Plant-Derived Inhibitors of AHL-Mediated Quorum Sensing in Bacteria: Modes of Action
Abstract
:1. Introduction
2. Methodology for the Search and Study of Plant-Derived QS Inhibitors
3. Plant-Derived Molecules that Affect AHL-Mediated QS in Bacteria
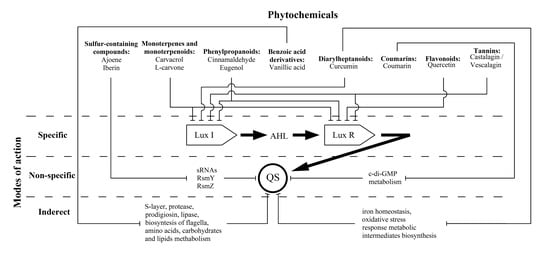
3.1. Sulphur-Containing Compounds
3.2. Monoterpenes and Monoterpenoids
3.3. Phenylpropanoids
3.4. Benzoic acid Derivatives
3.5. Diarylheptanoids
3.6. Coumarins
3.7. Flavonoids
3.8. Tannins
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fuqua, W.C.; Winans, S.C.; Greenberg, E.P. Quorum sensing in bacteria: The LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 1994, 176, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Schuster, M.; Joseph Sexton, D.; Diggle, S.P.; Peter Greenberg, E. Acyl-Homoserine Lactone Quorum Sensing: From Evolution to Application. Annu. Rev. Microbiol. 2013, 67, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Finch, R.G.; Pritchard, D.I.; Bycroft, B.W.; Williams, P.; Stewart, G.S. Quorum sensing: A novel target for anti-infective therapy. J. Antimicrob. Chemother. 1998, 42, 569–571. [Google Scholar] [CrossRef] [PubMed]
- Nadell, C.D.; Xavier, J.B.; Levin, S.A.; Foster, K.R. The evolution of quorum sensing in bacterial biofilms. PLoS Biol. 2008, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Kamaeva, A.; Vasilchenko, A.; Deryabin, D. Atomic Force Microscopy Reveals a Morphological Differentiation of Chromobacterium violaceum Cells Associated with Biofilm Development and Directed by N-Hexanoyl-L-Homoserine Lactone. PLoS ONE 2014, 9, 103741. [Google Scholar] [CrossRef]
- Whiteley, M.; Diggle, S.P.; Greenberg, E.P. Progress in and promise of bacterial quorum sensing research. Nature 2017, 551, 313–320. [Google Scholar] [CrossRef]
- Defoirdt, T. Quorum-Sensing Systems as Targets for Antivirulence Therapy. Trends Microbiol. 2018, 26, 313–328. [Google Scholar] [CrossRef]
- Rémy, B.; Mion, S.; Plener, L.; Elias, M.; Chabrière, E.; Daudé, D. Interference in Bacterial Quorum Sensing: A Biopharmaceutical Perspective. Front. Pharmacol. 2018, 9, 203. [Google Scholar] [CrossRef]
- Asfour, H.Z. Anti-Quorum Sensing Natural Compounds. J. Microsc. Ultrastruct. 2018, 6, 1–10. [Google Scholar] [CrossRef]
- Bouyahya, A.; Dakka, N.; Et-Touys, A.; Abrini, J.; Bakri, Y. Medicinal plant products targeting quorum sensing for combating bacterial infections. Asian Pac. J. Trop. Med. 2017, 10, 729–743. [Google Scholar] [CrossRef]
- Vattem, D.A.; Mihalik, K.; Crixell, S.H.; Mclean, R. Dietary phytochemicals as quorum sensing inhibitors. Fitoterapia 2007, 78, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Tolmacheva, A.A.; Rogozhin, E.A.; Deryabin, D.G. Antibacterial and quorum sensing regulatory activities of some traditional Eastern-European medicinal plants. Acta Pharm. 2014, 64, 173–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mcclean, K.H.; Winson, M.K.; Fish, L.; Taylor, A.; Chhabra, S.R.; Cámara, M.; Daykin, M.; Lamb, J.H.; Swift, S.; Bycroft, B.W.; et al. Quorum sensing and Chromobacterium violaceum: Exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 1997, 143, 3703–3711. [Google Scholar] [CrossRef] [PubMed]
- Adonizio, A.L.; Downum, K.; Bennett, B.C.; Mathee, K. Anti-quorum sensing activity of medicinal plants in southern Florida. J. Ethnopharmacol. 2006, 105, 427–435. [Google Scholar] [CrossRef]
- Deryabin, D.G.; Tolmacheva, A.A. Antibacterial and Anti-Quorum Sensing Molecular Composition Derived from Quercus cortex (Oak bark) Extract. Molecules 2015, 20, 17093–17108. [Google Scholar] [CrossRef]
- Winson, M.K.; Swift, S.; Fish, L.; Throup, J.P.; Jørgensen, F.; Chhabra, S.R.; Bycroft, B.W.; Williams, P.; Stewart, G.S.A.B. Construction and analysis of luxCDABE-based plasmid sensors for investigating N-acyl homoserine lactone-mediated quorum sensing. FEMS Microbiol. Lett. 1998, 163, 185–192. [Google Scholar] [CrossRef]
- Andersen, J.B.; Heydorn, A.; Hentzer, M.; Eberl, L.; Geisenberger, O.; Christensen, B.B.; Molin, S.; Givskov, M. gfp-based N-acyl homoserine-lactone sensor systems for detection of bacterial communication. Appl. Environ. Microbiol. 2001, 67, 575–585. [Google Scholar] [CrossRef]
- Stover, C.K.; Pham, X.Q.; Erwin, A.L.; Mizoguchi, S.D.; Warrener, P.; Hickey, M.J.; Brinkman, F.S.; Hufnagle, W.O.; Kowalik, D.J.; Lagrou, M.; et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 2000, 406, 959–964. [Google Scholar] [CrossRef]
- Koh, C.-L.; Sam, C.-K.; Yin, W.-F.; Tan, L.Y.; Krishnan, T.; Chong, Y.M.; Chan, K.-G. Plant-derived natural products as sources of anti-quorum sensing compounds. Sensors 2013, 13, 6217–6228. [Google Scholar] [CrossRef]
- Adonizio, A.; Dawlaty, J.; Ausubel, F.M.; Clardy, J.; Mathee, K. Ellagitannins from Conocarpus erectus exhibit anti-quorum sensing activity against Pseudomonas aeruginosa. Planta Med. 2008, 74, PB28. [Google Scholar] [CrossRef]
- Kong, C.; Eng, S.-A.; Lim, M.-P.; Nathan, S. Beyond Traditional Antimicrobials: A Caenorhabditis elegans Model for Discovery of Novel Anti-infectives. Front. Microbiol. 2016, 7, 1956. [Google Scholar] [CrossRef] [PubMed]
- van der Worp, H.B.; Howells, D.W.; Sena, E.S.; Porritt, M.J.; Rewell, S.; O’Collins, V.; Macleod, M.R. Can Animal Models of Disease Reliably Inform Human Studies? PLoS Med. 2010, 7, e1000245. [Google Scholar] [CrossRef] [PubMed]
- Duskaev, G.; Deryabin, D.; Karimov, I.; Kosyan, D.B.; Notova, S.V. Assessment of (In vitro) toxicity of quorum-sensing inhibitor molecules of Quercus cortex. J. Pharm. Sci. Res. 2018, 10, 91–95. [Google Scholar]
- Ta, C.A.K.; Arnason, J.T. Mini Review of Phytochemicals and Plant Taxa with Activity as Microbial Biofilm and Quorum Sensing Inhibitors. Molecules 2016, 21, 29. [Google Scholar] [CrossRef]
- Byeon, J.-Y.; Sim, J.; Ryu, E.-J.; Sim, J.; Lee, H.; Cho, K.-H.; Choi, B.-K.; Lee, J. In Silico Development of Quorum-Sensing Inhibitors. Bull. Korean Chem. Soc. 2017, 38, 728–734. [Google Scholar] [CrossRef]
- Aydemir, D.H.; Çifci, G.; Aviyente, V.; Boşgelmez-Tinaz, G. Quorum-sensing inhibitor potential of trans-anethole aganist Pseudomonas aeruginosa. J. Appl. Microbiol. 2018, 125, 731–739. [Google Scholar] [CrossRef]
- Swatton, J.E.; Davenport, P.W.; Maunders, E.A.; Griffin, J.L.; Lilley, K.S.; Welch, M. Impact of Azithromycin on the Quorum Sensing-Controlled Proteome of Pseudomonas aeruginosa. PLoS ONE 2016, 11, 0147698. [Google Scholar] [CrossRef]
- Ahmed, S.A.K.S.; Rudden, M.; Smyth, T.J.; Dooley, J.S.G.; Marchant, R.; Banat, I.M. Natural quorum sensing inhibitors effectively downregulate gene expression of Pseudomonas aeruginosa virulence factors. Appl. Microbiol. Biotechnol. 2019, 103, 3521–3535. [Google Scholar] [CrossRef]
- Asfahl, K.L.; Schuster, M. Additive Effects of Quorum Sensing Anti-Activators on Pseudomonas aeruginosa Virulence Traits and Transcriptome. Front. Microbiol. 2018, 8, 2654. [Google Scholar] [CrossRef]
- Wagner, V.E.; Bushnell, D.; Passador, L.; Brooks, A.I.; Iglewski, B.H. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: Effects of growth phase and environment. J. Bacteriol. 2003, 185, 2080–2095. [Google Scholar] [CrossRef]
- Doleman, J.F.; Grisar, K.; Liedekerke, L.V.; Saha, S.; Roe, M.; Tapp, H.S.; Mithen, R.F. The contribution of alliaceous and cruciferous vegetables to dietary sulphur intake. Food Chem. 2017, 234, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Cady, N.C.; McKean, K.A.; Behnke, J.; Kubec, R.; Mosier, A.P.; Kasper, S.H.; Burz, D.S.; Musah, R.A. Inhibition of Biofilm Formation, Quorum Sensing and Infection in Pseudomonas aeruginosa by Natural Products-Inspired Organosulfur Compounds. PLoS ONE 2012, 7, 38492. [Google Scholar] [CrossRef] [PubMed]
- Fong, J.; Yuan, M.; Jakobsen, T.H.; Mortensen, K.T.; Santos, M.M.S.D.; Chua, S.L.; Yang, L.; Tan, C.H.; Nielsen, T.E.; Givskov, M. Disulfide Bond-Containing Ajoene Analogues As Novel Quorum Sensing Inhibitors of Pseudomonas aeruginosa. J. Med. Chem. 2017, 60, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-R.; Ma, Y.-K.; Shi, Q.-S.; Xie, X.-B.; Sun, T.-L.; Peng, H.; Huang, X.-M. Diallyl disulfide from garlic oil inhibits Pseudomonas aeruginosa virulence factors by inactivating key quorum sensing genes. Appl. Microbiol. Biotechnol. 2018, 102, 7555–7564. [Google Scholar] [CrossRef] [PubMed]
- Ganin, H.; Rayo, J.; Amara, N.; Levy, N.; Krief, P.; Meijler, M.M. Sulforaphane and erucin, natural isothiocyanates from broccoli, inhibit bacterial quorum sensing. MedChemComm 2012, 4, 175–179. [Google Scholar] [CrossRef]
- Jakobsen, T.H.; Bragason, S.K.; Phipps, R.K.; Christensen, L.D.; van Gennip, M.; Alhede, M.; Skindersoe, M.; Larsen, T.O.; Høiby, N.; Bjarnsholt, T.; et al. Food as a Source for Quorum Sensing Inhibitors: Iberin from Horseradish Revealed as a Quorum Sensing Inhibitor of Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2012, 78, 2410–2421. [Google Scholar] [CrossRef]
- Tan, S.Y.-Y.; Liu, Y.; Chua, S.L.; Vejborg, R.M.; Jakobsen, T.H.; Chew, S.C.; Li, Y.; Nielsen, T.E.; Tolker-Nielsen, T.; Yang, L.; et al. Comparative Systems Biology Analysis To Study the Mode of Action of the Isothiocyanate Compound Iberin on Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 6648–6659. [Google Scholar] [CrossRef]
- Jakobsen, T.H.; Warming, A.N.; Vejborg, R.M.; Moscoso, J.A.; Stegger, M.; Lorenzen, F.; Rybtke, M.; Andersen, J.B.; Petersen, R.; Andersen, P.S.; et al. A broad range quorum sensing inhibitor working through sRNA inhibition. Sci. Rep. 2017, 7, 9857. [Google Scholar] [CrossRef]
- Myszka, K.; Czaczyk, K.; Majcher, M.; Schmidt, M.T.; Olkowicz, M.; Juzwa, W. Inhibition of quorum sensing-related biofilm of Pseudomonas fluorescens KM121 by Thymus vulgare essential oil and its major bioactive compounds. Int. Biodeterior. Biodegrad. 2016, 114, 252–259. [Google Scholar] [CrossRef]
- Burt, S.A.; Ojo-Fakunle, V.T.A.; Woertman, J.; Veldhuizen, E.J.A. The Natural Antimicrobial Carvacrol Inhibits Quorum Sensing in Chromobacterium violaceum and Reduces Bacterial Biofilm Formation at Sub-Lethal Concentrations. PLoS ONE 2014, 9, 93414. [Google Scholar] [CrossRef]
- Tapia-Rodriguez, M.R.; Hernandez-Mendoza, A.; Gonzalez-Aguilar, G.A.; Martinez-Tellez, M.A.; Martins, C.M.; Ayala-Zavala, J.F. Carvacrol as potential quorum sensing inhibitor of Pseudomonas aeruginosa and biofilm production on stainless steel surfaces. Food Control 2017, 75, 255–261. [Google Scholar] [CrossRef]
- Joshi, J.R.; Khazanov, N.; Senderowitz, H.; Burdman, S.; Lipsky, A.; Yedidia, I. Plant phenolic volatiles inhibit quorum sensing in pectobacteria and reduce their virulence by potential binding to ExpI and ExpR proteins. Sci. Rep. 2016, 6, 38126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Mei, Y.; He, B.; Sun, X.; Li, J. Reducing Quorum Sensing-Mediated Virulence Factor Expression and Biofilm Formation in Hafnia alvei by Using the Potential Quorum Sensing Inhibitor l-Carvone. Front. Microbiol. 2019, 9, 3324. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-Y.; Krishnan, T.; Wang, H.; Chen, Y.; Yin, W.-F.; Chong, Y.-M.; Tan, L.Y.; Chong, T.M.; Chan, K.-G. Non-antibiotic quorum sensing inhibitors acting against N-acyl homoserine lactone synthase as druggable target. Sci. Rep. 2015, 4, 7245. [Google Scholar] [CrossRef]
- Brackman, G.; Defoirdt, T.; Miyamoto, C.; Bossier, P.; Van Calenbergh, S.; Nelis, H.; Coenye, T. Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence in Vibrio spp. by decreasing the DNA-binding activity of the quorum sensing response regulator LuxR. BMC Microbiol. 2008, 8, 149. [Google Scholar] [CrossRef]
- Zhou, L.; Zheng, H.; Tang, Y.; Yu, W.; Gong, Q. Eugenol inhibits quorum sensing at sub-inhibitory concentrations. Biotechnol. Lett. 2013, 35, 631–637. [Google Scholar] [CrossRef]
- Rathinam, P.; Vijay Kumar, H.S.; Viswanathan, P. Eugenol exhibits anti-virulence properties by competitively binding to quorum sensing receptors. Biofouling 2017, 33, 624–639. [Google Scholar] [CrossRef]
- Lou, Z.; Letsididi, K.S.; Yu, F.; Pei, Z.; Wang, H.; Letsididi, R. Inhibitive Effect of Eugenol and Its Nanoemulsion on Quorum Sensing–Mediated Virulence Factors and Biofilm Formation by Pseudomonas aeruginosa. J. Food Prot. 2019, 82, 379–389. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, W.; Odah, K.A.; Kong, L.; Ma, H. Transcriptome Analysis Reveals AI-2 Relevant Genes of Multi-Drug Resistant Klebsiella pneumoniae in Response to Eugenol at Sub-MIC. Front. Microbiol. 2019, 10, 1159. [Google Scholar] [CrossRef]
- Katebian, L.; Gomez, E.; Skillman, L.; Li, D.; Ho, G.; Jiang, S.C. Inhibiting quorum sensing pathways to mitigate seawater desalination RO membrane biofouling. Desalination 2016, 393, 135–143. [Google Scholar] [CrossRef]
- Hossain, Md.A.; Lee, S.-J.; Park, N.-H.; Mechesso, A.F.; Birhanu, B.T.; Kang, J.; Reza, Md.A.; Suh, J.-W.; Park, S.-C. Impact of phenolic compounds in the acyl homoserine lactone-mediated quorum sensing regulatory pathways. Sci. Rep. 2017, 7, 10618. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.R.T.; Lou, Z.; Yu, F.; Wang, P.; Wang, H. Anti-quorum sensing and anti-biofilm activity of Amomum tsaoko (Amommum tsao-ko Crevost et Lemarie) on foodborne pathogens. Saudi J. Biol. Sci. 2017, 24, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Sethupathy, S.; Prasath, K.G.; Ananthi, S.; Mahalingam, S.; Balan, S.Y.; Pandian, S.K. Proteomic analysis reveals modulation of iron homeostasis and oxidative stress response in Pseudomonas aeruginosa PAO1 by curcumin inhibiting quorum sensing regulated virulence factors and biofilm production. J. Proteom. 2016, 145, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on Natural Coumarin Lead Compounds for Their Pharmacological Activity. BioMed Res. Int. 2013, 2013, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Molina, E.; Yordi, E.G. Coumarins—An Important Class of Phytochemicals. In Phytochemicals-Isolation, Characterisation and Role in Human Health; Rao, A.V., Rao, L.G., Eds.; InTech: Rijeka, Croatia, 2015; ISBN 978-953-51-2170-1. [Google Scholar]
- D’Almeida, R.E.; Molina, R.D.I.; Viola, C.M.; Luciardi, M.C.; Peñalver, C.N.; Bardón, A.; Arena, M.E. Comparison of seven structurally related coumarins on the inhibition of Quorum sensing of Pseudomonas aeruginosa and Chromobacterium violaceum. Bioorganic Chem. 2017, 73, 37–42. [Google Scholar] [CrossRef]
- Zeng, Z.; Qian, L.; Cao, L.; Tan, H.; Huang, Y.; Xue, X.; Shen, Y.; Zhou, S. Virtual screening for novel quorum sensing inhibitors to eradicate biofilm formation of Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2008, 79, 119–126. [Google Scholar] [CrossRef]
- Chua, S.L.; Liu, Y.; Li, Y.; Ting, H.J.; Kohli, G.S.; Cai, Z.; Suwanchaikasem, P.; Goh, K.K.K.; Ng, S.P.; Tolker-Nielsen, T.; et al. Reduced Intracellular c-di-GMP Content Increases Expression of Quorum Sensing-Regulated Genes in Pseudomonas aeruginosa. Front. Cell. Infect. Microbiol. 2017, 7, 451. [Google Scholar] [CrossRef]
- Ueda, A.; Wood, T.K. Connecting Quorum Sensing, c-di-GMP, Pel Polysaccharide, and Biofilm Formation in Pseudomonas aeruginosa through Tyrosine Phosphatase TpbA (PA3885). PLoS Pathog. 2009, 5, 1000483. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, 47. [Google Scholar] [CrossRef]
- Ververidis, F.; Trantas, E.; Douglas, C.; Vollmer, G.; Kretzschmar, G.; Panopoulos, N. Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I: Chemical diversity, impacts on plant biology and human health. Biotechnol. J. 2007, 2, 1214–1234. [Google Scholar] [CrossRef]
- Manner, S.; Fallarero, A. Screening of Natural Product Derivatives Identifies Two Structurally Related Flavonoids as Potent Quorum Sensing Inhibitors against Gram-Negative Bacteria. Int. J. Mol. Sci. 2018, 19, 1346. [Google Scholar] [CrossRef] [PubMed]
- Paczkowski, J.E.; Mukherjee, S.; McCready, A.R.; Cong, J.-P.; Aquino, C.J.; Kim, H.; Henke, B.R.; Smith, C.D.; Bassler, B.L. Flavonoids Suppress Pseudomonas aeruginosa Virulence through Allosteric Inhibition of Quorum-sensing Receptors. J. Biol. Chem. 2017, 292, 4064–4076. [Google Scholar] [CrossRef] [PubMed]
- Bele, A.A.; Jadhav, V.M.; Kadam, V.J. Potential of Tannnins: A Review. Asian J. Plant Sci. 2010, 9, 209–214. [Google Scholar]
- Schofield, P.; Mbugua, D.M.; Pell, A.N. Analysis of condensed tannins: A review. Anim. Feed Sci. Technol. 2001, 91, 21–40. [Google Scholar] [CrossRef]
- Li, G.; Yan, C.; Xu, Y.; Feng, Y.; Wu, Q.; Lv, X.; Yang, B.; Wang, X.; Xia, X. Punicalagin inhibits Salmonella virulence factors and has anti-quorum-sensing potential. Appl. Environ. Microbiol. 2014, 80, 6204–6211. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, L.; Gao, J.; Liu, X.; Feng, Y.; Wu, Q.; Baloch, A.B.; Cui, L.; Xia, X. Tannin-Rich Fraction from Pomegranate Rind Inhibits Quorum Sensing in Chromobacterium violaceum and Biofilm Formation in Escherichia coli. Foodborne Pathog. Dis. 2016, 13, 28–35. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Truchado, P.; Larrosa, M.; Espín, J.C.; Tomás-Barberán, F.A.; Allende, A.; García-Conesa, M.T. Urolithins, ellagitannin metabolites produced by colon microbiota, inhibit Quorum Sensing in Yersinia enterocolitica: Phenotypic response and associated molecular changes. Food Chem. 2012, 132, 1465–1474. [Google Scholar] [CrossRef]
- Maisuria, V.B.; Los Santos, Y.L.; Tufenkji, N.; Déziel, E. Cranberry-derived proanthocyanidins impair virulence and inhibit quorum sensing of Pseudomonas aeruginosa. Sci. Rep. 2016, 6, 30169. [Google Scholar] [CrossRef]
- Boutanaev, A.M.; Moses, T.; Zi, J.; Nelson, D.R.; Mugford, S.T.; Peters, R.J.; Osbourn, A. Investigation of terpene diversification across multiple sequenced plant genomes. Proc. Natl. Acad. Sci. USA 2015, 112, E81–E88. [Google Scholar] [CrossRef]
- Luís, Â.; Duarte, A.; Pereira, L.; Domingues, F. Chemical Profiling and Evaluation of Antioxidant and Anti-Microbial Properties of Selected Commercial Essential Oils: A Comparative Study. Medicines 2017, 4, 36. [Google Scholar] [CrossRef]
- Luís, Â.; Duarte, A.; Gominho, J.; Domingues, F.; Duarte, A.P. Chemical composition, antioxidant, antibacterial and anti-quorum sensing activities of Eucalyptus globulus and Eucalyptus radiata essential oils. Ind. Crops Prod. 2016, 79, 274–282. [Google Scholar] [CrossRef]
- Luciardi, M.C.; Blázquez, M.A.; Cartagena, E.; Bardón, A.; Arena, M.E. Mandarin essential oils inhibit quorum sensing and virulence factors of Pseudomonas aeruginosa. LWT Food Sci. Technol. 2016, 68, 373–380. [Google Scholar] [CrossRef]
- Snoussi, M.; Noumi, E.; Punchappady-Devasya, R.; Trabelsi, N.; Kanekar, S.; Nazzaro, F.; Fratianni, F.; Flamini, G.; De Feo, V.; Al-Sieni, A. Antioxidant properties and anti-quorum sensing potential of Carum copticum essential oil and phenolics against Chromobacterium violaceum. J. Food Sci. Technol. 2018, 55, 2824–2832. [Google Scholar] [CrossRef]
- Hahlbrock, K.; Scheel, D. Physiology and Molecular Biology of Phenylpropanoid Metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 347–369. [Google Scholar] [CrossRef]
- del Olmo, A.; Calzada, J.; Nuñez, M. Benzoic acid and its derivatives as naturally occurring compounds in foods and as additives: Uses, exposure, and controversy. Crit. Rev. Food Sci. Nutr. 2017, 57, 3084–3103. [Google Scholar] [CrossRef]
- Ponnusamy, K.; Paul, D.; Kweon, J.H. Inhibition of Quorum Sensing Mechanism and Aeromonas hydrophila Biofilm Formation by Vanillin. Environ. Eng. Sci. 2009, 26, 1359–1363. [Google Scholar] [CrossRef]
- Sethupathy, S.; Ananthi, S.; Selvaraj, A.; Shanmuganathan, B.; Vigneshwari, L.; Balamurugan, K.; Mahalingam, S.; Pandian, S.K. Vanillic acid from Actinidia deliciosa impedes virulence in Serratia marcescens by affecting S-layer, flagellin and fatty acid biosynthesis proteins. Sci. Rep. 2017, 7, 16328. [Google Scholar] [CrossRef]
- Brahmachari, G. Chemistry and Pharmacology of Naturally Occurring Bioactive Compounds. In Chemistry and Pharmacology of Naturally Occurring Bioactive Compounds; Brahmachari, G., Ed.; Taylor & Francis Group: Abingdon, UK, 2013; pp. 1–8. ISBN 978-1-4398-9167-4. [Google Scholar]
- Ilic-Tomic, T.; Sokovic, M.; Vojnovic, S.; Ciric, A.; Veljic, M.; Nikodinovic-Runic, J.; Novakovic, M. Diarylheptanoids from Alnus viridis ssp. viridis and Alnus glutinosa: Modulation of Quorum Sensing Activity in Pseudomonas aeruginosa. Planta Med. 2016, 83, 117–125. [Google Scholar] [CrossRef]
- Ding, T.; Li, T.; Wang, Z.; Li, J. Curcumin liposomes interfere with quorum sensing system of Aeromonas sobria and in silico analysis. Sci. Rep. 2017, 7, 8612. [Google Scholar] [CrossRef]
- Bahari, S.; Zeighami, H.; Mirshahabi, H.; Roudashti, S.; Haghi, F. Inhibition of Pseudomonas aeruginosa quorum sensing by subinhibitory concentrations of curcumin with gentamicin and azithromycin. J. Glob. Antimicrob. Resist. 2017, 10, 21–28. [Google Scholar] [CrossRef]
- Girennavar, B.; Cepeda, M.L.; Soni, K.A.; Vikram, A.; Jesudhasan, P.; Jayaprakasha, G.K.; Pillai, S.D.; Patil, B.S. Grapefruit juice and its furocoumarins inhibits autoinducer signaling and biofilm formation in bacteria. Int. J. Food Microbiol. 2008, 125, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Barranquero, J.A.; Reen, F.J.; McCarthy, R.R.; O’Gara, F. Deciphering the role of coumarin as a novel quorum sensing inhibitor suppressing virulence phenotypes in bacterial pathogens. Appl. Microbiol. Biotechnol. 2015, 99, 3303–3316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sass, A.; Acker, H.V.; Wille, J.; Verhasselt, B.; Nieuwerburgh, F.V.; Kaever, V.; Crabbé, A.; Coenye, T. Coumarin Reduces Virulence and Biofilm Formation in Pseudomonas aeruginosa by Affecting Quorum Sensing, Type III Secretion and C-di-GMP Levels. Front. Microbiol. 2018, 9, 1952. [Google Scholar] [CrossRef] [PubMed]
- Vasavi, H.S.; Arun, A.B.; Rekha, P.D. Anti-quorum sensing activity of flavonoid-rich fraction from Centella asiatica L. against Pseudomonas aeruginosa PAO1. J. Microbiol. Immunol. Infect. 2016, 49, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Pan, Y.; Li, X.; Jie, J.; Zeng, M. Chemical composition, antimicrobial and anti-quorum sensing activities of pummelo peel flavonoid extract. Ind. Crops Prod. 2017, 109, 862–868. [Google Scholar] [CrossRef]
- Vasavi, H.S.; Arun, A.B.; Rekha, P.-D. Anti-quorum sensing activity of Psidium guajava L. flavonoids against Chromobacterium violaceum and Pseudomonas aeruginosa PAO1: Quorum sensing inhibition by P. guajava. Microbiol. Immunol. 2014, 58, 286–293. [Google Scholar] [CrossRef]
- Skogman, M.; Kanerva, S.; Manner, S.; Vuorela, P.; Fallarero, A. Flavones as Quorum Sensing Inhibitors Identified by a Newly Optimized Screening Platform Using Chromobacterium violaceum as Reporter Bacteria. Molecules 2016, 21, 1211. [Google Scholar] [CrossRef]
- Gopu, V.; Meena, C.K.; Shetty, P.H. Quercetin Influences Quorum Sensing in Food Borne Bacteria: In-Vitro and In-Silico Evidence. PLoS ONE 2015, 10, 0134684. [Google Scholar] [CrossRef]
- Quecan, B.X.V.; Santos, J.T.C.; Rivera, M.L.C.; Hassimotto, N.M.A.; Almeida, F.A.; Pinto, U.M. Effect of Quercetin Rich Onion Extracts on Bacterial Quorum Sensing. Front. Microbiol. 2019, 10, 867. [Google Scholar] [CrossRef]
- Grabski, H.; Hunanyan, L.; Tiratsuyan, S.; Vardapetyan, H. Interaction of Quercetin with Transcriptional Regulator LasR of Pseudomonas Aeruginosa: Mechanistic Insights of the Inhibition of Virulence through Quorum Sensing; Bioinformatics; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 2017. [Google Scholar]
- Brillouet, J.-M.; Romieu, C.; Schoefs, B.; Solymosi, K.; Cheynier, V.; Fulcrand, H.; Verdeil, J.-L.; Conéjéro, G. The tannosome is an organelle forming condensed tannins in the chlorophyllous organs of Tracheophyta. Ann. Bot. 2013, 112, 1003–1014. [Google Scholar] [CrossRef] [Green Version]
- Adonizio, A.; Leal, S.M.; Ausubel, F.M.; Mathee, K. Attenuation of Pseudomonas aeruginosa virulence by medicinal plants in a Caenorhabditis elegans model system. J. Med. Microbiol. 2008, 57, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Adonizio, A.; Kong, K.-F.; Mathee, K. Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa by South Florida plant extracts. Antimicrob. Agents Chemother. 2008, 52, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Bhathena, Z. Broad Spectrum Anti-Quorum Sensing Activity of Tannin-Rich Crude Extracts of Indian Medicinal Plants. Scientifica 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarabhai, S.; Sharma, P.; Capalash, N. Ellagic Acid Derivatives from Terminalia chebula Retz. Downregulate the Expression of Quorum Sensing Genes to Attenuate Pseudomonas aeruginosa PAO1 Virulence. PLoS ONE 2013, 8, 53441. [Google Scholar] [CrossRef]
- Ulrey, R.K.; Barksdale, S.M.; Zhou, W.; van Hoek, M.L. Cranberry proanthocyanidins have anti-biofilm properties against Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2014, 14, 499. [Google Scholar] [CrossRef]
- Kalia, V.C.; Patel, S.K.S.; Kang, Y.C.; Lee, J.-K. Quorum sensing inhibitors as antipathogens: Biotechnological applications. Biotechnol. Adv. 2019, 37, 68–90. [Google Scholar] [CrossRef]
- McKenney, E.S.; Kendall, M.M. Microbiota and pathogen ‘pas de deux’: Setting up and breaking down barriers to intestinal infection. Pathog. Dis. 2016, 74, 051. [Google Scholar] [CrossRef]
- Njeru, S.; Matasyohb, J.; Mwanikic, C.; Maina, M.; Kobiad, G. A Review of some Phytochemicals commonly found in Medicinal Plants. Int. J. Med. Plant 2013, 105, 135–140. [Google Scholar]
- Ansari, F.; Ahmad, I. Quorum Sensing in Phytopathogenic Bacteria and Its Relevance in Plant Health. In Biotechnological Applications of Quorum Sensing Inhibitors; Springer: Berlin, Germany, 2018; pp. 351–370. ISBN 978-981-10-9025-7. [Google Scholar]
- Joshi, C.; Patel, P.; Kothari, V. Anti-infective potential of hydroalcoholic extract of Punica granatum peel against gram-negative bacterial pathogens. F1000Research 2019, 8, 70. [Google Scholar] [CrossRef]

| IUPAC (Trivial) Names of the Compounds Structural Formulas | Plants Source | Anti-QS Effects | Modes of Action | References |
|---|---|---|---|---|
| 1. Sulfur-Containing Compounds | ||||
(E)-1-(Prop-2-enyldisulfanyl)-3-prop-2-enylsulfinylprop-1-ene (Ajoene) | Allium sativum L. (garliс) | Inhibition of QS-regulated virulence factors (elastase, rhamnolipid, pyocyanin) and biofilm formation in P. aeruginosa; inhibition of P. aeruginosa infection in a murine model | Lowering expression of GacA-dependent small regulatory RNAs (RsmY and RsmZ) | [33,37,38] |
1-isothiocyanato-3-methylsulfinylpropane (Iberin) | Armoracia rusticana G.Gaertn., B.Mey., Scherb. (horseradish), Brassicaceae species | Blocking of QS-regulated features, matrix rhamnolypid biosynthesis and biofilm formation in P. aeruginosa | Inhibition of GacA-dependent small regulatory RNAs (RsmY and RsmZ); lowering the abundance of the LadS protein (GacS activator) | [34,36,37,38] |
| 2. Monoterpenes and Monoterpenoids | ||||
2-methyl-5-propan-2-ylphenol (Carvacrol) | Origanum vulgare L. (oregano), Thymus vulgaris L. and other Lamiaceae species | Inhibition of QS-regulated violacein biosynthesis and chitinase activity in C.violaceum; inhibition of QS-regulated pyocyanin production and biofilm formation in P. aeruginosa; lowering of AHL production and QS-controlled genes expression in Pectobacterium spp. | Binding to LuxI-type synthase (ExpI) and LuxR-type transcriptional regulator (ExpR) | [39,40,41,42] |
2-methyl-5-prop-1-en-2-ylcyclohex-2-en-1-one (l-carvone) | Carum carvi L. (caraway), Mentha spicata L. (spearmint) | Inhibition of QS-regulated swinging/swarming motility and reduction of AHL production in H.alvei | Binding to LuxI-type synthase (HalI) and LuxR-type transcriptional regulator (HalR) | [43] |
| 3. Phenylpropanoids | ||||
(2E)-3-Phenylprop-2-enal (Cinnamaldehyde) | Cinnamomum species, Hyacinthus orientalis L. (chinese cassia), Pogostemon cablin Benth. (patchouli) | Inhibition of QS-regulated virulence factors (protease, elastase, pyocyanin) and biofilm formation in P. aeruginosa; inhibition of QS-controlled extracellular protease, /swarming and biofilm formation in P. fluorescens; lowering of AHL production; pleiotropic effects on AI-2 mediated QS | Binding to LuxR-type receptor proteins and/or interaction with LuxI-type synthase (LasI) | [28,44,45,46] |
2-Methoxy-4-(prop-2-en-1-yl)phenol (Eugenol) | Syzygium aromaticum L., Myrtales species | Inhibitory effects on QS-biosensors; inhibition of QS-regulated elastase, protease, pyocyanin and pyoverdine biosynthesis in P. aeruginosa; inhibition of biofilm formation, matrix polysaccharides and rhamnolipid production;lowering of QS signaling molecules and QS-controlled genes expression in Pectobacterium spp.;pleiotropic effects on AI-2 mediated QS | Binding to LuxR-type proteins (LasR and ExpR); down-regulation of AHL synthases genes (lasI and rhlI); interaction with LuxI-type synthase (ExpI) | [47,48,49] |
| 4. Benzoic Acid Derivatives | ||||
4-hydroxy-3-methoxybenzoic acid (Vanillic acid)
 | Actinidia deliciosa (A.Chev.) C.F.Liang, A.R.Ferguson (kiwifruit) | Inhibition of QS-regulated virulence and biofilm formation in S. marcescens; increased survival of C. elegans upon S. marcescens infection | Changing the proteins content involved in S-layers, protease, prodigiosin and lipase production, targeting flagella, amino acids, carbohydrates and fatty acids biosynthesis | [50,51] |
| 5. Diarylheptanoids | ||||
(1E,6E)-1,7-Bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione (Curcumin) | Curcuma longa L. (turmeric) | Inhibition of QS-regulated violacein biosynthesis in C. violaceum and virulence factors production in Vibrio sp. and S. marcescens; inhibition of pyocyanin biosynthesis, elastase/protease activity, and biofilm formation in P. aeruginosa; reduction P. aeruginosa pathogenicity in C. elegans infection models; lowering AHL production in P. aeruginosa and A. sobria | Interaction with LuxI-type synthases; down-regulation of LuxI-type and LuxR-type proteins genes (lasI, lasR, rhlI and rhlR); targeting iron homeostasis, oxidative stress response and biosynthesis of metabolic intermediates involved in virulence factors production | [52,53,54,55] |
| 6. Coumarins | ||||
2H-chromen-2-one (Coumarin) | Dipteryx odorata (Aubl.) Willd. (tonka bean), Anthoxanthum odoratum L., Galium odoratum L., Hierochloe odorata L., Cinnamomum verum J.Presl and Melilotus officinalis L. | Inhibitory effects on QS-biosensors; inhibition of violacein biosynthesis in C. violaceum; inhibition of QS-regulated, phenazines production, swarming motility and biofilm formation in P. aeruginosa | Reduction of c-di-GMP metabolism | [56,57,58,59,60,61] |
| 7. Flavonoids | ||||
2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one (Quercetin) | Allium cepa L. (red onion), Brassica oleracea L. (leaf cabbage or “kale”), many others fruits, vegetables, leaves, and grains | Inhibition of QS-regulated bioluminescence in V. harveyi; Inhibition of violacein production in C. violaceum; Inhibition of pyocyanin production, proteolytic, elastolytic activities, swarming motility and biofilm formation in P. aeruginosa | Binding to LuxR-type receptor proteins (LasR, RhlR and CviR) | [62,63,64,65] |
| 8. Tannins | ||||
(46R)-7,8,9,12,13,14,25,26,27,30, 31,32,35,36,37,46-hexadecahydroxy-3,18,21,41,43-pentaoxanonacyclo[27.13.3.138,42.02,20.05,10.011,16.023,28.033,45.034,39]hexatetraconta-5,7,9,11,13,15,23,25,27,29(45),30,32,34(39),35,37-pentadecaene-4,17,22,40,44-pentone(Castalagin: R1=OH, R2=H/Vescalagin: R1=H, R2=OH) | Conocarpus erectus L, Anogeissus leiocarpa (DC.) Guill. & Perr., Terminalia avicennioides Guill., Perr. Fl. Seneg. Tent. | Inhibition of QS-controlled genes and QS-regulated virulence factors in P. aeruginosa; lowering of AHL production; increased survival of C. elegans upon P. aeruginosa infection | Interaction with QS-related proteins (including LuxI-type syntheses) via precipitation mechanism; bioactivity may be explained by secondary hydrolyzed and metabolized products | [45,66,67,68,69] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deryabin, D.; Galadzhieva, A.; Kosyan, D.; Duskaev, G. Plant-Derived Inhibitors of AHL-Mediated Quorum Sensing in Bacteria: Modes of Action. Int. J. Mol. Sci. 2019, 20, 5588. https://doi.org/10.3390/ijms20225588
Deryabin D, Galadzhieva A, Kosyan D, Duskaev G. Plant-Derived Inhibitors of AHL-Mediated Quorum Sensing in Bacteria: Modes of Action. International Journal of Molecular Sciences. 2019; 20(22):5588. https://doi.org/10.3390/ijms20225588
Chicago/Turabian StyleDeryabin, Dmitry, Anna Galadzhieva, Dianna Kosyan, and Galimjan Duskaev. 2019. "Plant-Derived Inhibitors of AHL-Mediated Quorum Sensing in Bacteria: Modes of Action" International Journal of Molecular Sciences 20, no. 22: 5588. https://doi.org/10.3390/ijms20225588