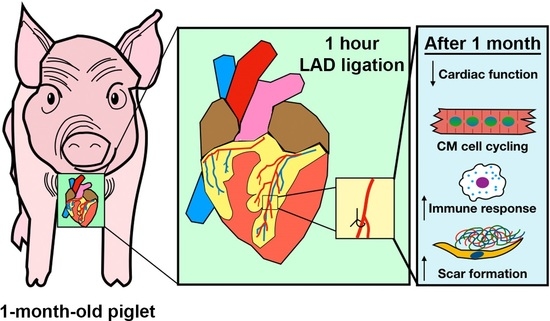
Scar Formation with Decreased Cardiac Function Following Ischemia/Reperfusion Injury in 1 Month Old Swine
Abstract
:1. Introduction
2. Materials and Methods
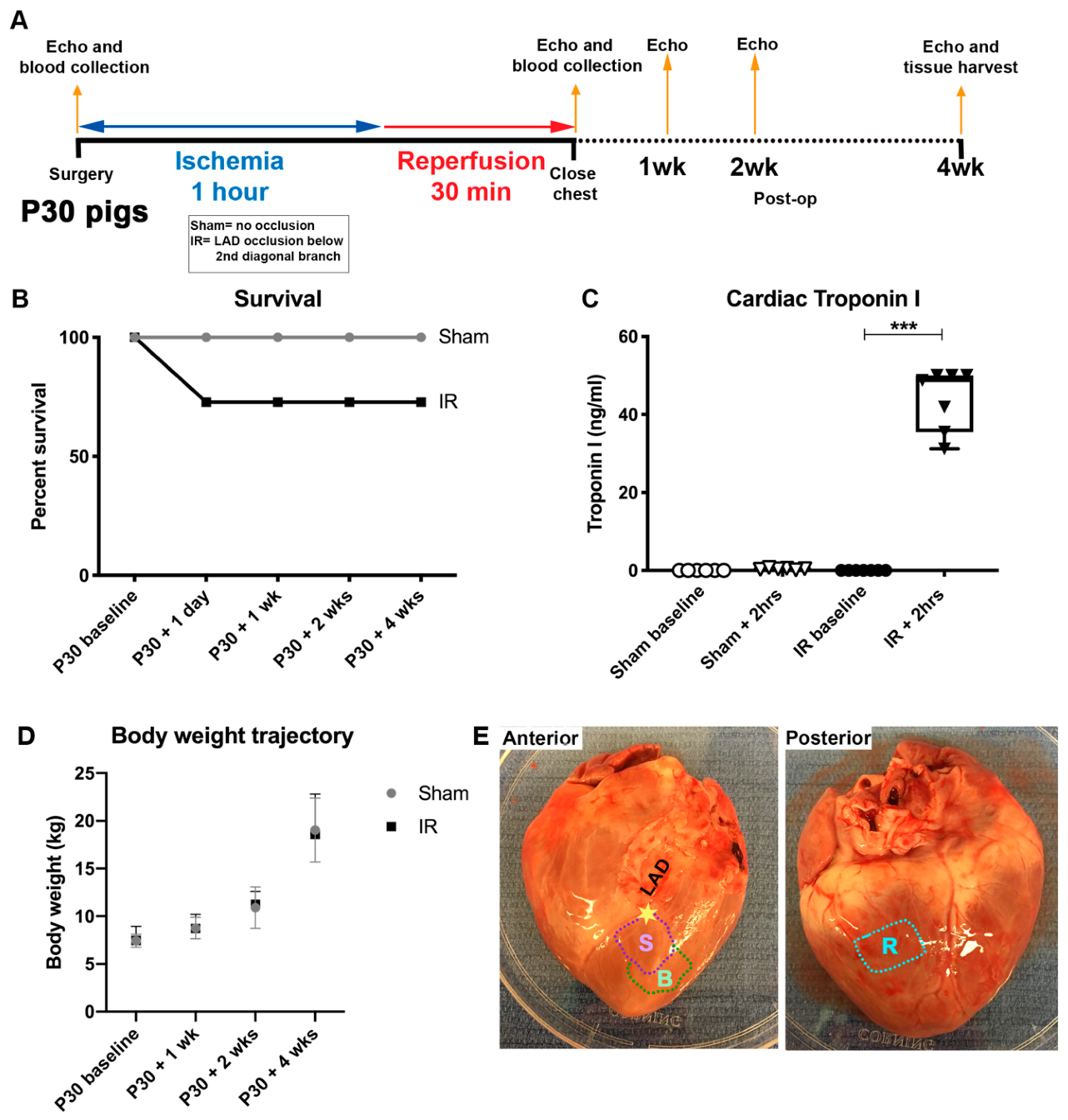
2.1. Ischemia/Reperfusion (IR) Cardiac Injury
2.2. Serum Cardiac Troponin I Determination
2.3. Echocardiography
2.4. Histology and Tissue Staining
2.4.1. Masson’s Trichrome
2.4.2. Immunohistochemistry
2.4.3. Lectin-DAB Staining
2.4.4. Cell Death Detection
2.4.5. Collagen Hybridizing Peptide Detection
2.5. RNA Analysis
2.5.1. RNA-Sequencing
2.5.2. Gene Expression Data Analysis
2.5.3. Gene Ontology and Pathway Analysis
2.6. Statistical Analysis
3. Results
3.1. Cardiac Ischemia/Reperfusion (IR) Injury, via Temporary Occlusion of the LAD, Produces Myocardial Tissue Damage in P30 Pigs
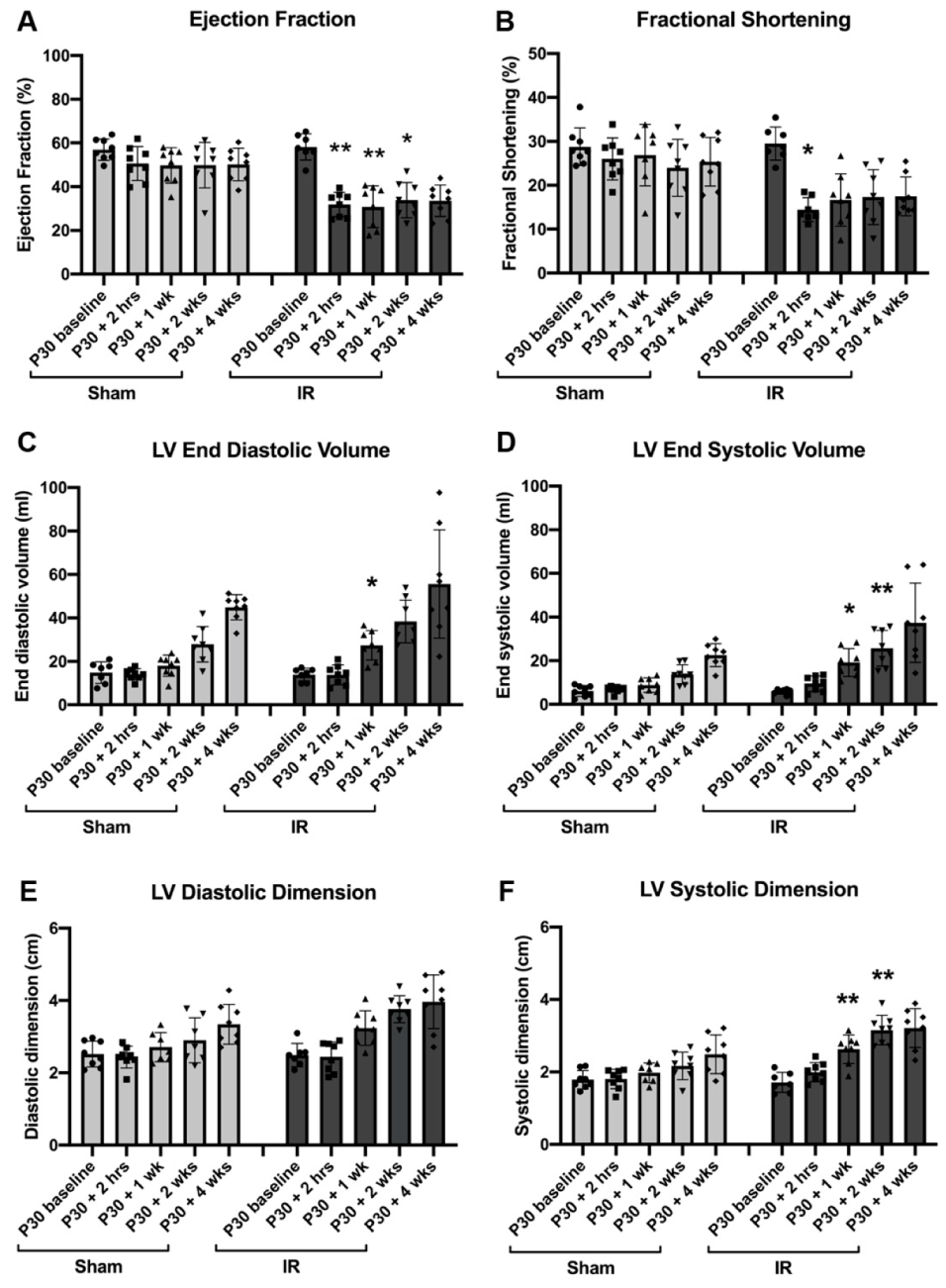
3.2. Cardiac Function Declines Post-Ischemic Injury in P30 Pigs
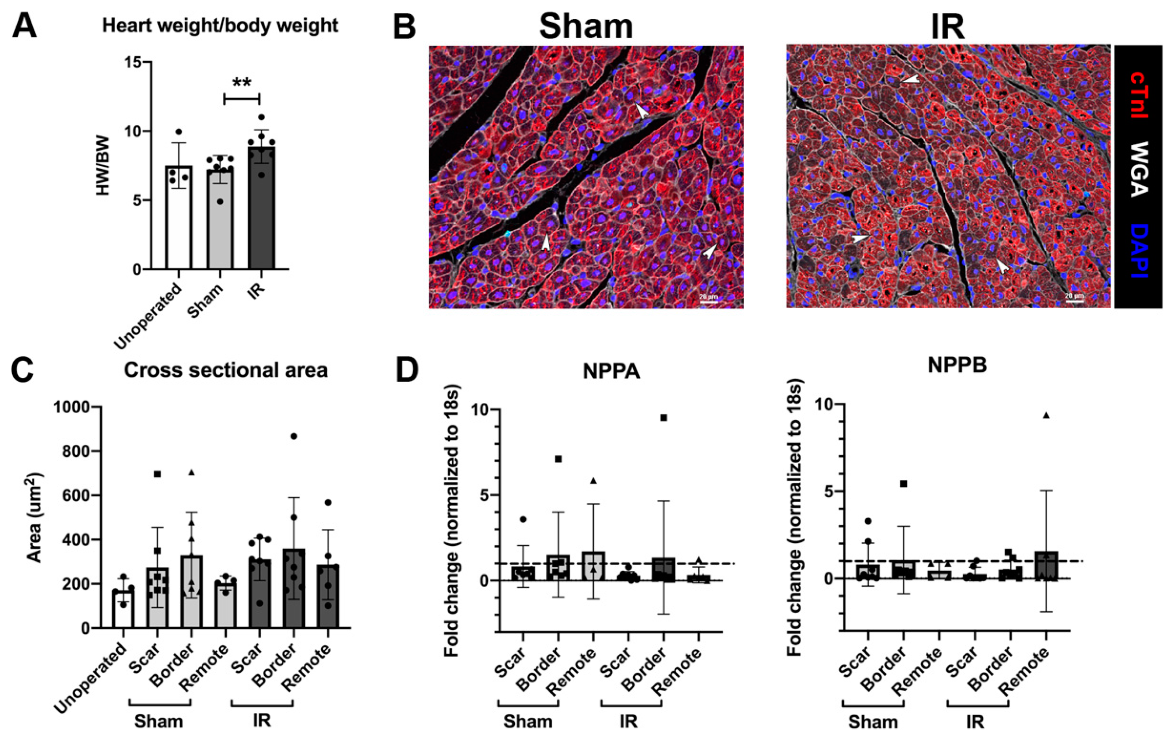
3.3. Cardiomyocyte Hypertrophy Is Not Induced with IR Injury, 4 Weeks Following Surgery
3.4. Cardiomyocyte Cell Cycling Activity Is Unchanged Following P30 Ischemia/Reperfusion Cardiac Injury
3.5. Vessel Density and Cell Death Are Unchanged Following P30 Ischemia/Reperfusion Cardiac Injury
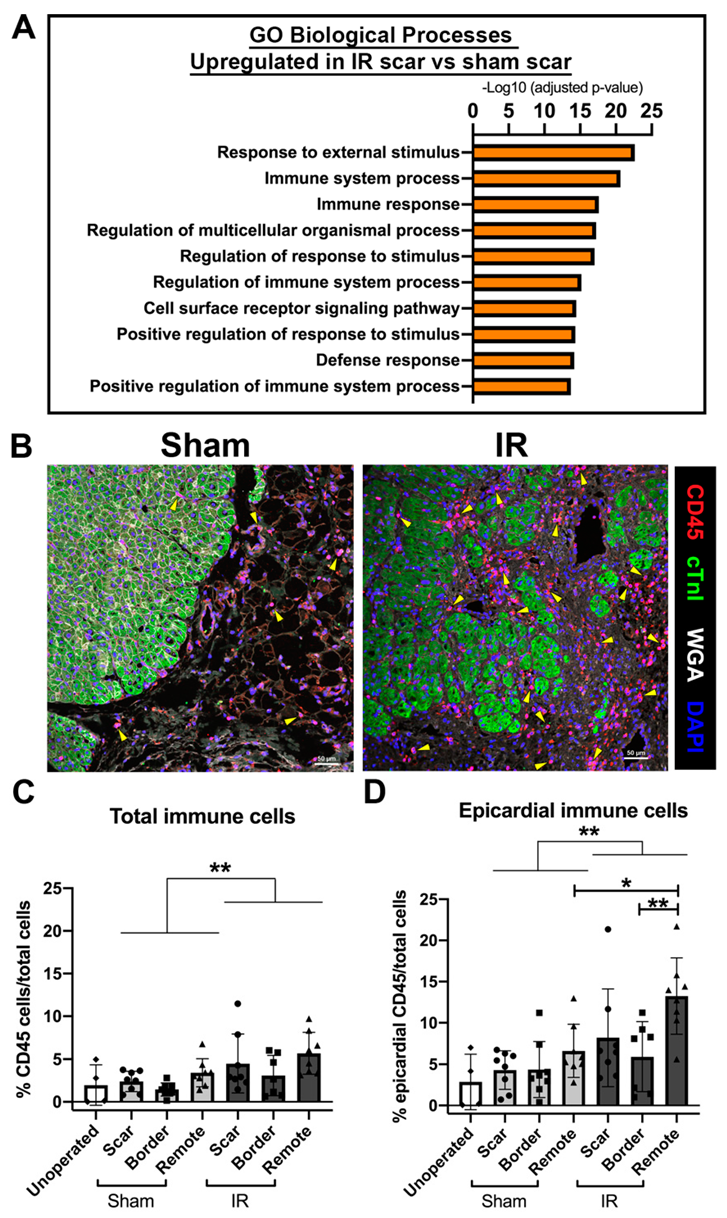
3.6. The Immune Response Is Upregulated at 4 Weeks Following Cardiac Ischemic Injury
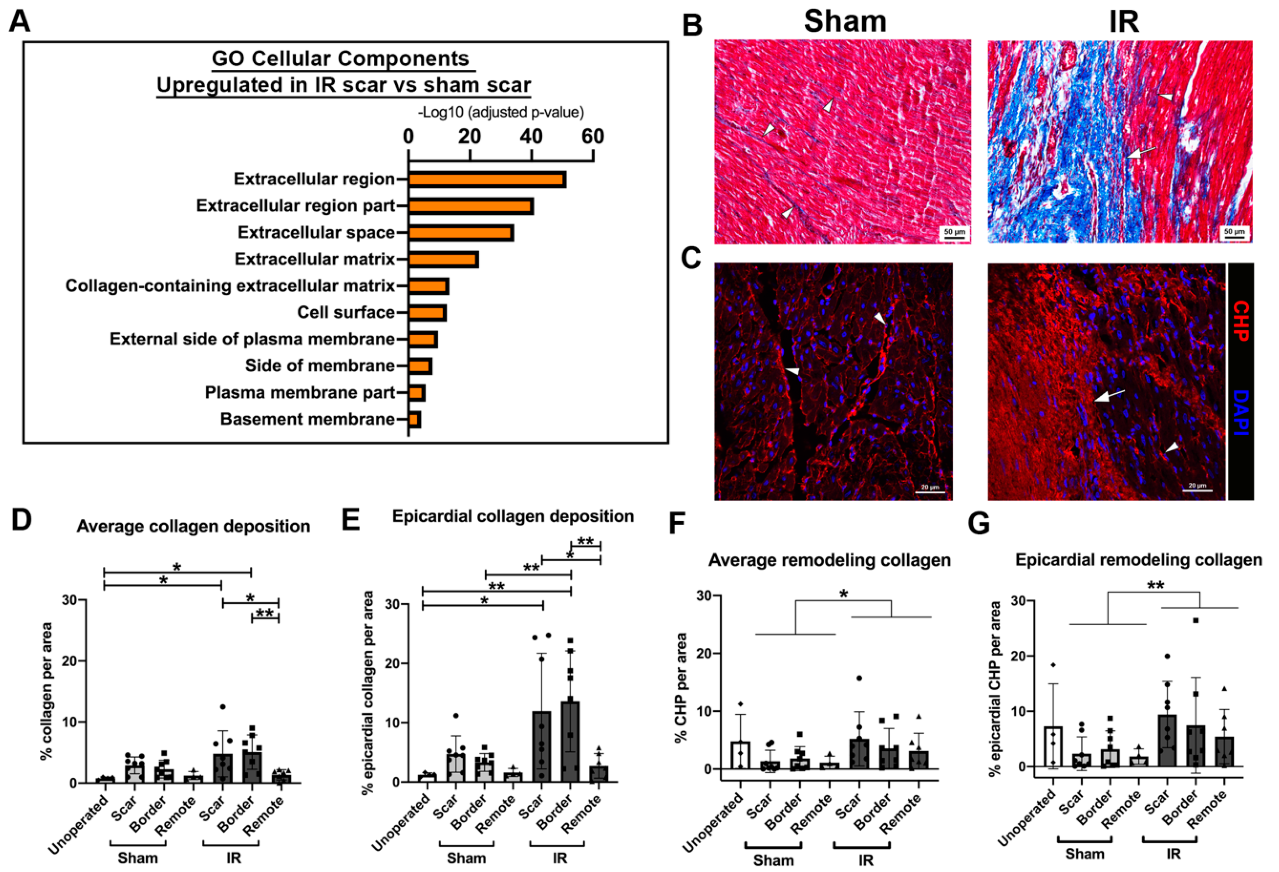
3.7. Myocardial Scarring Is Apparent 4 Weeks Following IR Cardiac Injury in P30 Pigs
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Velayutham, N.; Agnew, E.J.; Yutzey, K.E. Postnatal Cardiac Development and Regenerative Potential in Large Mammals. Pediatr. Cardiol. 2019, 40, 1345–1358. [Google Scholar] [CrossRef] [PubMed]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Hill, J.A.; Richardson, J.A.; Olson, E.N.; Sadek, H.A. Transient regenerative potential of the neonatal mouse heart. Science 2011, 331, 1078–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Zhang, E.; Zhao, M.; Chong, Z.; Fan, C.; Tang, Y.; Hunter, J.D.; Borovjagin, A.V.; Walcott, G.P.; Chen, J.Y.; et al. Regenerative Potential of Neonatal Porcine Hearts. Circulation 2018, 138, 2809–2816. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; D’Agostino, G.; Loo, S.J.; Wang, C.X.; Su, L.P.; Tan, S.H.; Tee, G.Z.; Pua, C.J.; Pena, E.M.; Cheng, R.B.; et al. Early Regenerative Capacity in the Porcine Heart. Circulation 2018, 138, 2798–2808. [Google Scholar] [CrossRef]
- Foglia, M.J.; Poss, K.D. Building and re-building the heart by cardiomyocyte proliferation. Development 2016, 143, 729–740. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Wang, X.; Capasso, J.M.; Gerdes, A.M. Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. J. Mol. Cell. Cardiol. 1996, 28, 1737–1746. [Google Scholar] [CrossRef]
- Sengupta, A.; Kalinichenko, V.V.; Yutzey, K.E. FoxO1 and FoxM1 transcription factors have antagonistic functions in neonatal cardiomyocyte cell-cycle withdrawal and IGF1 gene regulation. Circ. Res. 2013, 112, 267–277. [Google Scholar] [CrossRef] [Green Version]
- Senyo, S.E.; Steinhauser, M.L.; Pizzimenti, C.L.; Yang, V.K.; Cai, L.; Wang, M.; Wu, T.D.; Guerquin-Kern, J.L.; Lechene, C.P.; Lee, R.T. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 2013, 493, 433–436. [Google Scholar] [CrossRef] [Green Version]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Johnson, B.A.; Grinsfelder, D.; Canseco, D.; Mammen, P.P.; Rothermel, B.A.; Olson, E.N.; Sadek, H.A. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc. Natl. Acad. Sci. USA 2013, 110, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Haubner, B.J.; Adamowicz-Brice, M.; Khadayate, S.; Tiefenthaler, V.; Metzler, B.; Aitman, T.; Penninger, J.M. Complete cardiac regeneration in a mouse model of myocardial infarction. Aging 2012, 4, 966–977. [Google Scholar] [CrossRef] [Green Version]
- Nakada, Y.; Canseco, D.C.; Thet, S.; Abdisalaam, S.; Asaithamby, A.; Santos, C.X.; Shah, A.M.; Zhang, H.; Faber, J.E.; Kinter, M.T.; et al. Hypoxia induces heart regeneration in adult mice. Nature 2017, 541, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Velayutham, N.; Alfieri, C.M.; Agnew, E.J.; Riggs, K.W.; Baker, R.S.; Zafar, F.; Yutzey, K.E. Cardiomyocyte cell cycling, maturation, and growth by multinucleation in postnatal swine. bioRxiv 2019, 773101. [Google Scholar] [CrossRef]
- Patterson, M.; Barske, L.; Van Handel, B.; Rau, C.D.; Gan, P.; Sharma, A.; Parikh, S.; Denholtz, M.; Huang, Y.; Yamaguchi, Y.; et al. Frequency of mononuclear diploid cardiomyocytes underlies natural variation in heart regeneration. Nat. Genet. 2017, 49, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Ladage, D.; Tilemann, L.; Ishikawa, K.; Correll, R.N.; Kawase, Y.; Houser, S.R.; Molkentin, J.D.; Hajjar, R.J. Inhibition of PKCalpha/beta with ruboxistaurin antagonizes heart failure in pigs after myocardial infarction injury. Circ. Res. 2011, 109, 1396–1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabisonia, K.; Prosdocimo, G.; Aquaro, G.D.; Carlucci, L.; Zentilin, L.; Secco, I.; Ali, H.; Braga, L.; Gorgodze, N.; Bernini, F.; et al. MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs. Nature 2019, 569, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Malliaras, K.; Smith, R.R.; Kanazawa, H.; Yee, K.; Seinfeld, J.; Tseliou, E.; Dawkins, J.F.; Kreke, M.; Cheng, K.; Luthringer, D.; et al. Validation of contrast-enhanced magnetic resonance imaging to monitor regenerative efficacy after cell therapy in a porcine model of convalescent myocardial infarction. Circulation 2013, 128, 2764–2775. [Google Scholar] [CrossRef] [Green Version]
- Bagno, L.L.; Kanashiro-Takeuchi, R.M.; Suncion, V.Y.; Golpanian, S.; Karantalis, V.; Wolf, A.; Wang, B.; Premer, C.; Balkan, W.; Rodriguez, J.; et al. Growth hormone-releasing hormone agonists reduce myocardial infarct scar in swine with subacute ischemic cardiomyopathy. J. Am. Heart Assoc. 2015, 4, e001464. [Google Scholar] [CrossRef] [Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef]
- Risso, D.; Ngai, J.; Speed, T.P.; Dudoit, S. Normalization of RNA-seq data using factor analysis of control genes or samples. Nat. Biotechnol. 2014, 32, 896–902. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Bardes, E.E.; Aronow, B.J.; Jegga, A.G. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 2009, 37, W305–W311. [Google Scholar] [CrossRef] [PubMed]
- Munz, M.R.; Faria, M.A.; Monteiro, J.R.; Aguas, A.P.; Amorim, M.J. Surgical porcine myocardial infarction model through permanent coronary occlusion. Comp. Med. 2011, 61, 445–452. [Google Scholar] [PubMed]
- De Jong, R.; van Hout, G.P.; Houtgraaf, J.H.; Takashima, S.; Pasterkamp, G.; Hoefer, I.; Duckers, H.J. Cardiac function in a long-term follow-up study of moderate and severe porcine model of chronic myocardial infarction. Biomed. Res. Int. 2015, 2015, 209315. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, G.; Weil, B.R.; Young, R.F.; Fallavollita, J.A.; Canty, J.M., Jr. Nonocclusive multivessel intracoronary infusion of allogeneic cardiosphere-derived cells early after reperfusion prevents remote zone myocyte loss and improves global left ventricular function in swine with myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H345–H356. [Google Scholar] [CrossRef] [PubMed]
- Duran, J.M.; Taghavi, S.; Berretta, R.M.; Makarewich, C.A.; Sharp Iii, T.; Starosta, T.; Udeshi, F.; George, J.C.; Kubo, H.; Houser, S.R. A characterization and targeting of the infarct border zone in a swine model of myocardial infarction. Clin. Transl. Sci. 2012, 5, 416–421. [Google Scholar] [CrossRef]
- Sharp, T.E., III; Kubo, H.; Berretta, R.M.; Starosta, T.; Wallner, M.; Schena, G.J.; Hobby, A.R.; Yu, D.; Trappanese, D.M.; George, J.C.; et al. Protein Kinase C Inhibition With Ruboxistaurin Increases Contractility and Reduces Heart Size in a Swine Model of Heart Failure With Reduced Ejection Fraction. JACC Basic Transl. Sci. 2017, 2, 669–683. [Google Scholar] [CrossRef]
- Sharp, T.E., III; Schena, G.J.; Hobby, A.R.; Starosta, T.; Berretta, R.M.; Wallner, M.; Borghetti, G.; Gross, P.; Yu, D.; Johnson, J.; et al. Cortical Bone Stem Cell Therapy Preserves Cardiac Structure and Function After Myocardial Infarction. Circ. Res. 2017, 121, 1263–1278. [Google Scholar] [CrossRef]
- Prifti, E.; Di Lascio, G.; Harmelin, G.; Bani, D.; Briganti, V.; Veshti, A.; Bonacchi, M. Cellular cardiomyoplasty into infracted swine’s hearts by retrograde infusion through the venous coronary sinus: An experimental study. Cardiovasc. Revasc. Med. 2016, 17, 262–271. [Google Scholar] [CrossRef]
- Soonpaa, M.H.; Kim, K.K.; Pajak, L.; Franklin, M.; Field, L.J. Cardiomyocyte DNA synthesis and binucleation during murine development. Am. J. Physiol. 1996, 271, H2183–H2189. [Google Scholar] [CrossRef]
- Notari, M.; Ventura-Rubio, A.; Bedford-Guaus, S.J.; Jorba, I.; Mulero, L.; Navajas, D.; Marti, M.; Raya, A. The local microenvironment limits the regenerative potential of the mouse neonatal heart. Sci. Adv. 2018, 4, eaao5553. [Google Scholar] [CrossRef] [Green Version]
- Quaife-Ryan, G.A.; Sim, C.B.; Ziemann, M.; Kaspi, A.; Rafehi, H.; Ramialison, M.; El-Osta, A.; Hudson, J.E.; Porrello, E.R. Multicellular Transcriptional Analysis of Mammalian Heart Regeneration. Circulation 2017, 136, 1123–1139. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Xiang, F.L.; Braitsch, C.M.; Yutzey, K.E. Epicardium-derived fibroblasts in heart development and disease. J. Mol. Cell. Cardiol. 2016, 91, 23–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, B.; Honor, L.B.; He, H.; Ma, Q.; Oh, J.H.; Butterfield, C.; Lin, R.Z.; Melero-Martin, J.M.; Dolmatova, E.; Duffy, H.S.; et al. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J. Clin. Investig. 2011, 121, 1894–1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, A.I.; Porrello, E.R. Upsizing Neonatal Heart Regeneration. Circulation 2018, 138, 2817–2819. [Google Scholar] [CrossRef]
- Hashimoto, H.; Olson, E.N.; Bassel-Duby, R. Therapeutic approaches for cardiac regeneration and repair. Nat. Rev. Cardiol. 2018, 15, 585–600. [Google Scholar] [CrossRef]
- Lai, S.L.; Marin-Juez, R.; Stainier, D.Y.R. Immune responses in cardiac repair and regeneration: A comparative point of view. Cell. Mol. Life Sci. 2019, 76, 1365–1380. [Google Scholar] [CrossRef] [Green Version]


| RNA | Sequence 5′-3′ | |
|---|---|---|
| Forward | Reverse | |
| 18S | AATTCCGATAACGAACGAGACT | GGACATCTAAGGGCATCACAG |
| NPPA | TGAACCCAGCCCAGAGAGAT | CAGTCCACTCTGTGCTCCAA |
| NPPB | GTTGCTGCTAGGATGCCGTT | TACCTCCTGAGCACATTGCAGC |
| Parameter | Surgery | P30 Baseline | P30 + 2 h | P30 + 1 Week | P30 + 2 Weeks | P30 + 4 Weeks |
|---|---|---|---|---|---|---|
| Ventricular Septum Diastolic Thickness (cm) | Sham # | 0.43 ± 0.04 | 0.41 ± 0.09 | 0.51 ± 0.12 | 0.59 ± 0.07 | 0.59 ± 0.12 |
| IR | 0.48 ± 0.10 | 0.43 ± 0.06 | 0.47 ± 0.20 | 0.55 ± 0.07 | 0.58 ± 0.14 | |
| Ventricular Septum Systolic Thickness (cm) | Sham | 0.70 ± 0.15 | 0.61 ± 0.15 | 0.76 ± 0.10 | 0.93 ± 0.14 | 0.90 ± 0.27 |
| IR | 0.79 ± 0.14 | 0.53 ± 0.06 | 0.72 ± 0.24 | 0.70 ± 0.15 | 0.76 ± 0.26 | |
| LV Posterior Wall Diastolic Thickness (cm) | Sham | 0.44 ± 0.06 | 0.47 ± 0.03 | 0.46 ± 0.08 | 0.56 ± 0.09 | 0.56 ± 0.09 |
| IR | 0.42 ± 0.04 | 0.44 ± 0.06 | 0.47 ± 0.11 | 0.55 ± 0.14 | 0.55 ± 0.17 | |
| LV Posterior Wall Systolic Thickness (cm) | Sham | 0.62 ± 0.10 | 0.66 ± 0.07 | 0.74 ± 0.08 | 0.80 ± 0.10 | 0.88 ± 0.13 |
| IR | 0.67 ± 0.10 | 0.68 ± 0.11 | 0.75 ± 0.18 | 0.81 ± 0.21 | 0.88 ± 0.20 | |
| Stroke Volume (mL) | Sham | 8.27 ± 2.86 | 7.14 ± 1.70 | 9.21 ± 2.44 | 14.09 ± 5.70 | 22.35 ± 3.38 |
| IR | 8.16 ± 2.23 | 4.27 ± 1.38 ** | 8.23 ± 2.69 | 12.66 ± 3.14 | 18.24 ± 8.09 | |
| Heart Rate (bpm) | Sham | 145.28 ± 20.46 | 107.22 ± 10.05 | 177.50 ± 20.84 | 147.44 ± 27.85 | 131.11 ± 10.59 |
| IR | 149.72 ± 28.44 | 126.52 ± 34.15 | 183.71 ± 27.90 | 171.14 ± 13.37 * | 147.62 ± 18.11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agnew, E.J.; Velayutham, N.; Matos Ortiz, G.; Alfieri, C.M.; Hortells, L.; Moore, V.; Riggs, K.W.; Baker, R.S.; Gibson, A.M.; Ponny, S.R.; et al. Scar Formation with Decreased Cardiac Function Following Ischemia/Reperfusion Injury in 1 Month Old Swine. J. Cardiovasc. Dev. Dis. 2020, 7, 1. https://doi.org/10.3390/jcdd7010001
Agnew EJ, Velayutham N, Matos Ortiz G, Alfieri CM, Hortells L, Moore V, Riggs KW, Baker RS, Gibson AM, Ponny SR, et al. Scar Formation with Decreased Cardiac Function Following Ischemia/Reperfusion Injury in 1 Month Old Swine. Journal of Cardiovascular Development and Disease. 2020; 7(1):1. https://doi.org/10.3390/jcdd7010001
Chicago/Turabian StyleAgnew, Emma J, Nivedhitha Velayutham, Gabriela Matos Ortiz, Christina M Alfieri, Luis Hortells, Victoria Moore, Kyle W Riggs, R. Scott Baker, Aaron M Gibson, Sithara Raju Ponny, and et al. 2020. "Scar Formation with Decreased Cardiac Function Following Ischemia/Reperfusion Injury in 1 Month Old Swine" Journal of Cardiovascular Development and Disease 7, no. 1: 1. https://doi.org/10.3390/jcdd7010001