Therapeutic Potential of Lespedeza bicolor to Prevent Methylglyoxal-Induced Glucotoxicity in Familiar Diabetic Nephropathy
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals and Reagents
2.2. Preparation of LB Extract
2.3. Quantitative Study of the Phytochemicals in LB
2.4. Cell Culture
2.5. MTT Assay
2.6. Cell Apoptosis Assay
2.7. Measurement of Intracellular Oxidative Stress
2.8. Western Blotting
2.9. Animal Experiments
2.10. Oral Glucose Tolerance Test (OGTT)
2.11. Analysis of Plasma Lipids
2.12. Determination of MGO Levels in the Kidneys
2.13. Evaluation of the Levels of AGEs, IL-1β, and TNF-α in the Kidneys
2.14. Histology and Immunohistochemistry Studies
2.15. Statistical Analysis
3. Results
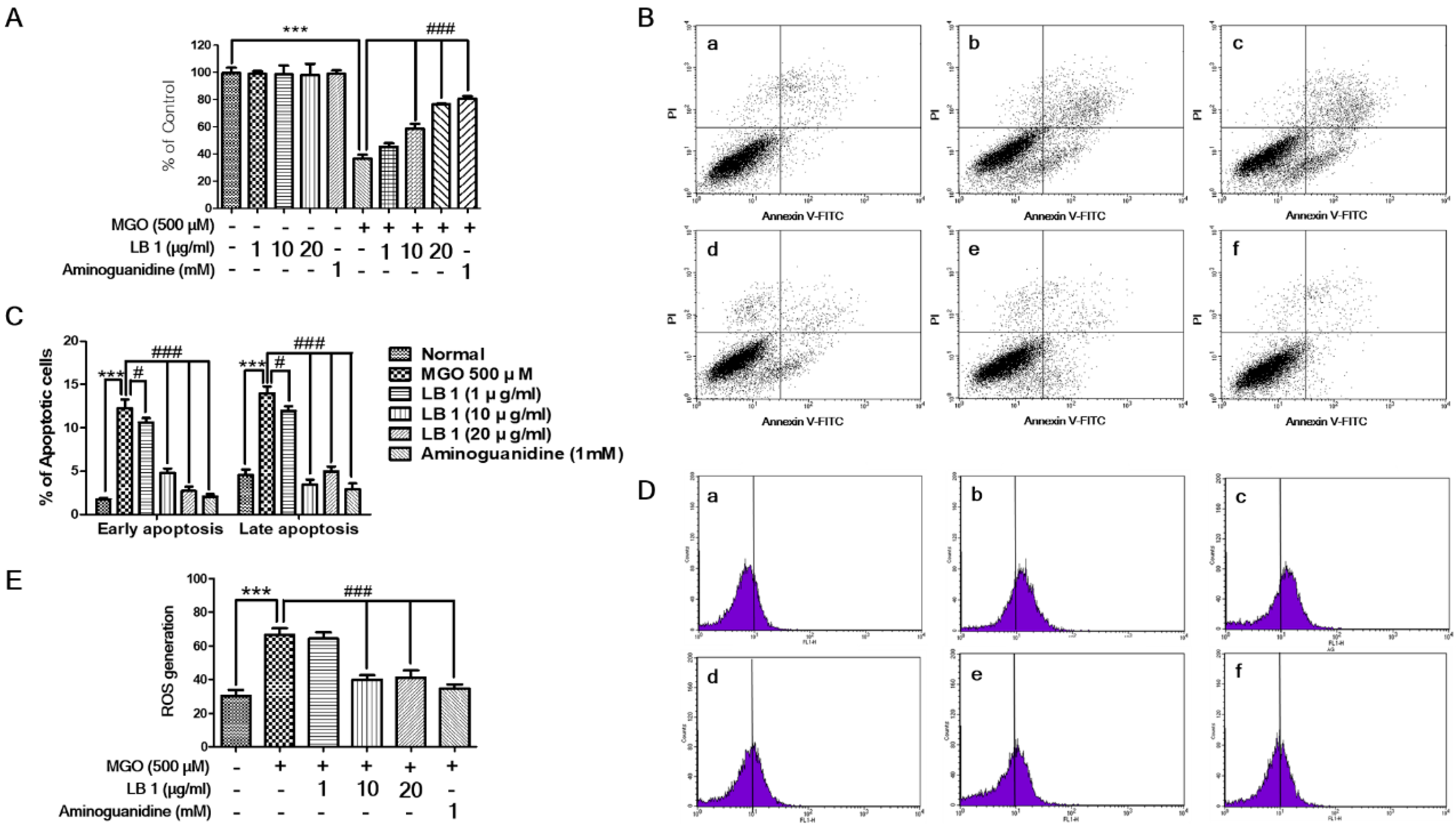
3.1. LB Pretreatment Attenuated MGO-Induced Cytotoxicity, Apoptosis, and Oxidative Stress in LLC-PK1 Cells
3.2. LB Pretreatment Upregulated Expression of Glo1 and Nrf2 in MGO-Treated LLC-PK1 Cells
3.3. Blood Glucose Tolerance
3.4. LB Pretreatment Decreased the Levels of Plasma Lipids in MGO-Treated Mice
3.5. LB Pretreatment Reduced MGO in the Kidneys of MGO-Treated Mice
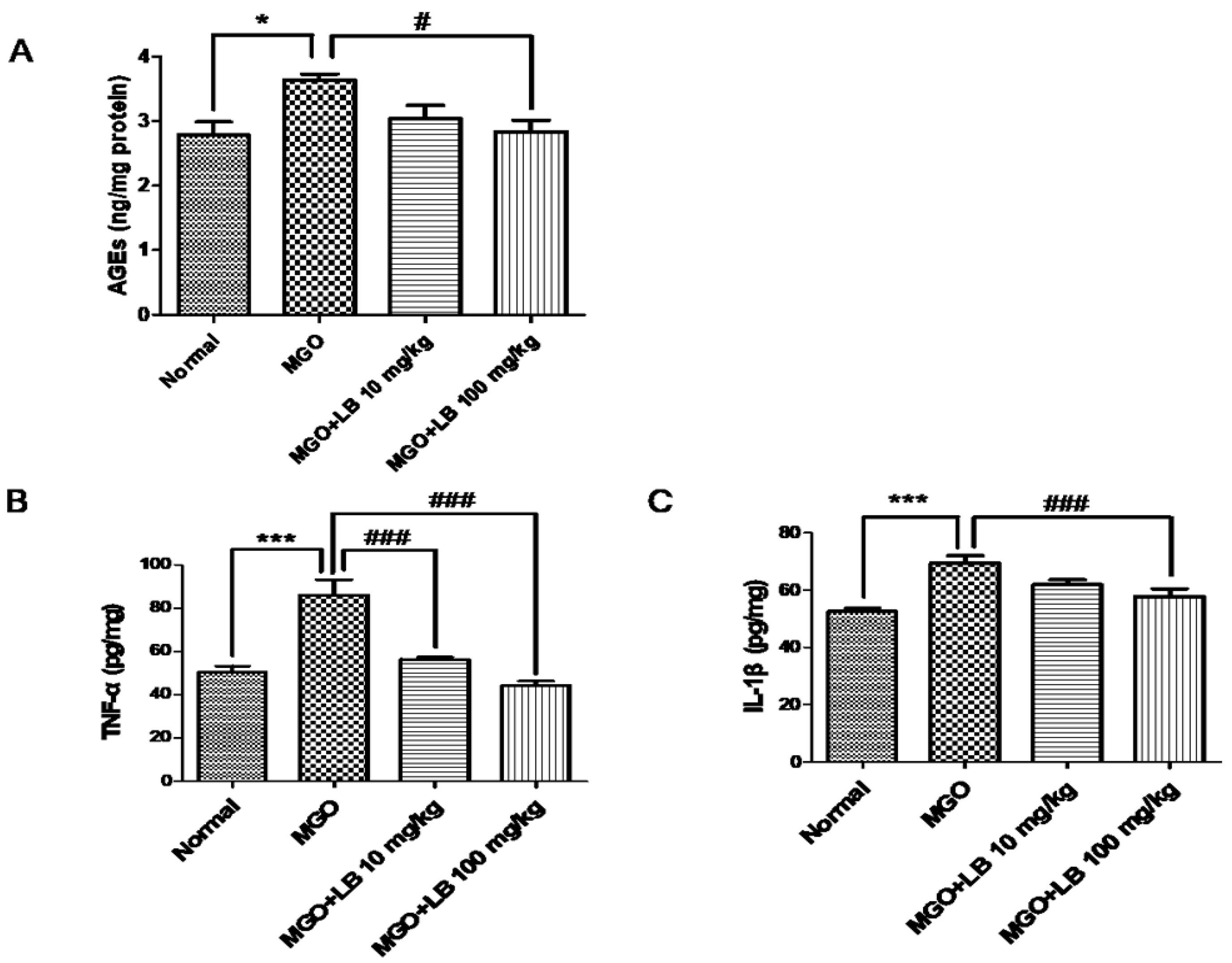
3.6. LB Pretreatment Decreased the Amount of AGEs in the Kidneys
3.7. LB Pretreatment Attenuated the Levels of Inflammatory Cytokines in the MGO-Treated Mice
3.8. LB Pretreatment Upregulated Glo1 and Nrf2 in the Kidneys of MGO-Treated Mice
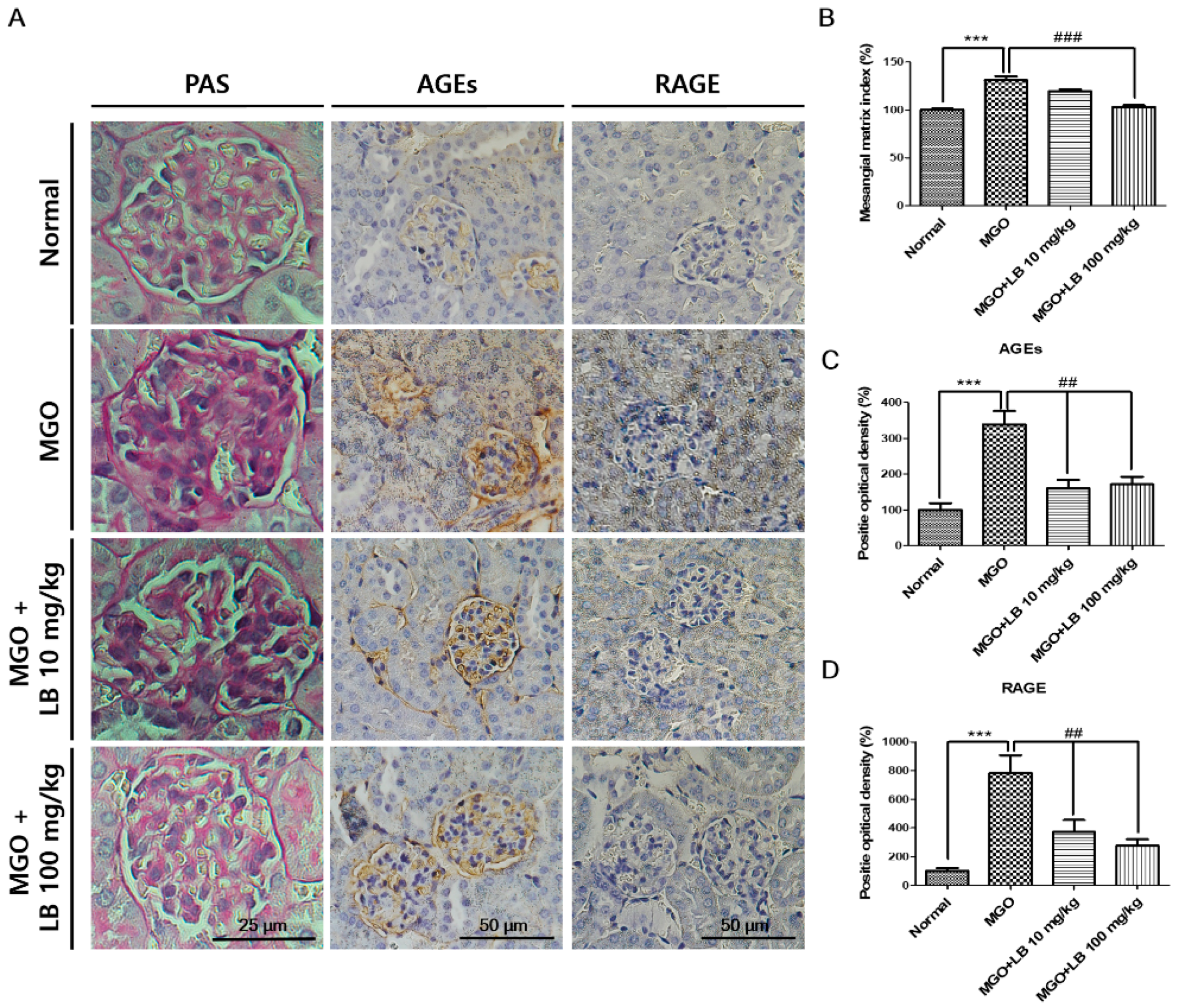
3.9. Pretreatment with LB Protected MGO-Treated Mice Against Renal Damage
3.10. HPLC Analysis of LB Extract
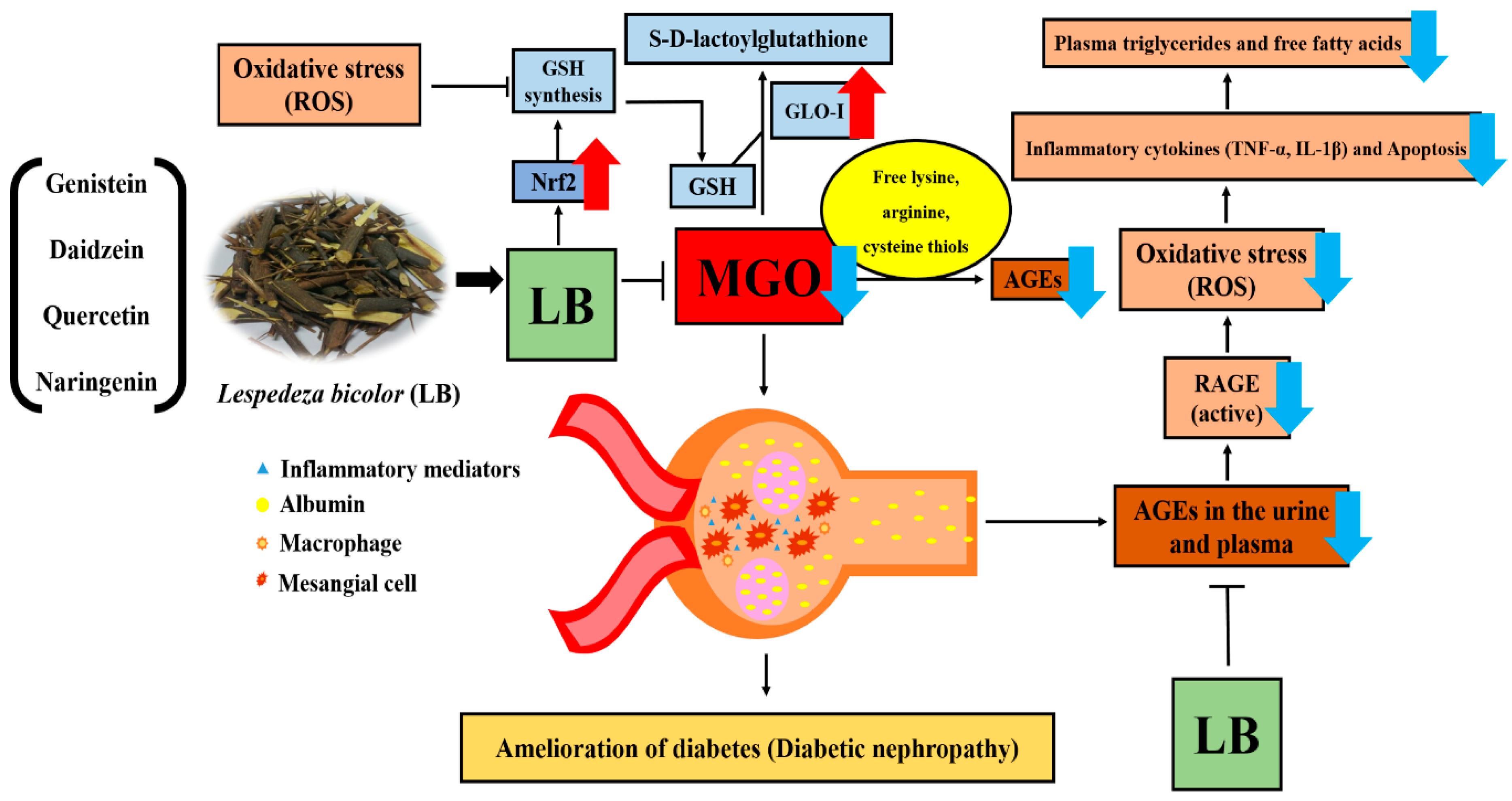
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| LB | Lespedeza bicolor |
| MGO | methylglyoxal |
| ROS | reactive oxygen species |
| AGEs | advanced glycation end-products |
| RAGE | receptor for advanced glycation end products |
| Glo1 | glyoxalase 1 |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| DM | diabetes mellitus |
| DN | diabetic nephropathy |
| ARE | antioxidant responsive element |
| o-PD | o-phenylenediamine |
| BSA | bovine serum albumin |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| DMSO | dimethyl sulfoxide |
| FBS | fetal bovine serum |
| DMEM | Dulbecco’s modified Eagle’s medium |
| 2-MQ | 2-methylquinoxaline |
| 5-MQ | 5-methylquinoxaline |
| BW | body weight |
| HPLC | high-performance liquid chromatography |
| FACS | fluorescence-activated cell sorting |
References
- Rathmann, W.; Giani, G. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 2568–2569. [Google Scholar] [CrossRef] [PubMed]
- Parveen, A.; Jin, M.; Kim, S.Y. Bioactive phytochemicals that regulate the cellular processes involved in diabetic nephropathy. Phytomedicine 2018, 39, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Shamsaldeen, Y.A.; Mackenzie, L.S.; Lione, L.A.; Benham, C.D. Methylglyoxal, a metabolite increased in diabetes is associated with insulin resistance, vascular dysfunction and neuropathies. Curr. Drug Metab. 2016, 17, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, O.; Niessen, P.M.; Ferreira, I.; Miyata, T.; Scheffer, P.G.; Teerlink, T.; Schrauwen, P.; Brownlee, M.; Stehouwer, C.D.; Schalkwijk, C.G. Overexpression of glyoxalase-I reduces hyperglycemia-induced levels of advanced glycation end products and oxidative stress in diabetic rats. J. Biol. Chem. 2011, 286, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Du, X.; D’Agati, V.D.; Milne, R.; Sui, G.; Geoffrion, M.; Brownlee, M. Knockdown of glyoxalase 1 mimics diabetic nephropathy in nondiabetic mice. Diabetes 2014, 63, 291–299. [Google Scholar] [CrossRef] [PubMed]
- De Haan, J.B. Nrf2 activators as attractive therapeutics for diabetic nephropathy. Diabetes 2011, 60, 2683–2684. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Thornalley, P.J. The critical role of methylglyoxal and glyoxalase 1 in diabetic nephropathy. Diabetes 2014, 63, 50–52. [Google Scholar] [CrossRef]
- Rajappa, R.; Bovilla, V.; Madhunapantula, S.V. Naturally Occurring Nrf2 Activators in the Management of Diabetes. Nutr. Food Sci. Int. J. 2017, 2, 555595. [Google Scholar]
- Liu, Y.; Ren, W.; Bai, Y.; Wan, L.; Sun, X.; Liu, Y.; Xiong, W.; Zhang, Y.-Y.; Zhou, L. Oxyresveratrol prevents murine H22 hepatocellular carcinoma growth and lymph node metastasis via inhibiting tumor angiogenesis and lymphangiogenesis. J. Nat. Med. 2018, 72, 481–492. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, L.; Zhou, Q.; Yan, S.; Li, Z.; Sheng, J.; Zhang, W. (+)-Catechin ameliorates diabetic nephropathy by trapping methylglyoxal in type 2 diabetic mice. Mol. Nutr. Food Res. 2014, 58, 2249–2260. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.H.; Hwang, Y.; Heo, S.J.; Jun, H.S. Diphlorethohydroxycarmalol Attenuates Methylglyoxal-Induced Oxidative Stress and Advanced Glycation End Product Formation in Human Kidney Cells. Oxidative Med. Cell. Longev. 2018, 2018, 3654095. [Google Scholar] [CrossRef] [PubMed]
- Do, M.H.; Lee, J.H.; Wahedi, H.M.; Pak, C.; Lee, C.H.; Yeo, E.J.; Lim, Y.; Ha, S.K.; Choi, I.; Kim, S.Y. Lespedeza bicolor ameliorates endothelial dysfunction induced by methylglyoxal glucotoxicity. Phytomedicine 2017, 36, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Hossaine, M.D.; Park, S.C. A potential anti-inflammation activity and depigmentation effect of Lespedeza bicolor extract and its fractions. Saudi J. Biol. Sci. 2016, 23, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Joo, E.-Y.; Kim, N.-W. Polyphenol contents and physiological activity of the Lespedeza bicolor extracts. Korean J. Food Preserv. 2006, 13, 616–622. [Google Scholar]
- Lee, J.K.; Kang, D.G.; Lee, H.S. Vascular relaxation induced by aqueous extract of Lespedeza cuneata via the NO-cGMP pathway. J. Nat. Med. 2012, 66, 17–24. [Google Scholar] [CrossRef]
- Ullah, S. Methanolic extract from Lespedeza bicolor: Potential candidates for natural antioxidant and anticancer agent. J. Tradit. Chin. Med. 2017, 37, 444–451. [Google Scholar] [CrossRef]
- Woo, H.S.; Kim, D.W.; Curtis-Long, M.J.; Lee, B.W.; Lee, J.H.; Kim, J.Y.; Kang, J.E.; Park, K.H. Potent inhibition of bacterial neuraminidase activity by pterocarpans isolated from the roots of Lespedeza bicolor. Bioorganic Med. Chem. Lett. 2011, 21, 6100–6103. [Google Scholar] [CrossRef]
- Park, S.Y.; Kwon, S.J.; Lim, S.S.; Kim, J.-K.; Lee, K.W.; Park, J.H.Y. Licoricidin, an active compound in the hexane/ethanol extract of Glycyrrhiza Uralensis, inhibits lung metastasis of 4T1 Murine mammary carcinoma cells. Int. J. Mol. Sci. 2016, 17, 934. [Google Scholar] [CrossRef]
- Sharma, B.R.; Rhyu, D.Y. Lespedeza davurica (Lax.) Schindl. extract protects against cytokine-induced beta-cell damage and streptozotocin-induced diabetes. BioMed Res. Int. 2015, 2015, 169256. [Google Scholar] [CrossRef]
- Park, H.Y.; Kim, G.B.; Kwon, Y.S. Two new dihydrofuranoisoflavanones from the leaves of Lespedeza maximowiczi and their inhibitory effect on the formation of advanced glycation end products. Arch. Pharmacal Res. 2010, 33, 1159–1163. [Google Scholar] [CrossRef]
- Dhar, A.; Desai, K.; Liu, J.; Wu, L. Methylglyoxal, protein binding and biological samples: Are we getting the true measure? J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.R.; Kim, M.S.; Yokozawa, T.; Rhyu, D.Y. Anti-oxidant and anti-diabetic activities of Lespedeza cuneata water extract. J. Med. Plants Res. 2014, 8, 935–941. [Google Scholar] [Green Version]
- Mori-Hongo, M.; Takimoto, H.; Katagiri, T.; Kimura, M.; Ikeda, Y.; Miyase, T. Melanin synthesis inhibitors from Lespedeza floribunda. J. Nat. Prod. 2009, 72, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Maximov, O.B.; Kulesh, N.I.; Stepanenko, L.S.; Dmitrenok, P.S. New prenylated isoflavanones and other constituents of Lespedeza bicolor. Fitoterapia 2004, 75, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Sha, J.; Sui, B.; Su, X.; Meng, Q.; Zhang, C. Alteration of oxidative stress and inflammatory cytokines induces apoptosis in diabetic nephropathy. Mol. Med. Rep. 2017, 16, 7715–7723. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Cheng, Y.Q.; Liu, X.L.; Hao, Y.C.; Li, Y.; Zhu, X.; Zhang, F.; Yin, X.X. Mangiferin Upregulates Glyoxalase 1 Through Activation of Nrf2/ARE Signaling in Central Neurons Cultured with High Glucose. Mol. Neurobiol. 2017, 54, 4060–4070. [Google Scholar] [CrossRef]
- Xue, M.; Rabbani, N.; Momiji, H.; Imbasi, P.; Anwar, M.M.; Kitteringham, N.; Park, B.K.; Souma, T.; Moriguchi, T.; Yamamoto, M. Transcriptional control of glyoxalase 1 by Nrf2 provides a stress-responsive defence against dicarbonyl glycation. Biochem. J. 2012, 443, 213–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.-W.; Zhu, X.; Yang, Q.-Q.; Lu, Q.; Wang, J.-Y.; Li, H.-P.; Wei, Y.-Q.; Yin, J.-L.; Yin, X.-X. Suppression of methylglyoxal hyperactivity by mangiferin can prevent diabetes-associated cognitive decline in rats. Psychopharmacology 2013, 228, 585–594. [Google Scholar] [CrossRef]
- Yan, S.F.; Ramasamy, R.; Schmidt, A.M. The RAGE axis a fundamental mechanism signaling danger to the vulnerable vasculature. Circ. Res. 2010, 106, 842–853. [Google Scholar] [CrossRef]
- Kaur, C.; Ghosh, A.; Pareek, A.; Sopory, S.K.; Singla-Pareek, S.L. Glyoxalases and stress tolerance in plants. Biochem. Soc. Trans. 2014, 42, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Hao, J.L.; Xie, T.; Malik, T.H.; Lu, C.B.; Liu, C.; Shu, C.; Lu, C.W.; Zhou, D.D. Nrf2 as a target for prevention of age-related and diabetic cataracts by against oxidative stress. Aging Cell 2017, 16, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-H.; Hsu, W.-H.; Hsu, Y.-W.; Pan, T.-M. Dimerumic acid attenuates receptor for advanced glycation endproducts signal to inhibit inflammation and diabetes mediated by Nrf2 activation and promotes methylglyoxal metabolism into d-lactic acid. Free Radic. Biol. Med. 2013, 60, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.-H.; Lee, B.-H.; Chang, Y.-Y.; Hsu, Y.-W.; Pan, T.-M. A novel natural Nrf2 activator with PPARγ-agonist (monascin) attenuates the toxicity of methylglyoxal and hyperglycemia. Toxicol. Appl. Pharmacol. 2013, 272, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J. The importance of free fatty acids in the development of Type 2 diabetes. Diabet. Med. 2007, 24, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.; Dhar, I.; Jiang, B.; Desai, K.M.; Wu, L. Chronic methylglyoxal infusion by minipump causes pancreatic β-cell dysfunction and induces type 2 diabetes in Sprague-Dawley rats. Diabetes 2011, 60, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-K.; Chen, S.-M.; Li, Y.-C.; Huang, T.-C.; Lee, J.-A. Low-molecular-weight chitosan scavenges methylglyoxal and N ε-(carboxyethyl) lysine, the major factors contributing to the pathogenesis of nephropathy. SpringerPlus 2015, 4, 312. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.; Vannucci, S.J.; Du Yan, S.S.; Herold, K.; Yan, S.F.; Schmidt, A.M. Advanced glycation end products and RAGE: A common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology 2005, 15, 16R–28R. [Google Scholar] [CrossRef]
- Lu, J.; Randell, E.; Han, Y.; Adeli, K.; Krahn, J.; Meng, Q.H. Increased plasma methylglyoxal level, inflammation, and vascular endothelial dysfunction in diabetic nephropathy. Clin. Biochem. 2011, 44, 307–311. [Google Scholar] [CrossRef]
- Bierhaus, A.; Humpert, P.M.; Morcos, M.; Wendt, T.; Chavakis, T.; Arnold, B.; Stern, D.M.; Nawroth, P.P. Understanding RAGE, the receptor for advanced glycation end products. J. Mol. Med. 2005, 83, 876–886. [Google Scholar] [CrossRef]
- Alexiou, P.; Chatzopoulou, M.; Pegklidou, K.; Demopoulos, V. RAGE: A multi-ligand receptor unveiling novel insights in health and disease. Curr. Med. Chem. 2010, 17, 2232–2252. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.R.; Liu, D. Anti-diabetic functions of soy isoflavone genistein: Mechanisms underlying its effects on pancreatic beta-cell function. Food Funct. 2013, 4, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Ashour, M.B.; Abdel-Moneim, A.; Ahmed, O.M. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J. Diabetes Complicat. 2012, 26, 483–490. [Google Scholar] [CrossRef] [PubMed]


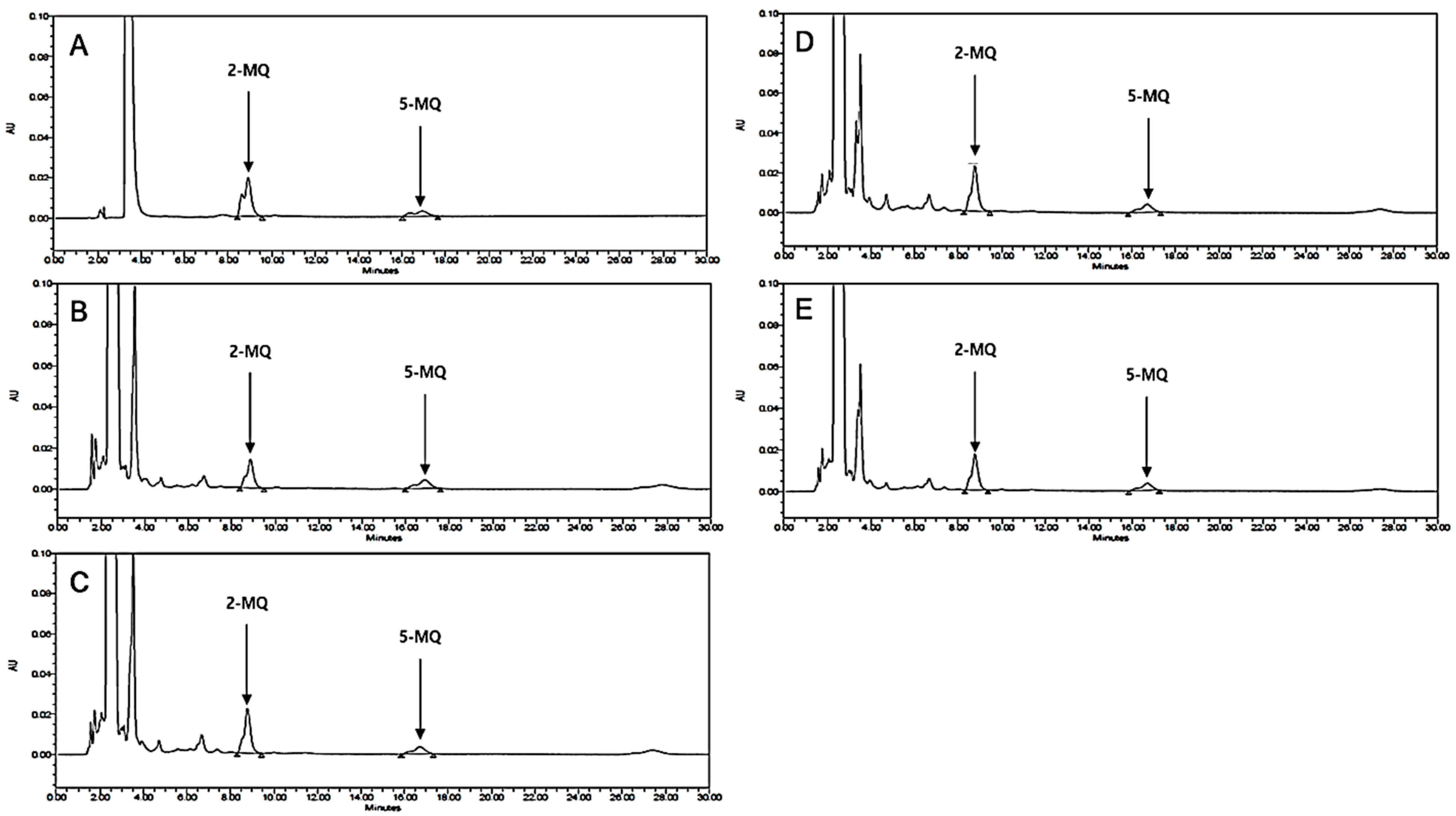

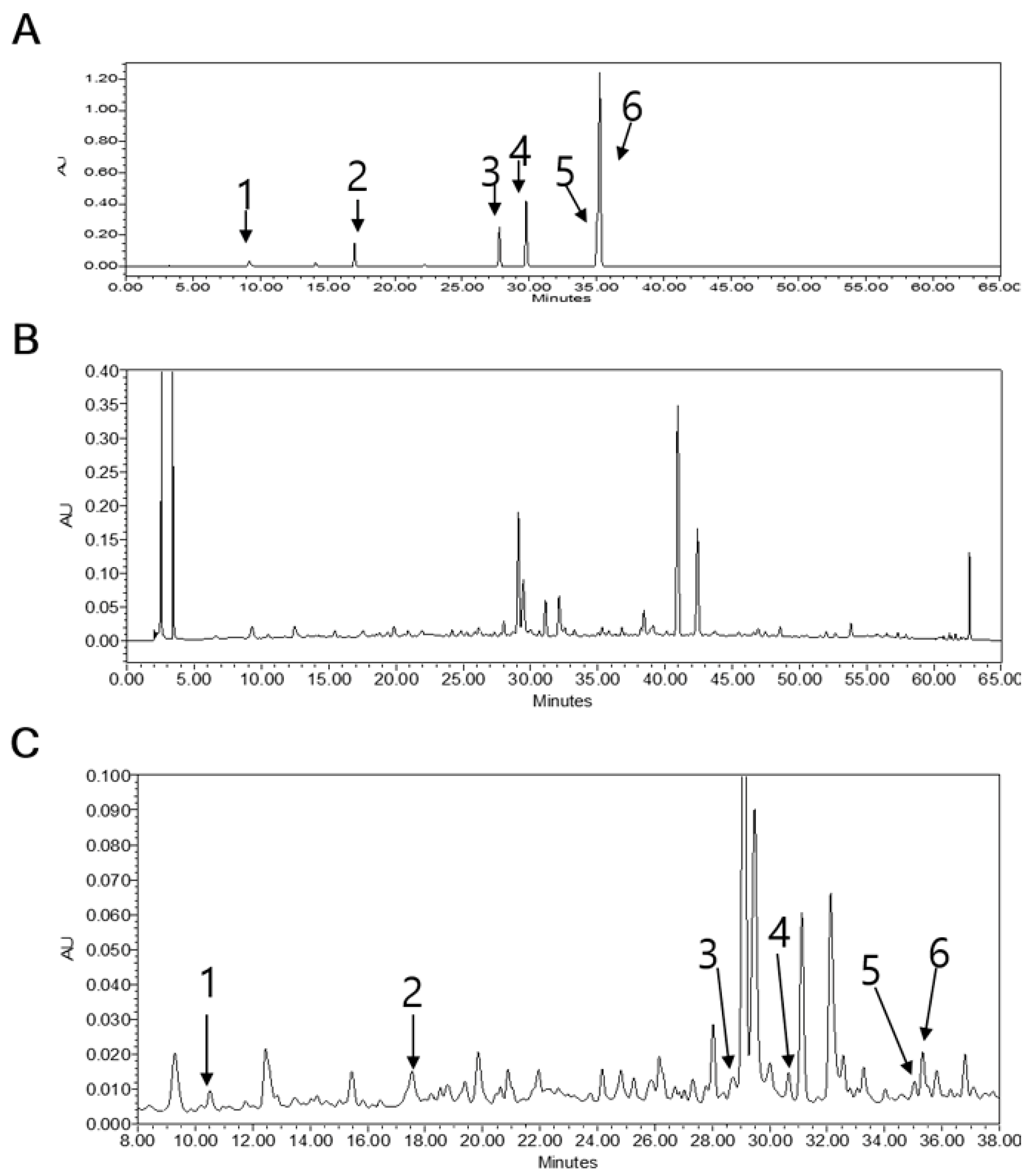
| Sample | Concentration (nmol/mg Protein) |
|---|---|
| Normal | 0.972 ± 0.148 |
| MGO (300 mg/kg) | 1.704 ± 0.053 ** |
| MGO (300 mg/kg) + LB (10 mg/kg) | 1.680 ± 0.063 |
| MGO (300 mg/kg) + LB (100 mg/kg) | 1.152 ± 0.050 ## |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do, M.H.; Lee, J.H.; Cho, K.; Kang, M.C.; Subedi, L.; Parveen, A.; Kim, S.Y. Therapeutic Potential of Lespedeza bicolor to Prevent Methylglyoxal-Induced Glucotoxicity in Familiar Diabetic Nephropathy. J. Clin. Med. 2019, 8, 1138. https://doi.org/10.3390/jcm8081138
Do MH, Lee JH, Cho K, Kang MC, Subedi L, Parveen A, Kim SY. Therapeutic Potential of Lespedeza bicolor to Prevent Methylglyoxal-Induced Glucotoxicity in Familiar Diabetic Nephropathy. Journal of Clinical Medicine. 2019; 8(8):1138. https://doi.org/10.3390/jcm8081138
Chicago/Turabian StyleDo, Moon Ho, Jae Hyuk Lee, Kyohee Cho, Min Cheol Kang, Lalita Subedi, Amna Parveen, and Sun Yeou Kim. 2019. "Therapeutic Potential of Lespedeza bicolor to Prevent Methylglyoxal-Induced Glucotoxicity in Familiar Diabetic Nephropathy" Journal of Clinical Medicine 8, no. 8: 1138. https://doi.org/10.3390/jcm8081138





