Repeated Vaginal Exposures to the Common Cosmetic and Household Preservative Methylisothiazolinone Induce Persistent, Mast Cell-Dependent Genital Pain in ND4 Mice
Abstract
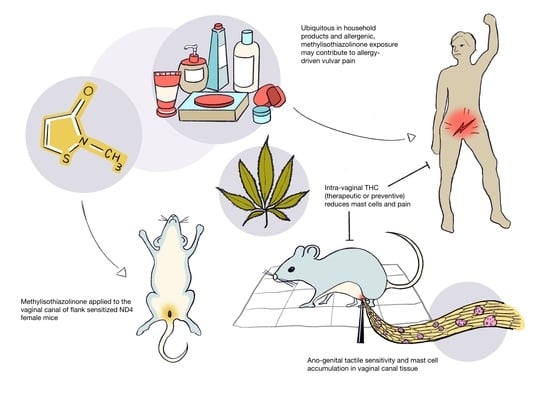
:1. Introduction
2. Results
2.1. Meta-Analysis of Patch-Testing Studies Reveals Widespread Sensitization to MI in Populations Tested in Europe and North America
2.2. Repeated Exposures to MI in the Vaginal Canal Induce Painful Ano-Genital Responses to Touch and Aberrant Mast Cell Accumulation in the Affected Tissues
2.3. Repeated Exposures to MI in the Vaginal Canal Induce Inflammatory Changes in the Vaginal Mucosa and in the Spinal Cord of Mice
2.4. Therapeutic Administration of THC in the Vaginal Canal after 10 MI Exposures Reduces Both Mast Cell Numbers as Well as Painful Sensitivity to Touch
2.5. Preventive Administration of THC in the Vaginal Canal before and during 10 MI Exposures Reduces Both Mast Cell Numbers as Well as Painful Sensitivity to Touch
3. Discussion
4. Materials and Methods
4.1. Meta-Analysis of MI Sensitization Studies
4.2. Animal Usage
4.3. MI Sensitization and Challenge
4.4. Ear Edema Measurements
4.5. THC Treatments
4.6. Tissue Collection and Storage
4.7. Tactile Sensitivity
4.8. Immunofluorescent Staining and Microscopy
4.9. RNA Isolation and Quantification of Gene Expression
4.10. Protein Quantification
4.11. Quantification of Eosinophil Activity
4.12. Vaginal 16S rRNA-Based Microbiome Profiling and Analysis
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reed, B.D.; Harlow, S.D.; Sen, A.; Legocki, L.J.; Edwards, R.M.; Arato, N.; Haefner, H.K. Prevalence and demographic characteristics of vulvodynia in a population-based sample. Am. J. Obstet. Gynecol. 2012, 206, 170. [Google Scholar] [CrossRef] [PubMed]
- Harlow, B.L.; Kunitz, C.G.; Nguyen, R.H.N.; Rydell, S.A.; Turner, R.M.; MacLehose, R.F. Prevalence of symptoms consistent with a diagnosis of vulvodynia: Population-based estimates from 2 geographic regions. Am. J. Obstet. Gynecol. 2014, 210, 40. [Google Scholar] [CrossRef] [PubMed]
- Harlow, B.L.; He, W.; Nguyen, R.H.N. Allergic reactions and risk of vulvodynia. Ann. Epidemiol. 2009, 19, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Weström, L.V.; Willén, R. Vestibular nerve fiber proliferation in vulvar vestibulitis syndrome. Obstet. Gynecol. 1998, 91, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, J.; Goldschmid, N.; Sabo, E. Hyperinnervation and mast cell activation may be used as histopathologic diagnostic criteria for vulvar vestibulitis. Gynecol. Obstet. Invest. 2004, 58, 171–178. [Google Scholar] [CrossRef]
- Bornstein, J.; Cohen, Y.; Zarfati, D.; Sela, S.; Ophir, E. Involvement of heparanase in the pathogenesis of localized vulvodynia. Int. J. Gynecol. Pathol. 2008, 27, 136–141. [Google Scholar] [CrossRef]
- Martinov, T.; Glenn-Finer, R.; Burley, S.; Tonc, E.; Balsells, E.; Ashbaugh, A.; Swanson, L.; Daughters, R.S.; Chatterjea, D. Contact hypersensitivity to oxazolone provokes vulvar mechanical hyperalgesia in mice. PLoS ONE 2013, 8, e78673. [Google Scholar] [CrossRef]
- Landry, J.; Martinov, T.; Mengistu, H.; Dhanwada, J.; Benck, C.J.; Kline, J.; Boo, B.; Swanson, L.; Tonc, E.; Daughters, R.; et al. Repeated hapten exposure induces persistent tactile sensitivity in mice modeling localized provoked vulvodynia. PLoS ONE 2017, 12, e0169672. [Google Scholar] [CrossRef]
- Boo, B.; Kamath, R.; Arriaga-Gomez, E.; Landry, J.; Emanuel, E.; Joo, S.; Saldías Montivero, M.; Martinov, T.; Fife, B.T.; Chatterjea, D. Tetrahydrocannabinol Reduces Hapten-Driven Mast Cell Accumulation and Persistent Tactile Sensitivity in Mouse Model of Allergen-Provoked Localized Vulvodynia. Int. J. Mol. Sci. 2019, 20, 2163. [Google Scholar] [CrossRef]
- Schwensen, J.F.; Lundov, M.D.; Bossi, R.; Banerjee, P.; Giménez-Arnau, E.; Lepoittevin, J.P.; Lidén, C.; Uter, W.; Yazar, K.; White, I.R.; et al. Methylisothiazolinone and benzisothiazolinone are widely used in paint: A multicentre study of paints from five European countries. Contact Dermatitis 2015, 72, 127–138. [Google Scholar] [CrossRef]
- Yazar, K.; Lundov, M.D.; Faurschou, A.; Matura, M.; Boman, A.; Johansen, J.D.; Lidén, C. Methylisothiazolinone in rinse-off products causes allergic contact dermatitis: A repeated open-application study. Br. J. Dermatol. 2015, 173, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Lundov, M.D.; Opstrup, M.S.; Johansen, J.D. Methylisothiazolinone contact allergy--growing epidemic. Contact Derm. 2013, 69, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Popple, A.; Williams, J.; Maxwell, G.; Gellatly, N.; Dearman, R.J.; Kimber, I. T lymphocyte dynamics in methylisothiazolinone-allergic patients. Contact Derm. 2016, 75, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lundov, M.D.; Krongaard, T.; Menné, T.L.; Johansen, J.D. Methylisothiazolinone contact allergy: A review. Br. J. Dermatol. 2011, 165, 1178–1182. [Google Scholar] [CrossRef] [PubMed]
- Vij, A.; Sood, A.; Piliang, M.; Mesinkovska, N.A. Infection or allergy? The multifaceted nature of vulvar dermatoses. Int. J. Women’s Dermatol. 2015, 1, 170–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marfatia, Y.S.; Patel, D.; Menon, D.; Naswa, S. Genital contact allergy: A diagnosis missed. Indian J. Sex. Transm. Dis. AIDS 2016, 37, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Reed, B.D.; McKee, K.S.; Plegue, M.A.; Park, S.K.; Haefner, H.K.; Harlow, S.D. Environmental Exposure History and Vulvodynia Risk: A Population-Based Study. J. Women’s Health 2019, 28, 69–76. [Google Scholar] [CrossRef]
- Hannuksela, M. Rapid increase in contact allergy to Kathon CG in Finland. Contact Derm. 1986, 15, 211–214. [Google Scholar] [CrossRef]
- De Groot, A.C.; Weyland, J.W. Kathon CG: A review. J. Am. Acad. Dermatol. 1988, 18, 350–358. [Google Scholar] [CrossRef]
- Bruze, M.; Dahlquist, I.; Fregert, S.; Gruvberger, B.; Persson, K. Contact allergy to the active ingredients of Kathon CG. Contact Derm. 1987, 16, 183–188. [Google Scholar] [CrossRef]
- Fregert, S.; Trulson, L.; Zimerson, E. Contact allergic reactions to diphenylthiourea and phenylisothiocyanate in PVC adhesive tape. Contact Derm. 1982, 8, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Klaassen, C.D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; Alan Andersen, F. Final report of the safety assessment of methylisothiazolinone. Int. J. Toxicol. 2010, 29, 187S–213S. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, A.; Lipp, M.; Jacob, S.E. Appropriate Testing of Isothiazolinones in Children. Pediatr. Dermatol. 2017, 34, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Castanedo-Tardana, M.P.; Zug, K.A. Methylisothiazolinone. Dermatitis 2013, 24, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Basketter, D.A.; Angelini, G.; Ingber, A.; Kern, P.S.; Menné, T. Nickel, chromium and cobalt in consumer products: Revisiting safe levels in the new millennium. Contact Derm. 2003, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Devos, F.C.; Pollaris, L.; Van Den Broucke, S.; Seys, S.; Goossens, A.; Nemery, B.; Hoet, P.H.M.; Vanoirbeek, J.A.J. Methylisothiazolinone: Dermal and respiratory immune responses in mice. Toxicol. Lett. 2015, 235, 179–188. [Google Scholar] [CrossRef]
- Mathias, C.B.; Freyschmidt, E.-J.; Caplan, B.; Jones, T.; Poddighe, D.; Xing, W.; Harrison, K.L.; Gurish, M.F.; Oettgen, H.C. IgE influences the number and function of mature mast cells, but not progenitor recruitment in allergic pulmonary inflammation. J. Immunol. 2009, 182, 2416–2424. [Google Scholar] [CrossRef]
- Bax, H.J.; Keeble, A.H.; Gould, H.J. Cytokinergic IgE Action in Mast Cell Activation. Front. Immunol. 2012, 3, 229. [Google Scholar] [CrossRef] [Green Version]
- DiSabato, D.; Quan, N.; Godbout, J.P. Neuroinflammation: The Devil is in the Details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef]
- Zhou, Y.-Q.; Liu, Z.; Liu, Z.-H.; Chen, S.-P.; Li, M.; Shahveranov, A.; Ye, D.-W.; Tian, Y.-K. Interleukin-6: An emerging regulator of pathological pain. J. Neuroinflamm. 2016, 13, 141. [Google Scholar] [CrossRef]
- Nguyen, R.H.; Swanson, D.; Harlow, B.L. Urogenital infections in relation to the occurrence of vulvodynia. J. Reprod. Med. 2009, 54, 385–392. [Google Scholar] [PubMed]
- Khandekar, M.; Brady, S.S.; Rydell, S.A.; Turner, R.M.; Schreiner, P.J.; Harlow, B.L. Early-life Chronic Stressors, Rumination, and the Onset of Vulvodynia. J. Sex. Med. 2019, 16, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Khandekar, M.; Brady, S.S.; Stewart, E.G.; Harlow, B.L. Is chronic stress during childhood associated with adult-onset vulvodynia? J. Women’s Health (Larchmt) 2014, 23, 649–656. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Consumers: Commission Improves Safety of Cosmetics. European Commission Press Release Database. 2014. Available online: https://europa.eu/rapid/press-release_IP-14-1051_en.htm (accessed on 13 September 2019).
- Majeed, S.K. Mast cell distribution in mice. Arzneimittelforschung 1994, 44, 1170–1173. [Google Scholar]
- Shafik, A.; El-Sibai, E.; Shafik, I.; Shafik, A. Immunohistochemical identification of the pacemaker cajal cells in the normal human vagina. Arch. Gynecol. Obstet. 2005, 272, 13–16. [Google Scholar] [CrossRef]
- Han, H.; Park, S.J.; Ahn, H.; Ryu, J.S. Involvement of mast cells in inflammation induced by Trichomonas vaginalis via crosstalk with vaginal epithelial cells. Parasite Immunol. 2012, 34, 8–14. [Google Scholar] [CrossRef]
- Gendrin, C.; Vorhagen, J.; Ngo, L.; Whidbey, C.; Boldenow, E.; Santana-Ufret, V.; Clauson, M.; Burnside, K.; Galloway, D.P.; Waldorf, K.A.; et al. Mast cell degranulation by a hemolytic lipid toxin decreased GBS colonization and infection. Sci. Adv. 2015, 1, e1400225. [Google Scholar] [CrossRef]
- Renga, G.; Borghi, M.; Oikonomou, V.; Mosci, P.; Bartoli, A.; Renauld, J.; Romani, L.; Costantini, C. IL-9 Integrates the Host-Candida Cross-Talk in Vulvovaginal Candidiasis to Balance Inflammation and Tolerance. Front. Immuol. 2018, 9. [Google Scholar] [CrossRef]
- Papoutsis, D.; Haefner, H.K.; Crum, C.P.; Opipari, A.W.; Reed, B.D. Vestibular mast cell density in vulvodynia: A case-controlled study. J. Low. Genit. Tract Dis. 2017, 20, 275–279. [Google Scholar] [CrossRef]
- Foster, D.C.; Hasday, J.D. Elevated tissue levels of interleukin-1 beta and tumor necrosis factor-alpha in vulvar vestibulitis. Obstet. Gynecol. 1997, 89, 291–296. [Google Scholar] [CrossRef]
- Foster, D.C.; Pierkarz, K.H.; Murant, T.I.; LaPoint, R.; Haidaris, C.G.; Phipps, R.P. Enhanced synthesis of proinflammatory cytokines by vulvar vestibular fibroblasts: Implications for vulvar vestibulitis. Am. J. Obstet. Gynecol. 2007, 196, 346. [Google Scholar] [CrossRef] [PubMed]
- Reed, B.D.; Plegue, M.A.; Sen, A.; Haefner, H.K.; Siddiqui, J.; Remick, D.G. Nerve Growth Factor and Selected Cytokines in Women with and Without Vulvodynia. J. Low. Genit. Tract Dis. 2018, 22, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Pascal, M.; Perez-Gordo, M.; Caballero, T.; Escribese, M.M.; Lopez Longo, M.N.; Luengo, O.; Manso, L.; Matheu, V.; Seoane, E.; Zamorano, M.; et al. Microbiome and Allergic Diseases. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Minerbi, A.; Gonzalez, E.; Brereton, N.J.B.; Anjarkouchian, A.; Dewar, K.; Fitzcharles, M.-A.; Chevalier, S.; Shir, Y. Altered microbiome composition in individuals with fibromyalgia. Pain 2019. [Google Scholar] [CrossRef]
- Chichlowski, M.; Rudolph, C. Visceral Pain and Gastrointestinal Microbiome. J. Neurogastroenterol. Motil. 2015, 21, 172–181. [Google Scholar] [CrossRef]
- Ling, Z.; Kong, J.; Liu, F.; Zhu, H.; Chen, X.; Wang, Y.; Li, L.; Nelson, K.E.; Xia, T.; Xiang, C. Molecular analysis of the diversity of vaginal microbiota associated with bacterial vaginosis. BMC Genom. 2010, 11, 488. [Google Scholar] [CrossRef]
- Fredricks, D.N.; Fiedler, T.L.; Marrazzo, J.M. Molecular identification of bacteria associated with bacterial vaginosis. N. Engl. J. Med. 2005, 3, 1899–1911. [Google Scholar] [CrossRef]
- Ziklo, N.; Vidgen, M.E.; Taing, K.; Huston, W.M.; Timms, P. Dysbiosis of the vaginal microbiota and higher vaginal kynurenine/tryptophan ratio reveals an association with Chlamydia trachomatis genital infections. Front. Cell. Infect. Microbiol. 2018, 8, 1. [Google Scholar] [CrossRef]
- Ata, B.; Yildiz, S.; Turkgeldi, E.; Brocal, V.P.; Dinleyici, E.C.; Moya, A.; Urman, B. The Endobiota Study: Comparison of Vaginal, Cervical and Gut Microbiota Between Women with Stage 3/4 Endometriosis and Healthy Controls. Sci. Rep. 2019, 9, 2204. [Google Scholar] [CrossRef]
- Greenbaum, S.; Greenbaum, G.; Moran-Gilad, J.; Weintraub, A.Y. Ecological dynamics of the vaginal microbiome in relation to health and disease. Am. J. Obstet. Gynecol. 2019, 220, 324–335. [Google Scholar] [CrossRef]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zolnoun, D.A.; Francisco, E.M.; Holden, J.K.; Dennis, R.G.; Tommerdahl, M. Altered central sensitization in subgroups of women with vulvodynia. Clin. J. Pain 2011, 27, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Nhu, Q.M.; Aceves, S.S. Tissue Remodeling in Chronic Eosinophilic Esophageal Inflammation: Parallels in Asthma and Therapeutic Perspectives. Front. Med. (Lausanne) 2017, 4, 128. [Google Scholar] [CrossRef] [PubMed]
- Vallvé-Juanico, J.; Houshdaran, S.; Giudice, L.C. The endometrial immune environment of women with endometriosis. Hum. Reprod. Update 2019, 25, 565–592. [Google Scholar] [CrossRef] [PubMed]
- Nyirjesy, P. Chronic vulvovaginal candidiasis. Am. Fam. Physician 2001, 63, 697–702. [Google Scholar] [PubMed]
- Black, C.A.; Rohan, L.C.; Cost, M.; Watkins, S.C.; Draviam, R.; Alber, S.; Edwards, R.P. Vaginal mucosa serves as an inductive site for tolerance. J. Immunol. 2000, 165, 5077–5083. [Google Scholar] [CrossRef] [PubMed]
- Abrams, R. Growing Scrutiny for an Allergy Trigger Used in Personal Care Products. The New York Times. 24 January 2015. Available online: https://www.nytimes.com/2015/01/24/business/allergy-trigger-found-in-many-personal-care-items-comes-under-greater-scrutiny.html (accessed on 3 September 2019).
- Wickham, H.; Henry, L. Tidyr: Easily Tidy Data with ‘spread()’ and ‘gather()’ Functions, R package version 0.6.3, last modified September 1, 2017. Available online: https://CRAN.R-project.org/package=tidyr (accessed on 10 September 2019).
- Kahle, D.; Wickham, H. ggmap: Spatial Visualization with ggplot2. R J. 2013, 5, 144–161. [Google Scholar] [CrossRef] [Green Version]
- Harrison, M.; O’Brien, A.; Adams, L.; Cowin, G.; Ruitenberg, M.J.; Sengul, G.; Watson, C. Vertebral landmarks for the identification of spinal cord segments in the mouse. Neuroimage 2013, 68, 22–29. [Google Scholar] [CrossRef]
- Farmer, M.A.; Taylor, A.M.; Bailey, A.L.; Tuttle, A.H.; MacIntyre, L.C.; Milagrosa, Z.E.; Crissman, H.P.; Bennett, G.J.; Ribeiro-da-Silva, A.; Binik, Y.M.; et al. Repeated vulvovaginal fungal infections cause persistent pain in a mouse model of vulvodynia. Sci. Transl. Med. 2011, 3, 101ra91. [Google Scholar] [CrossRef]
- Kakurai, M.; Monteforte, R.; Suto, H.; Tsai, M.; Nakae, S.; Galli, S.J. Mast Cell-Derived Tumor Necrosis Factor Can Promote Nerve Fiber Elongation in the Skin during Contact Hypersensitivity in Mice. Am. J. Pathol. 2006, 169, 1713–1721. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Gohl, D.M.; Vangay, P.; Garbe, J.; MacLean, A.; Hauge, A.; Becker, A.; Gould, T.J.; Clayton, J.B.; Johnson, T.J.; Hunter, R.; et al. Systematic improvement of amplicon marker gene methods for increased accuracy in microbiome studies. Nat. Biotechnol. 2016, 34, 942–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Ghalith, G.A.; Hillmann, B.; Ang, K.; Shields-Cutler, R.; Knights, D. SHI7 Is a Self-Learning Pipeline for Multipurpose Short-Read DNA Quality Control. mSystems 2018, 3, e00202-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Shields-Cutler, R.R.; Al-Ghalith, G.A.; Yassour, M.; Knights, D. Splinectome R Enables Group Comparisons in Longitudinal Microbiome Studies. Front. Microbiol. 2018, 9, 785. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arriaga-Gomez, E.; Kline, J.; Emanuel, E.; Neamonitaki, N.; Yangdon, T.; Zacheis, H.; Pasha, D.; Lim, J.; Bush, S.; Boo, B.; et al. Repeated Vaginal Exposures to the Common Cosmetic and Household Preservative Methylisothiazolinone Induce Persistent, Mast Cell-Dependent Genital Pain in ND4 Mice. Int. J. Mol. Sci. 2019, 20, 5361. https://doi.org/10.3390/ijms20215361
Arriaga-Gomez E, Kline J, Emanuel E, Neamonitaki N, Yangdon T, Zacheis H, Pasha D, Lim J, Bush S, Boo B, et al. Repeated Vaginal Exposures to the Common Cosmetic and Household Preservative Methylisothiazolinone Induce Persistent, Mast Cell-Dependent Genital Pain in ND4 Mice. International Journal of Molecular Sciences. 2019; 20(21):5361. https://doi.org/10.3390/ijms20215361
Chicago/Turabian StyleArriaga-Gomez, Erica, Jaclyn Kline, Elizabeth Emanuel, Nefeli Neamonitaki, Tenzin Yangdon, Hayley Zacheis, Dogukan Pasha, Jinyoung Lim, Susan Bush, Beebie Boo, and et al. 2019. "Repeated Vaginal Exposures to the Common Cosmetic and Household Preservative Methylisothiazolinone Induce Persistent, Mast Cell-Dependent Genital Pain in ND4 Mice" International Journal of Molecular Sciences 20, no. 21: 5361. https://doi.org/10.3390/ijms20215361