Li, B.; Chaurasia, S.S.; Shui, G.; Wenk, M.R. Meibum Lipid Composition in Asians with Dry Eye Disease. PLoS ONE 2011, 6, e24339. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.M.; Tong, L.; Duan, X.; Acharya, U.R.; Tan, J.H.; Petznick, A.; Wenk, M.R.; Shui, G. Longitudinal changes in tear fluid lipidome brought about by eyelid-warming treatment in a cohort of meibomian gland dysfunction. J. Lipid Res. 2014, 55, 1959–1969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, W.; Lian, C.; Ying, L.; Kim, G.E.; You, I.C.; Park, S.H.; Yoon, K.C. Expression of Lipid Peroxidation Markers in the Tear Film and Ocular Surface of Patients with Non-Sjogren Syndrome: Potential Biomarkers for Dry Eye Disease. Curr. Eye Res. 2016, 41, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Hagan, S.; Martin, E.; Enríquez-De-Salamanca, A. Tear fluid biomarkers in ocular and systemic disease: Potential use for predictive, preventive and personalised medicine. EPMA J. 2016, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Azkargorta, M.; Soria, J.; Acera, A.; Iloro, I.; Elortza, F. Human tear proteomics and peptidomics in ophthalmology: Toward the translation of proteomic biomarkers into clinical practice. J. Proteom. 2017, 150, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Du, C.-X.; Pan, X.-D. The use of in-strip digestion for fast proteomic analysis on tear fluid from dry eye patients. PLoS ONE 2018, 13, e0200702. [Google Scholar] [CrossRef]
- Aass, C.; Norheim, I.; Eriksen, E.F.; Thorsby, P.M.; Pepaj, M. Single unit filter-aided method for fast proteomic analysis of tear fluid. Anal. Biochem. 2015, 480, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Li, J.; Guo, T.; Ghosh, S.; Koh, S.K.; Tian, D.; Zhang, L.; Jia, D.; Beuerman, R.W.; Aebersold, R.; et al. Global Metabonomic and Proteomic Analysis of Human Conjunctival Epithelial Cells (IOBA-NHC) in Response to Hyperosmotic Stress. J. Proteome Res. 2015, 14, 3982–3995. [Google Scholar] [CrossRef]
- Perumal, N.; Funke, S.; Wolters, D.; Pfeiffer, N.; Grus, F.H. Characterization of human reflex tear proteome reveals high expression of lacrimal proline-rich protein 4 (PRR4). Proteomics 2015, 15, 3370–3381. [Google Scholar] [CrossRef] [PubMed]
- Bartalena, L.; Fatourechi, V. Extrathyroidal manifestations of Graves’ disease: A 2014 update. J. Endocrinol. Investig. 2014, 37, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Matheis, N.; Grus, F.H.; Breitenfeld, M.; Knych, I.; Funke, S.; Pitz, S.; Ponto, K.A.; Pfeiffer, N.; Kahaly, G.J. Proteomics differentiate between thyroid-associated orbitopathy and dry eye syndrome. Investig. Opthalmology Vis. Sci. 2015, 56, 2649. [Google Scholar] [CrossRef] [PubMed]
- Vissink, A.; Bootsma, H.; Spijkervet, F.K.; Hu, S.; Wong, D.T.; Kallenberg, C.G. Current and Future Challenges in Primary Sjogren’s Syndrome. Curr. Pharm. Biotechnol. 2012, 13, 2026–2045. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Sheng, M.; Li, J.; Yan, G.; Lin, A.; Li, M.; Wang, W.; Chen, Y. Tear proteomic analysis of Sjögren syndrome patients with dry eye syndrome by two-dimensional-nano-liquid chromatography coupled with tandem mass spectrometry. Sci. Rep. 2014, 4, 5772. [Google Scholar] [CrossRef] [PubMed]
- Cocho, L.; Fernandez, I.; Calonge, M.; Martínez, V.; González-García, M.J.; Caballero, D.; López-Corral, L.; García-Vázquez, C.; Vazquez, L.; Stern, M.E.; et al. Gene Expression–Based Predictive Models of Graft Versus Host Disease–Associated Dry Eye. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4570. [Google Scholar] [CrossRef] [PubMed]
- Cocho, L.; Fernandez, I.; Calonge, M.; Martínez, V.; González-García, M.J.; Caballero, D.; López-Corral, L.; García-Vázquez, C.; Vazquez, L.; Stern, M.E.; et al. Biomarkers in Ocular Chronic Graft Versus Host Disease: Tear Cytokine- and Chemokine-Based Predictive Model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.W.; Han, S.J.; Song, M.K.; Kim, T.-I.; Kim, E.K.; Min, Y.H.; Cheong, J.-W.; Seo, K.Y. Tear Cytokines as Biomarkers for Chronic Graft-versus-Host Disease. Boil. Blood Marrow Transplant. 2015, 21, 2079–2085. [Google Scholar] [CrossRef] [Green Version]
- Giannaccare, G.; Bonifazi, F.; Sessa, M.; Fresina, M.; Arpinati, M.; Bandini, G.; Versura, P. Dry Eye Disease Is Already Present in Hematological Patients before Hematopoietic Stem Cell Transplantation. Cornea 2016, 35, 638–643. [Google Scholar] [CrossRef]
- Schaumberg, D.A.; Sullivan, D.A.; Dana, M.R. Epidemiology of dry eye syndrome. Adv. Exp. Med. Biol. 2002, 506, 989–998. [Google Scholar]
- Lelli, G.J.; Musch, D.C.; Gupta, A.; Farjo, Q.A.; Nairus, T.M.; Mian, S.I. Ophthalmic Cyclosporine Use in Ocular GVHD. Cornea 2006, 25, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Malta, J.B.; Soong, H.K.; Shtein, R.M.; Musch, D.C.; Rhoades, W.; Sugar, A.; Mian, S.I. Treatment of Ocular Graft-Versus-Host Disease with Topical Cyclosporine 0.05%. Cornea 2010, 29, 1392–1396. [Google Scholar] [CrossRef] [PubMed]
- DiMasi, J.A.; Grabowski, H.G.; Hansen, R.W. Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 2016, 47, 20–33. [Google Scholar] [PubMed] [Green Version]
- Zhang, B.; Radisic, M. Organ-on-a-chip devices advance to market. Lab Chip 2017, 17, 2395–2420. [Google Scholar] [CrossRef] [PubMed]
- Baker, M. Tissue models: A living system on a chip. Nature 2011, 471, 661. [Google Scholar] [CrossRef]
- Seo, J.; Byun, W.Y.; Frank, A.; Massaro-Giordano, M.; Lee, V.; Bunya, V.Y.; Huh, D. Human blinking ‘eye-on-a-chip’. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3872. [Google Scholar]
- Foster, J.W.; Wahlin, K.; Adams, S.M.; Birk, D.E.; Zack, D.J.; Chakravarti, S. Cornea organoids from human induced pluripotent stem cells. Sci. Rep. 2017, 7, 41286. [Google Scholar] [CrossRef]
- Susaimanickam, P.J.; Maddileti, S.; Pulimamidi, V.K.; Boyinpally, S.R.; Naik, R.R.; Naik, M.N.; Reddy, G.B.; Sangwan, V.S.; Mariappan, I. Generating minicorneal organoids from human induced pluripotent stem cells. Development 2017, 144, 2338–2351. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.; Byun, W.Y.; Alisafaei, F.; Georgescu, A.; Yi, Y.-S.; Massaro-Giordano, M.; Shenoy, V.B.; Lee, V.; Bunya, V.Y.; Huh, D. Multiscale reverse engineering of the human ocular surface. Nat. Med. 2019, 25, 1310–1318. [Google Scholar] [CrossRef]
- Coursey, T.G.; Henriksson, J.T.; Marcano, D.C.; Shin, C.S.; Isenhart, L.C.; Ahmed, F.; De Paiva, C.S.; Pflugfelder, S.C.; Acharya, G. Dexamethasone nanowafer as an effective therapy for dry eye disease. J. Control. Release 2015, 213, 168–174. [Google Scholar] [CrossRef] [Green Version]
- Bian, F.; Shin, C.S.; Wang, C.; Pflugfelder, S.C.; Acharya, G.; De Paiva, C.S. Dexamethasone Drug Eluting Nanowafers Control Inflammation in Alkali-Burned Corneas Associated with Dry Eye. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3222–3230. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Fraga, J.; López-Miguel, A.; González-García, M.J.; Fernández, I.; López-de-la-Rosa, A.; Enríquez-de-Salamanca, A.; Stern, M.E.; Calonge, M. Topical fluorometholone protects the ocular surface of dry eye patients from desiccating stress: A randomized controlled clinical trial. Ophthalmology 2016, 123, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Edman, M.C.; Janga, S.R.; Yarber, F.; Meng, Z.; Klinngam, W.; Bushman, J.; Ma, T.; Liu, S.; Louie, S. Rapamycin eye drops suppress lacrimal gland inflammation in a murine model of Sjögren’s syndrome. Investig. Ophthalmol. Vis. Sci. 2017, 58, 372–385. [Google Scholar] [CrossRef]
- Simopoulos, A. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Deinema, L.A.; Vingrys, A.J.; Wong, C.Y.; Jackson, D.C.; Chinnery, H.R.; Downie, L.E. A Randomized, Double-Masked, Placebo-Controlled Clinical Trial of Two Forms of Omega-3 Supplements for Treating Dry Eye Disease. Ophthalmology 2017, 124, 43–52. [Google Scholar] [CrossRef]
- Andrade, A.S.; Salomon, T.B.; Behling, C.S.; Mahl, C.D.; Hackenhaar, F.S.; Putti, J.; Benfato, M.S. Alpha-lipoic acid restores tear production in an animal model of dry eye. Exp. Eye Res. 2014, 120, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, R.; Kumar, P.; Phogat, H.; Kaur, A.; Kumar, M. Oral omega-3 fatty acids treatment in computer vision syndrome related dry eye. Contact Lens Anterior Eye 2015, 38, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Choi, J.-H.; Oh, H.-J.; Park, S.-H.; Lee, J.-B.; Yoon, K.C. Effects of Eye Drops Containing a Mixture of Omega-3 Essential Fatty Acids and Hyaluronic Acid on the Ocular Surface in Desiccating Stress-induced Murine Dry Eye. Curr. Eye Res. 2014, 39, 871–878. [Google Scholar] [CrossRef]
- Lembach, C.; Fogt, J.; Kowalski, M.; King-Smith, P.E.; Epitropoulos, A.; Hendershot, A.; Maszczak, J.; Jones-Jordan, L.; Barr, J. Tear lipid layer thickness with eye drops in meibomian gland dysfunction. Clin. Ophthalmol. 2016, 10, 2237–2243. [Google Scholar]
- Wang, M.T.M.; Jaitley, Z.; Lord, S.M.; Craig, J.P. Comparison of Self-Applied Heat Therapy for Meibomian Gland Dysfunction. Optom. Vis. Sci. 2015, 92, e321–e326. [Google Scholar] [CrossRef]
- Kim, Y.J.; Ryu, J.S.; Park, S.Y.; Lee, H.J.; Ko, J.H.; Kim, M.K.; Wee, W.R.; Oh, J.Y. Comparison of Topical Application of TSG-6, Cyclosporine, and Prednisolone for Treating Dry Eye. Cornea 2016, 35, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Sawazaki, R.; Ishihara, T.; Usui, S.; Hayashi, E.; Tahara, K.; Hoshino, T.; Higuchi, A.; Nakamura, S.; Tsubota, K.; Mizushima, T. Diclofenac Protects Cultured Human Corneal Epithelial Cells Against Hyperosmolarity and Ameliorates Corneal Surface Damage in a Rat Model of Dry Eye. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2547–2556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, P.; Rupenthal, I.D. Modern approaches to the ocular delivery of cyclosporine A. Drug Discov. Today 2016, 21, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; De Paiva, C.S.; Villarreal, A.L.; Stern, M.E. Effects of Sequential Artificial Tear and Cyclosporine Emulsion Therapy on Conjunctival Goblet Cell Density and Transforming Growth Factor-β2 Production. Cornea 2008, 27, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Macri, A.; Scanarotti, C.; Bassi, A.M.; Giuffrida, S.; Sangalli, G.; Traverso, C.E.; Iester, M. Evaluation of oxidative stress levels in the conjunctival epithelium of patients with or without dry eye, and dry eye patients treated with preservative-free hyaluronic acid 0.15% and vitamin B12 eye drops. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Zernii, E.Y.; Gancharova, O.S.; Baksheeva, V.E.; Golovastova, M.O.; Kabanova, E.I.; Savchenko, M.S.; Tiulina, V.V.; Sotnikova, L.F.; Zamyatnin, A.A.; Philippov, P.P.; et al. Mitochondria-Targeted Antioxidant SkQ1 Prevents Anesthesia-Induced Dry Eye Syndrome. Oxidative Med. Cell. Longev. 2017, 2017, 9281519. [Google Scholar] [CrossRef] [PubMed]
- Bremond-Gignac, D.; Gicquel, J.-J.; Chiambaretta, F. Pharmacokinetic evaluation of diquafosol tetrasodium for the treatment of Sjögren’s syndrome. Expert Opin. Drug Metab. Toxicol. 2014, 10, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Simsek, C.; Kojima, T.; Higa, K.; Kawashima, M.; Dogru, M.; Shimizu, T.; Tsubota, K.; Shimazaki, J. The effects of 3% diquafosol sodium eye drop application on meibomian gland and ocular surface alterations in the Cu, Zn-superoxide dismutase-1 (Sod1) knockout mice. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 739–750. [Google Scholar] [CrossRef]
- Contreras-Ruiz, L.; Masli, S. Immunomodulatory Cross-Talk between Conjunctival Goblet Cells and Dendritic Cells. PLoS ONE 2015, 10, e0120284. [Google Scholar] [CrossRef]
- Tan, X.; Chen, Y.; Foulsham, W.; Amouzegar, A.; Inomata, T.; Liu, Y.; Chauhan, S.K.; Dana, R. The immunoregulatory role of corneal epithelium-derived thrombospondin-1 in dry eye disease. Ocul. Surf. 2018, 16, 470–477. [Google Scholar] [CrossRef]
- Ratay, M.L.; Glowacki, A.J.; Balmert, S.C.; Acharya, A.P.; Polat, J.; Andrews, L.P.; Fedorchak, M.V.; Schuman, J.S.; Vignali, D.A.; Little, S.R. Treg-recruiting microspheres prevent inflammation in a murine model of dry eye disease. J. Control. Release 2017, 258, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.; Tsung, A.; Billiar, T. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic. Biol. Med. 2010, 48, 1121–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Saldanha, I.J.; Li, T.; Yang, C.; Owczarzak, J.; Williamson, P.R.; Dickersin, K. Clinical trials and systematic reviews addressing similar interventions for the same condition do not consider similar outcomes to be important: A case study in HIV/AIDS. J. Clin. Epidemiol. 2017, 84, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Novack, G.D.; Asbell, P.; Barabino, S.; Bergamini, M.V.; Ciolino, J.B.; Foulks, G.N.; Goldstein, M.; Lemp, M.A.; Schrader, S.; Woods, C.; et al. TFOS DEWS II Clinical Trial Design Report. Ocul. Surf. 2017, 15, 629–649. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heidari, M.; Noorizadeh, F.; Wu, K.; Inomata, T.; Mashaghi, A. Dry Eye Disease: Emerging Approaches to Disease Analysis and Therapy. J. Clin. Med. 2019, 8, 1439. https://doi.org/10.3390/jcm8091439
Heidari M, Noorizadeh F, Wu K, Inomata T, Mashaghi A. Dry Eye Disease: Emerging Approaches to Disease Analysis and Therapy. Journal of Clinical Medicine. 2019; 8(9):1439. https://doi.org/10.3390/jcm8091439
Chicago/Turabian StyleHeidari, Mostafa, Farsad Noorizadeh, Kevin Wu, Takenori Inomata, and Alireza Mashaghi. 2019. "Dry Eye Disease: Emerging Approaches to Disease Analysis and Therapy" Journal of Clinical Medicine 8, no. 9: 1439. https://doi.org/10.3390/jcm8091439




