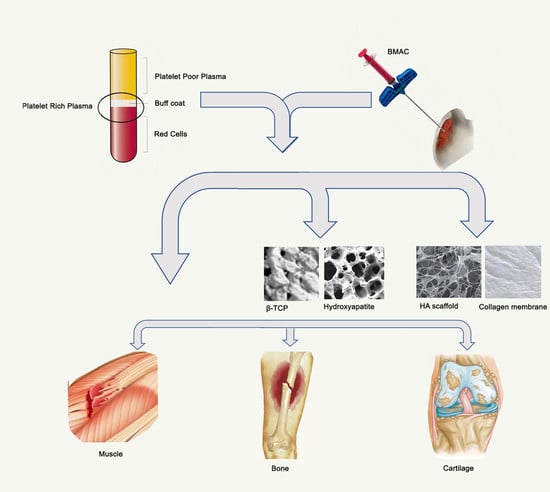
PRP and BMAC for Musculoskeletal Conditions via Biomaterial Carriers
Abstract
:1. Introduction
2. Platelet-Rich Plasma (PRP)
3. Bone Marrow Aspirate Concentrate (BMAC)
4. Biomaterials
4.1. Collagen
4.2. Hyaluronic Acid (HA)
4.3. Poly (Lactic-Co-Glycolic) Acid (PLGA)
4.4. Cartilage
5. Conclusions
Funding
Conflicts of Interest
References
- Weinstein, S.L. The Burden of Musculoskeletal Conditions. J. Bone Joint Surg. Am. 2016, 98, 1331. [Google Scholar] [CrossRef] [PubMed]
- Huard, J.; Lu, A.; Mu, X.; Guo, P.; Li, Y. Muscle Injuries and Repair: What’s New on the Horizon! Cells Tissues Organs 2016, 202, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Huard, J.; Li, Y.; Fu, F.H. Muscle injuries and repair: Current trends in research. J. Bone Joint Surg. Am. 2002, 84-a, 822–832. [Google Scholar] [CrossRef]
- Jarvinen, T.A.; Jarvinen, T.L.; Kaariainen, M.; Kalimo, H.; Jarvinen, M. Muscle injuries: Biology and treatment. Am. J. Sports Med. 2005, 33, 745–764. [Google Scholar] [CrossRef]
- Morelli, K.M.; Brown, L.B.; Warren, G.L. Effect of NSAIDs on Recovery from Acute Skeletal Muscle Injury: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2018, 46, 224–233. [Google Scholar] [CrossRef]
- Lepley, L.K.; Butterfield, T.A. Shifting the Current Clinical Perspective: Isolated Eccentric Exercise as an Effective Intervention to Promote the Recovery of Muscle After Injury. J. Sport Rehabil. 2017, 26, 122–130. [Google Scholar] [CrossRef]
- Bourne, M.N.; Williams, M.D.; Opar, D.A.; Al Najjar, A.; Kerr, G.K.; Shield, A.J. Impact of exercise selection on hamstring muscle activation. Br. J. Sports Med. 2017, 51, 1021–1028. [Google Scholar] [CrossRef]
- Hauger, A.V.; Reiman, M.P.; Bjordal, J.M.; Sheets, C.; Ledbetter, L.; Goode, A.P. Neuromuscular electrical stimulation is effective in strengthening the quadriceps muscle after anterior cruciate ligament surgery. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 399–410. [Google Scholar] [CrossRef]
- Erickson, M.L.; Ryan, T.E.; Backus, D.; McCully, K.K. Endurance neuromuscular electrical stimulation training improves skeletal muscle oxidative capacity in individuals with motor-complete spinal cord injury. Muscle Nerve 2017, 55, 669–675. [Google Scholar] [CrossRef]
- Oyaizu, T.; Enomoto, M.; Yamamoto, N.; Tsuji, K.; Horie, M.; Muneta, T.; Sekiya, I.; Okawa, A.; Yagishita, K. Hyperbaric oxygen reduces inflammation, oxygenates injured muscle, and regenerates skeletal muscle via macrophage and satellite cell activation. Sci. Rep. 2018, 8, 1288. [Google Scholar] [CrossRef]
- Staples, J.R.; Clement, D.B.; Taunton, J.E.; McKenzie, D.C. Effects of hyperbaric oxygen on a human model of injury. Am. J. Sports Med. 1999, 27, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef] [PubMed]
- Madrigal, J.L.; Stilhano, R.; Silva, E.A. Biomaterial-Guided Gene Delivery for Musculoskeletal Tissue Repair. Tissue Eng. Part B Rev. 2017, 23, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, J.C.; Vunjak-Novakovic, G. Should we use cells, biomaterials, or tissue engineering for cartilage regeneration? Stem Cell Res. Ther. 2016, 7, 56. [Google Scholar] [CrossRef]
- Vinatier, C.; Guicheux, J. Cartilage tissue engineering: From biomaterials and stem cells to osteoarthritis treatments. Ann. Phys. Rehabil. Med. 2016, 59, 139–144. [Google Scholar] [CrossRef]
- Gomoll, A.H.; Minas, T. The quality of healing: Articular cartilage. Wound Repair Regen. 2014, 22 (Suppl. 1), 30–38. [Google Scholar] [CrossRef]
- Thomas, A.C.; Hubbard-Turner, T.; Wikstrom, E.A.; Palmieri-Smith, R.M. Epidemiology of Posttraumatic Osteoarthritis. J. Athl. Train. 2017, 52, 491–496. [Google Scholar] [CrossRef]
- Ziegler, C.G.; Van Sloun, R.; Gonzalez, S.; Whitney, K.E.; DePhillipo, N.N.; Kennedy, M.I.; Dornan, G.J.; Evans, T.A.; Huard, J.; LaPrade, R.F. Characterization of Growth Factors, Cytokines, and Chemokines in Bone Marrow Concentrate and Platelet-Rich Plasma: A Prospective Analysis. Am. J. Sports Med. 2019, 47, 2174–2187. [Google Scholar] [CrossRef]
- Denapoli, P.M.; Stilhano, R.S.; Ingham, S.J.; Han, S.W.; Abdalla, R.J. Platelet-Rich Plasma in a Murine Model: Leukocytes, Growth Factors, Flt-1, and Muscle Healing. Am. J. Sports Med. 2016, 44, 1962–1971. [Google Scholar] [CrossRef]
- Marx, R.E. Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant. Dent. 2001, 10, 225–228. [Google Scholar] [CrossRef]
- DeLong, J.M.; Russell, R.P.; Mazzocca, A.D. Platelet-rich plasma: The PAW classification system. Arthroscopy 2012, 28, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.; Subcommittee on Platelet Physiology. The use of platelets in regenerative medicine and proposal for a new classification system: guidance from the SSC of the ISTH. J. Thromb Haemost. 2018, 16, 1895–1900. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, B.; Tol, J.L.; Almusa, E.; Boukarroum, S.; Eirale, C.; Farooq, A.; Whiteley, R.; Chalabi, H. Platelet-rich plasma does not enhance return to play in hamstring injuries: A randomised controlled trial. Br. J. Sports Med. 2015, 49, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Reurink, G.; Goudswaard, G.J.; Moen, M.H.; Weir, A.; Verhaar, J.A.; Bierma-Zeinstra, S.M.; Maas, M.; Tol, J.L.; Dutch, H.I.T.s.I. Rationale, secondary outcome scores and 1-year follow-up of a randomised trial of platelet-rich plasma injections in acute hamstring muscle injury: The Dutch Hamstring Injection Therapy study. Br. J. Sports Med. 2015, 49, 1206–1212. [Google Scholar] [CrossRef]
- Sanchez, M.; Anitua, E.; Azofra, J.; Aguirre, J.J.; Andia, I. Intra-articular injection of an autologous preparation rich in growth factors for the treatment of knee OA: A retrospective cohort study. Clin. Exp. Rheumatol. 2008, 26, 910–913. [Google Scholar]
- Kon, E.; Buda, R.; Filardo, G.; Di Martino, A.; Timoncini, A.; Cenacchi, A.; Fornasari, P.M.; Giannini, S.; Marcacci, M. Platelet-rich plasma: Intra-articular knee injections produced favorable results on degenerative cartilage lesions. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 472–479. [Google Scholar] [CrossRef]
- Kon, E.; Mandelbaum, B.; Buda, R.; Filardo, G.; Delcogliano, M.; Timoncini, A.; Fornasari, P.M.; Giannini, S.; Marcacci, M. Platelet-Rich Plasma Intra-Articular Injection Versus Hyaluronic Acid Viscosupplementation as Treatments for Cartilage Pathology: From Early Degeneration to Osteoarthritis. Arthrosc. J. Arthrosc. Relat. Surg. 2011, 27, 1490–1501. [Google Scholar] [CrossRef]
- Lee, G.W.; Son, J.H.; Kim, J.D.; Jung, G.H. Is platelet-rich plasma able to enhance the results of arthroscopic microfracture in early osteoarthritis and cartilage lesion over 40 years of age? Eur J. Orthop. Surg. Traumatol. 2013, 23, 581–587. [Google Scholar] [CrossRef]
- Patel, S.; Dhillon, M.S.; Aggarwal, S.; Marwaha, N.; Jain, A. Treatment with Platelet-Rich Plasma Is More Effective Than Placebo for Knee Osteoarthritis A Prospective, Double-Blind, Randomized Trial. Am. J. Sports Med. 2013, 41, 356–364. [Google Scholar] [CrossRef]
- Duif, C.; Vogel, T.; Topcuoglu, F.; Spyrou, G.; von Schulze Pellengahr, C.; Lahner, M. Does intraoperative application of leukocyte-poor platelet-rich plasma during arthroscopy for knee degeneration affect postoperative pain, function and quality of life? A 12-month randomized controlled double-blind trial. Arch. Orthop. Trauma Surg. 2015, 135, 971–977. [Google Scholar] [CrossRef]
- Filardo, G.; Di Matteo, B.; Di Martino, A.; Merli, M.L.; Cenacchi, A.; Fornasari, P.; Marcacci, M.; Kon, E. Platelet-Rich Plasma Intra-articular Knee Injections Show No Superiority Versus Viscosupplementation: A Randomized Controlled Trial. Am. J. Sports Med. 2015, 43, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.; Delgado, D.; Sanchez, P.; Muinos-Lopez, E.; Paiva, B.; Granero-Molto, F.; Prosper, F.; Pompei, O.; Perez, J.C.; Azofra, J.; et al. Combination of Intra-Articular and Intraosseous Injections of Platelet Rich Plasma for Severe Knee Osteoarthritis: A Pilot Study. Biomed. Res. Int. 2016, 2016, 4868613. [Google Scholar] [CrossRef] [PubMed]
- Gormeli, G.; Gormeli, C.A.; Ataoglu, B.; Colak, C.; Aslanturk, O.; Ertem, K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: A randomized, double-blind, placebo-controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, R.; Kulinski, K.; Kozar-Kaminska, K.; Wielgus, M.; Langner, M.; Wasko, M.K.; Kowalczewski, J.; Pomianowski, S. A Prospective, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Study Evaluating Meniscal Healing, Clinical Outcomes, and Safety in Patients Undergoing Meniscal Repair of Unstable, Complete Vertical Meniscal Tears (Bucket Handle) Augmented with Platelet-Rich Plasma. Biomed. Res. Int. 2018, 2018, 9315815. [Google Scholar] [CrossRef]
- Kaminski, R.; Maksymowicz-Wleklik, M.; Kulinski, K.; Kozar-Kaminska, K.; Dabrowska-Thing, A.; Pomianowski, S. Short-Term Outcomes of Percutaneous Trephination with a Platelet Rich Plasma Intrameniscal Injection for the Repair of Degenerative Meniscal Lesions. A Prospective, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Study. Int. J. Mol. Sci. 2019, 20, 856. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E.; Carlson, E.R.; Eichstaedt, R.M.; Schimmele, S.R.; Strauss, J.E.; Georgeff, K.R. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998, 85, 638–646. [Google Scholar] [CrossRef]
- Rodriguez, A.; Anastassov, G.E.; Lee, H.; Buchbinder, D.; Wettan, H. Maxillary sinus augmentation with deproteinated bovine bone and platelet rich plasma with simultaneous insertion of endosseous implants. J. Oral Maxillofac Surg. 2003, 61, 157–163. [Google Scholar] [CrossRef]
- Daif, E.T. Effect of autologous platelet-rich plasma on bone regeneration in mandibular fractures. Dent. Traumatol. 2013, 29, 399–403. [Google Scholar] [CrossRef]
- Anitua, E.; Murias-Freijo, A.; Alkhraisat, M.H.; Orive, G. Clinical, radiographical, and histological outcomes of plasma rich in growth factors in extraction socket: A randomized controlled clinical trial. Clin. Oral Investig. 2015, 19, 589–600. [Google Scholar] [CrossRef]
- Malhotra, R.; Kumar, V.; Garg, B.; Singh, R.; Jain, V.; Coshic, P.; Chatterjee, K. Role of autologous platelet-rich plasma in treatment of long-bone nonunions: A prospective study. Musculoskelet. Surg. 2015, 99, 243–248. [Google Scholar] [CrossRef]
- Tabrizi, R.; Karagah, T.; Shahidi, S.; Zare, N. Does platelet-rich plasma enhance healing in the idiopathic bone cavity? A single-blind randomized clinical trial. Int. J. Oral Maxillofac. Surg. 2015, 44, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Ghaffarpasand, F.; Shahrezaei, M.; Dehghankhalili, M. Effects of Platelet Rich Plasma on Healing Rate of Long Bone Non-union Fractures: A Randomized Double-Blind Placebo Controlled Clinical Trial. Bull. Emerg. Trauma 2016, 4, 134–140. [Google Scholar] [PubMed]
- Castillo-Cardiel, G.; Medina-Quintana, V.M.; Lomeli-Enriquez, M.; Medrano-Munoz, F.; Guerrero-Velazquez, C.; Contreras-Lopez, C.K.; Fuentes-Orozco, C.; Irusteta-Jimenez, L.; Michel-Espinoza, L.R.; Gonzalez-Ojeda, A. Platelet-rich plasma and its effect in bone regeneration in mandibular fractures. Controlled clinical trial. Gac. Med. Mex. 2017, 153, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Olivo, C.; Garza-Borjon, A.; Simental-Mendia, M.; Vilchez-Cavazos, F.; Tamez-Mata, Y.; Pena-Martinez, V. Delayed union of humeral shaft fractures: Comparison of autograft with and without platelet-rich plasma treatment: A randomized, single blinded clinical trial. Arch. Orthop. Trauma Surg. 2017, 137, 1247–1252. [Google Scholar] [CrossRef]
- Al Hamid, M.S.; Mohamed Ali, M.R.; Yusof, A.; George, J.; Lee, L.P. Platelet-rich plasma injections for the treatment of hamstring injuries: A randomized controlled trial. Am. J. Sports Med. 2014, 42, 2410–2418. [Google Scholar] [CrossRef]
- Bubnov, R.; Yevseenko, V.; Semeniv, I. Ultrasound guided injections of platelets rich plasma for muscle injury in professional athletes. Comparative study. Med. Ultrason. 2013, 15, 101–105. [Google Scholar] [CrossRef]
- Martinez-Zapata, M.J.; Orozco, L.; Balius, R.; Soler, R.; Bosch, A.; Rodas, G.; Til, L.; Peirau, X.; Urrutia, G.; Gich, I.; et al. Efficacy of autologous platelet-rich plasma for the treatment of muscle rupture with haematoma: A multicentre, randomised, double-blind, placebo-controlled clinical trial. Blood Transfus. 2016, 14, 245–254. [Google Scholar] [CrossRef]
- Rossi, L.A.; Molina Romoli, A.R.; Bertona Altieri, B.A.; Burgos Flor, J.A.; Scordo, W.E.; Elizondo, C.M. Does platelet-rich plasma decrease time to return to sports in acute muscle tear? A randomized controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 3319–3325. [Google Scholar] [CrossRef]
- Dutta, S.R.; Passi, D.; Singh, P.; Sharma, S.; Singh, M.; Srivastava, D. A randomized comparative prospective study of platelet-rich plasma, platelet-rich fibrin, and hydroxyapatite as a graft material for mandibular third molar extraction socket healing. Natl. J. Maxillofac. Surg. 2016, 7, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Filardo, G.; Kon, E.; Pereira Ruiz, M.T.; Vaccaro, F.; Guitaldi, R.; Di Martino, A.; Cenacchi, A.; Fornasari, P.M.; Marcacci, M. Platelet-rich plasma intra-articular injections for cartilage degeneration and osteoarthritis: Single- versus double-spinning approach. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 2082–2091. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Piatetzky, S., II; Petrakova, K.V. Osteogenesis in transplants of bone marrow cells. J. Embryol. Exp. Morphol. 1966, 16, 381–390. [Google Scholar] [PubMed]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.; Friedenstein, A.J. Stromal stem cells: Marrow-derived osteogenic precursors. Ciba Found. Symp. 1988, 136, 42–60. [Google Scholar] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Witwer, K.W.; Van Balkom, B.W.M.; Bruno, S.; Choo, A.; Dominici, M.; Gimona, M.; Hill, A.F.; De Kleijn, D.; Koh, M.; Lai, R.C.; et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J. Extracell. Vesicles 2019, 8, 1609206. [Google Scholar] [CrossRef] [Green Version]
- Cavinatto, L.; Hinckel, B.B.; Tomlinson, R.E.; Gupta, S.; Farr, J.; Bartolozzi, A.R. The Role of Bone Marrow Aspirate Concentrate for the Treatment of Focal Chondral Lesions of the Knee: A Systematic Review and Critical Analysis of Animal and Clinical Studies. Arthroscopy 2019, 35, 1860–1877. [Google Scholar] [CrossRef]
- Chahla, J.; Dean, C.S.; Moatshe, G.; Pascual-Garrido, C.; Serra Cruz, R.; LaPrade, R.F. Concentrated Bone Marrow Aspirate for the Treatment of Chondral Injuries and Osteoarthritis of the Knee: A Systematic Review of Outcomes. Orthop. J. Sports Med. 2016, 4, 2325967115625481. [Google Scholar] [CrossRef]
- Kim, J.D.; Lee, G.W.; Jung, G.H.; Kim, C.K.; Kim, T.; Park, J.H.; Cha, S.S.; You, Y.B. Clinical outcome of autologous bone marrow aspirates concentrate (BMAC) injection in degenerative arthritis of the knee. Eur. J. Orthop. Surg. Traumatol. 2014, 24, 1505–1511. [Google Scholar] [CrossRef]
- Themistocleous, G.S.; Chloros, G.D.; Kyrantzoulis, I.M.; Georgokostas, I.A.; Themistocleous, M.S.; Papagelopoulos, P.J.; Savvidou, O.D. Effectiveness of a single intra-articular bone marrow aspirate concentrate (BMAC) injection in patients with grade 3 and 4 knee osteoarthritis. Heliyon 2018, 4, e00871. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, S.A.; Kazmerchak, S.E.; Heckman, M.G.; Zubair, A.C.; O’Connor, M.I. A Prospective, Single-Blind, Placebo-Controlled Trial of Bone Marrow Aspirate Concentrate for Knee Osteoarthritis. Am. J. Sports Med. 2017, 45, 82–90. [Google Scholar] [CrossRef]
- Shapiro, S.A.; Arthurs, J.R.; Heckman, M.G.; Bestic, J.M.; Kazmerchak, S.E.; Diehl, N.N.; Zubair, A.C.; O’Connor, M.I. Quantitative T2 MRI Mapping and 12-Month Follow-up in a Randomized, Blinded, Placebo Controlled Trial of Bone Marrow Aspiration and Concentration for Osteoarthritis of the Knees. Cartilage 2018, 1947603518796142. [Google Scholar] [CrossRef] [PubMed]
- Burin, D.; Pyasik, M.; Ronga, I.; Cavallo, M.; Salatino, A.; Pia, L. “As long as that is my hand, that willed action is mine”: Timing of agency triggered by body ownership. Conscious. Cogn. 2018, 58, 186–192. [Google Scholar] [CrossRef]
- Karnovsky, S.C.; DeSandis, B.; Haleem, A.M.; Sofka, C.M.; O’Malley, M.; Drakos, M.C. Comparison of Juvenile Allogenous Articular Cartilage and Bone Marrow Aspirate Concentrate Versus Microfracture With and Without Bone Marrow Aspirate Concentrate in Arthroscopic Treatment of Talar Osteochondral Lesions. Foot Ankle Int. 2018, 39, 393–405. [Google Scholar] [CrossRef] [PubMed]
- DeSandis, B.A.; Haleem, A.M.; Sofka, C.M.; O’Malley, M.J.; Drakos, M.C. Arthroscopic Treatment of Osteochondral Lesions of the Talus Using Juvenile Articular Cartilage Allograft and Autologous Bone Marrow Aspirate Concentration. J. Foot Ankle Surg. 2018, 57, 273–280. [Google Scholar] [CrossRef]
- Hernigou, P.; Beaujean, F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin. Orthop. Relat. Res. 2002, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Hernigou, P.; Poignard, A.; Beaujean, F.; Rouard, H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J. Bone Joint Surg. Am. 2005, 87, 1430–1437. [Google Scholar] [CrossRef]
- Tabatabaee, R.M.; Saberi, S.; Parvizi, J.; Mortazavi, S.M.; Farzan, M. Combining Concentrated Autologous Bone Marrow Stem Cells Injection with Core Decompression Improves Outcome for Patients with Early-Stage Osteonecrosis of the Femoral Head: A Comparative Study. J. Arthroplasty 2015, 30, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Hauzeur, J.P.; De Maertelaer, V.; Baudoux, E.; Malaise, M.; Beguin, Y.; Gangji, V. Inefficacy of autologous bone marrow concentrate in stage three osteonecrosis: A randomized controlled double-blind trial. Int. Orthop. 2018, 42, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.; Templeman, D.; Weinlein, J.C. Nonunion of the Femur and Tibia: An Update. Orthop. Clin. North. Am. 2016, 47, 365–375. [Google Scholar] [CrossRef]
- Zura, R.; Xiong, Z.; Einhorn, T.; Watson, J.T.; Ostrum, R.F.; Prayson, M.J.; Della Rocca, G.J.; Mehta, S.; McKinley, T.; Wang, Z.; et al. Epidemiology of Fracture Nonunion in 18 Human Bones. JAMA Surg. 2016, 151, e162775. [Google Scholar] [CrossRef]
- Migonney, V. Biomaterials; Wiley-ISTE: London, UK, 2014. [Google Scholar]
- Helary, C.; Desimone, M.F. Recent advances in biomaterials for tissue engineering and controlled drug delivery. Curr. Pharm. Biotechnol. 2015, 16, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Hortensius, R.A.; Harley, B.A. Naturally derived biomaterials for addressing inflammation in tissue regeneration. Exp. Biol. Med. (Maywood) 2016, 241, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Kim, H.J.; Chung, J.Y.; Lee, J.H.; Young, S.B.; Kim, Y.H. Natural and synthetic biomaterials for controlled drug delivery. Arch. Pharm. Res. 2014, 37, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kasper, F.K.; Mikos, A.G. Biomaterials for tissue engineering. Ann. Biomed. Eng. 2014, 42, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1. [Google Scholar] [CrossRef]
- Williams, P.A.; Campbell, K.T.; Silva, E.A. Biomaterials and Cells for Revascularization; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Jabbarzadeh, E.; Starnes, T.; Khan, Y.M.; Jiang, T.; Wirtel, A.J.; Deng, M.; Lv, Q.; Nair, L.S.; Doty, S.B.; Laurencin, C.T. Induction of angiogenesis in tissue-engineered scaffolds designed for bone repair: A combined gene therapy-cell transplantation approach. Proc. Natl. Acad. Sci. USA 2008, 105, 11099–11104. [Google Scholar] [CrossRef]
- Duan, C.; Liu, J.; Yuan, Z.; Meng, G.; Yang, X.; Jia, S.; Zhang, J.; Chen, S. Adenovirus-mediated transfer of VEGF into marrow stromal cells combined with PLGA/TCP scaffold increases vascularization and promotes bone repair in vivo. Arch. Med. Sci. 2014, 10, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Hidaka, C.; Ibarra, C.; Hannafin, J.A.; Torzilli, P.A.; Quitoriano, M.; Jen, S.S.; Warren, R.F.; Crystal, R.G. Formation of vascularized meniscal tissue by combining gene therapy with tissue engineering. Tissue Eng. 2002, 8, 93–105. [Google Scholar] [CrossRef]
- Siclari, A.; Mascaro, G.; Gentili, C.; Kaps, C.; Cancedda, R.; Boux, E. Cartilage repair in the knee with subchondral drilling augmented with a platelet-rich plasma-immersed polymer-based implant. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 1225–1234. [Google Scholar] [CrossRef]
- Gigante, A.; Cecconi, S.; Calcagno, S.; Busilacchi, A.; Enea, D. Arthroscopic knee cartilage repair with covered microfracture and bone marrow concentrate. Arthrosc. Tech. 2012, 1, e175–e180. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Friess, W. Collagen—Biomaterial for drug delivery. Eur. J. Pharm. Biopharm. 1998, 45, 113–136. [Google Scholar] [CrossRef]
- Fang, D.; Jin, P.; Huang, Q.; Yang, Y.; Zhao, J.; Zheng, L. Platelet-rich plasma promotes the regeneration of cartilage engineered by mesenchymal stem cells and collagen hydrogel via the TGF-beta/SMAD signaling pathway. J. Cell Physiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, V.; Niculescu-Morzsa, E.; Bauer, C.; Lacza, Z.; Nehrer, S. Redifferentiation of Articular Chondrocytes by Hyperacute Serum and Platelet Rich Plasma in Collagen Type I Hydrogels. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef]
- Suri, S.; Han, L.H.; Zhang, W.; Singh, A.; Chen, S.; Schmidt, C.E. Solid freeform fabrication of designer scaffolds of hyaluronic acid for nerve tissue engineering. Biomed. Microdevices 2011, 13, 983–993. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Freymann, U.; Endres, M.; Neumann, K.; Scholman, H.J.; Morawietz, L.; Kaps, C. Expanded human meniscus-derived cells in 3-D polymer-hyaluronan scaffolds for meniscus repair. Acta Biomater. 2012, 8, 677–685. [Google Scholar] [CrossRef]
- Kruger, J.P.; Ketzmar, A.K.; Endres, M.; Pruss, A.; Siclari, A.; Kaps, C. Human platelet-rich plasma induces chondrogenic differentiation of subchondral progenitor cells in polyglycolic acid-hyaluronan scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 681–692. [Google Scholar] [CrossRef]
- Birdwhistell, K.E.; Karumbaiah, L.; Franklin, S.P. Sustained Release of Transforming Growth Factor-beta1 from Platelet-Rich Chondroitin Sulfate Glycosaminoglycan Gels. J. Knee Surg. 2018, 31, 410–415. [Google Scholar] [CrossRef]
- Beigi, M.H.; Atefi, A.; Ghanaei, H.R.; Labbaf, S.; Ejeian, F.; Nasr-Esfahani, M.H. Activated platelet-rich plasma improves cartilage regeneration using adipose stem cells encapsulated in a 3D alginate scaffold. J. Tissue Eng. Regen. Med. 2018, 12, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Mooney, D.J. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 1998, 16, 224–230. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers (Basel) 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Gopferich, A. Mechanisms of polymer degradation and erosion. Biomaterials 1996, 17, 103–114. [Google Scholar] [CrossRef]
- Ito, Y.; Kondo, S.; Chen, G.; Imanishi, Y. Patterned artificial juxtacrine stimulation of cells by covalently immobilized insulin. FEBS Lett. 1997, 403, 159–162. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.J.; Song, S.H.; Lee, D.S.; Park, T.G. Immobilization of cell adhesive RGD peptide onto the surface of highly porous biodegradable polymer scaffolds fabricated by a gas foaming/salt leaching method. Biomaterials 2004, 25, 5613–5620. [Google Scholar] [CrossRef]
- Ishida, K.; Kuroda, R.; Miwa, M.; Tabata, Y.; Hokugo, A.; Kawamoto, T.; Sasaki, K.; Doita, M.; Kurosaka, M. The regenerative effects of platelet-rich plasma on meniscal cells in vitro and its in vivo application with biodegradable gelatin hydrogel. Tissue Eng. 2007, 13, 1103–1112. [Google Scholar] [CrossRef]
- He, F.; Chen, Y.; Li, J.; Lin, B.; Ouyang, Y.; Yu, B.; Xia, Y.; Yu, B.; Ye, J. Improving bone repair of femoral and radial defects in rabbit by incorporating PRP into PLGA/CPC composite scaffold with unidirectional pore structure. J. Biomed. Mater. Res. A 2015, 103, 1312–1324. [Google Scholar] [CrossRef]
- Chen, G.; Liu, D.; Maruyama, N.; Ohgushi, H.; Tanaka, J.; Tateishi, T. Cell adhesion of bone marrow cells, chondrocytes, ligament cells and synovial cells on a PLGA–collagen hybrid mesh. Mater. Sci. Eng. C 2004, 6, 7. [Google Scholar] [CrossRef]
- Huang, W.; Carlsen, B.; Wulur, I.; Rudkin, G.; Ishida, K.; Wu, B.; Yamaguchi, D.T.; Miller, T.A. BMP-2 exerts differential effects on differentiation of rabbit bone marrow stromal cells grown in two-dimensional and three-dimensional systems and is required for in vitro bone formation in a PLGA scaffold. Exp. Cell Res. 2004, 299, 325–334. [Google Scholar] [CrossRef]
- Dallari, D.; Savarino, L.; Stagni, C.; Cenni, E.; Cenacchi, A.; Fornasari, P.M.; Albisinni, U.; Rimondi, E.; Baldini, N.; Giunti, A. Enhanced tibial osteotomy healing with use of bone grafts supplemented with platelet gel or platelet gel and bone marrow stromal cells. J. Bone Joint Surg. Am. 2007, 89, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
- Jager, M.; Herten, M.; Fochtmann, U.; Fischer, J.; Hernigou, P.; Zilkens, C.; Hendrich, C.; Krauspe, R. Bridging the gap: Bone marrow aspiration concentrate reduces autologous bone grafting in osseous defects. J. Orthop. Res. 2011, 29, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Krych, A.J.; Nawabi, D.H.; Farshad-Amacker, N.A.; Jones, K.J.; Maak, T.G.; Potter, H.G.; Williams, R.J., 3rd. Bone Marrow Concentrate Improves Early Cartilage Phase Maturation of a Scaffold Plug in the Knee: A Comparative Magnetic Resonance Imaging Analysis to Platelet-Rich Plasma and Control. Am. J. Sports Med. 2016, 44, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Sauerbier, S.; Stricker, A.; Kuschnierz, J.; Buhler, F.; Oshima, T.; Xavier, S.P.; Schmelzeisen, R.; Gutwald, R. In vivo comparison of hard tissue regeneration with human mesenchymal stem cells processed with either the FICOLL method or the BMAC method. Tissue Eng. Part C Methods 2010, 16, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Yassibag-Berkman, Z.; Tuncer, O.; Subasioglu, T.; Kantarci, A. Combined use of platelet-rich plasma and bone grafting with or without guided tissue regeneration in the treatment of anterior interproximal defects. J. Periodontol. 2007, 78, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Attia, A. Evaluation of Beta-tricalcium phosphate and platelets rich plasma in management of intrabony defects: Clinical and Radiographic study. Egypt. Dent. J. 2010, 56, 10. [Google Scholar]
- Saini, N.; Sikri, P.; Gupta, H. Evaluation of the relative efficacy of autologous platelet-rich plasma in combination with beta-tricalcium phosphate alloplast versus an alloplast alone in the treatment of human periodontal infrabony defects: A clinical and radiological study. Indian J. Dent. Res. 2011, 22, 107–115. [Google Scholar] [CrossRef]
- Ozdemir, B.; Okte, E. Treatment of intrabony defects with beta-tricalciumphosphate alone and in combination with platelet-rich plasma. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 976–983. [Google Scholar] [CrossRef]
- Okuda, K.; Tai, H.; Tanabe, K.; Suzuki, H.; Sato, T.; Kawase, T.; Saito, Y.; Wolff, L.F.; Yoshiex, H. Platelet-rich plasma combined with a porous hydroxyapatite graft for the treatment of intrabony periodontal defects in humans: A comparative controlled clinical study. J. Periodontol. 2005, 76, 890–898. [Google Scholar] [CrossRef]
- Vaishnavi, C.; Mohan, B.; Narayanan, L.L. Treatment of endodontically induced periapical lesions using hydroxyapatite, platelet-rich plasma, and a combination of both: An in vivo study. J. Conserv Dent. 2011, 14, 140–146. [Google Scholar] [CrossRef]
- Menezes, L.M.; Rao, J. Long-term clinical evaluation of platelet-rich plasma in the treatment of human periodontal intraosseous defects: A comparative clinical trial. Quintessence Int. 2012, 43, 571–582. [Google Scholar] [PubMed]
- Kutkut, A.; Andreana, S.; Kim, H.L.; Monaco, E., Jr. Extraction socket preservation graft before implant placement with calcium sulfate hemihydrate and platelet-rich plasma: A clinical and histomorphometric study in humans. J. Periodontol. 2012, 83, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Siclari, A.; Mascaro, G.; Gentili, C.; Cancedda, R.; Boux, E. A cell-free scaffold-based cartilage repair provides improved function hyaline-like repair at one year. Clin. Orthop. Relat. Res. 2012, 470, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Siclari, A.; Mascaro, G.; Kaps, C.; Boux, E. A 5-year follow-up after cartilage repair in the knee using a platelet-rich plasma-immersed polymer-based implant. Open Orthop. J. 2014, 8, 346–354. [Google Scholar] [CrossRef]
- Siclari, A.; Krueger, J.P.; Endres, M.; Boux, E. A 24-month follow-up after treatment of hallux rigidus with resection arthroplasty in combination with a resorbable polymer-based implant and platelet-rich plasma. Foot Ankle Surg. 2018, 24, 389–393. [Google Scholar] [CrossRef]
- Enea, D.; Cecconi, S.; Calcagno, S.; Busilacchi, A.; Manzotti, S.; Kaps, C.; Gigante, A. Single-stage cartilage repair in the knee with microfracture covered with a resorbable polymer-based matrix and autologous bone marrow concentrate. Knee 2013, 20, 562–569. [Google Scholar] [CrossRef]
- Giannini, S.; Buda, R.; Vannini, F.; Cavallo, M.; Grigolo, B. One-step bone marrow-derived cell transplantation in talar osteochondral lesions. Clin. Orthop. Relat. Res. 2009, 467, 3307–3320. [Google Scholar] [CrossRef]
- Buda, R.; Vannini, F.; Cavallo, M.; Grigolo, B.; Cenacchi, A.; Giannini, S. Osteochondral lesions of the knee: A new one-step repair technique with bone-marrow-derived cells. J. Bone Joint Surg. Am. 2010, 92 (Suppl. 2), 2–11. [Google Scholar] [CrossRef]
- Gobbi, A.; Whyte, G.P. One-Stage Cartilage Repair Using a Hyaluronic Acid-Based Scaffold with Activated Bone Marrow-Derived Mesenchymal Stem Cells Compared With Microfracture: Five-Year Follow-up. Am. J. Sports Med. 2016, 44, 2846–2854. [Google Scholar] [CrossRef]
- Gobbi, A.; Scotti, C.; Karnatzikos, G.; Mudhigere, A.; Castro, M.; Peretti, G.M. One-step surgery with multipotent stem cells and Hyaluronan-based scaffold for the treatment of full-thickness chondral defects of the knee in patients older than 45 years. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 2494–2501. [Google Scholar] [CrossRef]
- Dhollander, A.A.; De Neve, F.; Almqvist, K.F.; Verdonk, R.; Lambrecht, S.; Elewaut, D.; Verbruggen, G.; Verdonk, P.C. Autologous matrix-induced chondrogenesis combined with platelet-rich plasma gel: Technical description and a five pilot patients report. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Enea, D.; Cecconi, S.; Calcagno, S.; Busilacchi, A.; Manzotti, S.; Gigante, A. One-step cartilage repair in the knee: Collagen-covered microfracture and autologous bone marrow concentrate. A pilot study. Knee 2015, 22, 30–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gigante, A.; Calcagno, S.; Cecconi, S.; Ramazzotti, D.; Manzotti, S.; Enea, D. Use of collagen scaffold and autologous bone marrow concentrate as a one-step cartilage repair in the knee: Histological results of second-look biopsies at 1 year follow-up. Int. J. Immunopathol. Pharmacol. 2011, 24, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Giannini, S.; Buda, R.; Battaglia, M.; Cavallo, M.; Ruffilli, A.; Ramponi, L.; Pagliazzi, G.; Vannini, F. One-step repair in talar osteochondral lesions: 4-year clinical results and t2-mapping capability in outcome prediction. Am. J. Sports Med. 2013, 41, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, A.; Karnatzikos, G.; Scotti, C.; Mahajan, V.; Mazzucco, L.; Grigolo, B. One-Step Cartilage Repair with Bone Marrow Aspirate Concentrated Cells and Collagen Matrix in Full-Thickness Knee Cartilage Lesions: Results at 2-Year Follow-up. Cartilage 2011, 2, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, J.; Skowronski, R.; Rutka, M. Large cartilage lesions of the knee treated with bone marrow concentrate and collagen membrane—Results. Ortop. Traumatol. Rehabil. 2013, 15, 69–76. [Google Scholar] [CrossRef]
- Kenney, E.B.; Lekovic, V.; Han, T.; Carranza, F.A., Jr.; Dimitrijevic, B. The use of a porous hydroxylapatite implant in periodontal defects. I. Clinical results after six months. J. Periodontol. 1985, 56, 82–88. [Google Scholar] [CrossRef]
- Guarnieri, R.; Pecora, G.; Fini, M.; Aldini, N.N.; Giardino, R.; Orsini, G.; Piattelli, A. Medical grade calcium sulfate hemihydrate in healing of human extraction sockets: Clinical and histological observations at 3 months. J. Periodontol. 2004, 75, 902–908. [Google Scholar] [CrossRef]
- Hernigou, P.; Dubory, A.; Pariat, J.; Potage, D.; Roubineau, F.; Jammal, S.; Flouzat Lachaniette, C.H. Beta-tricalcium phosphate for orthopedic reconstructions as an alternative to autogenous bone graft. Morphologie 2017, 101, 173–179. [Google Scholar] [CrossRef]
- Guillaume, B. Filling bone defects with beta-TCP in maxillofacial surgery: A review. Morphologie 2017, 101, 113–119. [Google Scholar] [CrossRef]
- Nooeaid, P.; Salih, V.; Beier, J.P.; Boccaccini, A.R. Osteochondral tissue engineering: Scaffolds, stem cells and applications. J. Cell Mol. Med. 2012, 16, 2247–2270. [Google Scholar] [CrossRef] [PubMed]
- Temenoff, J.S.; Mikos, A.G. Review: Tissue engineering for regeneration of articular cartilage. Biomaterials 2000, 21, 431–440. [Google Scholar] [CrossRef]
- Buda, R.; Vannini, F.; Cavallo, M.; Baldassarri, M.; Natali, S.; Castagnini, F.; Giannini, S. One-step bone marrow-derived cell transplantation in talarosteochondral lesions: Mid-term results. Joints 2013, 1, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Etulain, J.; Mena, H.A.; Meiss, R.P.; Frechtel, G.; Gutt, S.; Negrotto, S.; Schattner, M. An optimised protocol for platelet-rich plasma preparation to improve its angiogenic and regenerative properties. Sci. Rep. 2018, 24, 1513. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Jo, S.; Mikos, A.G. Biomimetic materials for tissue engineering. Biomaterials 2003, 24, 4353–4364. [Google Scholar] [CrossRef]
| Tissue | Study | System | Anticoagulant | Classification * | Findings |
|---|---|---|---|---|---|
| Cartilage | Sanchez et al., 2008 [25] | Manual 640× g/8 min | Sodium citrate | II1 | (+) significant improvement of pain |
| Kon et al., 2010 [26] | Manual 1800 rpm/15 min 3500 rpm/10 min | Sodium citrate | IIB1 | (+) reduction of pain and improvement of knee function in younger patients with low degree of articular degeneration | |
| Kon et al., 2011 [27] | Manual 1480 rpm/6 min 3400 rpm/15 min | DNS | IIB1 | (+) PRP greater and longer efficacy than HA injection | |
| Lee et al., 2013 [28] | Magellan Autologous Platelet Separator (Medtronic Biologic Therapeutics and Diagnostics) | Sodium citrate | IB2 | (+) significant improvement in clinical results in early OA. | |
| Patel et al., 2013 [29] | Manual 1500 rpm/15 min Leukocyte filtered | Citrate phosphate dextrose | PRP-IIA1 | (+) effective to alleviate symptoms in early knee OA | |
| Duif et al., 2015 [30] | ACP-system (Arthrex) 1500 rpm/5 min | DNS | 2 | (+) pain reduction, gain knee function | |
| Filardo et al., 2015 [31] | Manual 1480 rpm/6 min 3400 rpm/15 min | DNS | Red-L-PRP-IIB1 | (=) PRP do not provide a superior clinical improvement when compared to HA | |
| Sanchez al, 2016 [32] | Manual 580× g/8 min | Sodium citrate | PRP-IIA1 | (+) multiple injections of PRP are useful in achieving better clinical results in early OA | |
| Gormeli et al., 2017 [33] | Manual 1500 rpm/6 min 3500 rpm/12 min | DNS | Red-L-PRP-IIB1 | (+) multiple injections of PRP are useful in achieving better clinical results in early OA | |
| Kaminski et al., 2018 [34] | DNS | DNS | II | (+) increased meniscus repair | |
| Kaminski et al., 2019 [35] | Manual 900 rpm/9 min 3200 rpm/15 min | DNS | IIB1 | (+) significant improvement in the rate of chronic meniscal tear healing | |
| Bone | Marx et al., 1998 [36] | Manual 5600 rpm and 2400 rpm | Citrate Dextrose | Red-L-PRP-IIB1 | (+) enhanced bone graft in mandibular fracture |
| Rodriguez et al., 2003 [37] | Smart Prep (Harvest Technologies) | DNS | II2 | (+) effective in maxillary sinus augmentation | |
| Daif et al., 2012 [38] | Manual 1200 rpm/20 min 2000 rpm/15 min | Sodium citrate | Red-L-PRP-IIB1 | (+) enhanced bone regeneration in mandibular fracture | |
| Anitua et al., 2015 [39] | Manual 580× g/8 min | Sodium citrate | I1 | (+) enhanced healing of extraction socket | |
| Malhotra et al., 2015 [40] | DNS | DNS | PRPIB | (+) fracture healing acceleration in nonunion fractures | |
| Tabrizi et al., 2015 [41] | Manual 800× g/5 min 1500× g/5 min | Citrate phosphate dextrose | II1 | (+) enhanced bone formation in bone cavity | |
| Ghaffarpasand et al., 2016 [42] | Gravitational Platelet Separation System (GPS III; BIOMET) 3200 rpm/15 min | Acid-citrate dextrose | B2 | (+) higher cure rate and less pain in non-union fractures | |
| Castillo-Cardiel et al., 2017 [43] | PRGF System III (BTI) 450× g/8 min | Sodium citrate | II2 | (+) increase of bone intensity and density in mandibular fractures | |
| Acosta-Olívio et al., 2017 [44] | Manual 1800 rpm/5 min 3200 rpm/3 min | Sodium citrate | II1 | (+) earlier bone consolidation in shaft fractures | |
| Muscle | Hamid et al., 2014 [45] | GPS III | DNS | Red-L-PRP-IIB2 | (+) PRP combined with rehabilitation program was more effective in treating hamstring injuries than rehabilitation program alone |
| Hamilton et al., 2015 [23] | GPS III 3200 rpm/15 min | Citrate dextrose | Red-L-PRPIB2 | (−) no benefit of PRP injection compared to rehabilitation in athletes | |
| Reurink et al., 2015 [24] | ACP-system | EDTA | PRP-IA2 | (−) no benefit of PRP injections compared with placebo in patients with acute hamstring | |
| Bubnov et al., 2016 [46] | Manual DNS | DNS | 1 | (+) injections of PRP under ultrasound guidance had higher level of pain relief, physical recovery, and faster regeneration compared with conventional conservative treatment in acute muscle trauma in professional athletes | |
| Martinez-zapata et al., 2016 [47] | Platelet apheresis | DNS | PRP-IIB3 | (−) PRP did not improve the time to healing compared to that in the control group | |
| Rossi et al., 2017 [48] | Manual 1400 rpm/3 min 3000 rpm/4 min | EDTA | I1 | (+) PRP injection combined with a rehabilitation program me shortened time to return to sports compared to a rehabilitation programme only |
| Tissue | Study | BMAC preparation | # Progenitor Cells Injected | Follow-up | Findings |
|---|---|---|---|---|---|
| Cartilage | Kim et al., 2014 [58] | SmartPReP2 Bone Marrow Procedure Pack BMAC2 kits (Harvest Technology) | DNS | 12 months | (+) 41 patients injected with BMAC. VAS showed significant pre- to postoperative improvement. All functional scores were increased after the procedure. Better outcomes were obtained in early to moderate stages of OA than more advanced stages. (OA of the knees) |
| Shapiro et al., 2016 [60] | Filtered 170 µm Magellan Autologous Platelet Separator System (Arteriocyte) | MSC = 3.44 × 104 HSC = 4.62 × 106 | 6 months | (=) 25 patients, 13 were injected with BMAC in their right knee and placebo in the left knee and 12 received the opposite. No differences were observed between placebo and treated knee. (OA of the knees) | |
| Desandis et al., 2017 [64] | DNS | DNS | 16.7 months | (+) 46 patients treated with JACI–BMAC were retrospectively evaluated. The mean questionnaire SF-12v2 and FAOS improved significantly from pre- to postoperatively. Of the 46 patients in the study, 22 had postoperative MRI scans that could be scored. MOCART score was 46.8. (Osteochondral Lesions of the Talus) | |
| Shapiro et al., 2018 [61] | Filtered 170 µm Magellan Autologous Platelet Separator System (Arteriocyte) | MSC = 3.44 × 104 HSC = 4.62 × 106 | 12 months | (=) 25 patients, 13 were injected with BMAC in their right knee and placebo in the left knee and 12 received the opposite. BMAC did not show superior results compared to saline group. (OA of the Knees) | |
| Themistocleous et al., 2018 [59] | 2800 rpm/15 min | DNS | 11 months | (+) 121 patients treated with BMAC were retrospectively evaluated. NPS decreased 8.33 preoperatively to 4.49 postoperatively (p < 0.001). The mean Oxford knee score (OKS) increased from 20.20 pre-operatively to 32.92 postoperatively (p < 0.001). (OA of the Knees) | |
| Karnovsky et al., 2018 [63] | Magellan Autologous Platelet Separator (Anteriocyte Medical Systems) | DNS | 28.1 months | (−) 30 patients treated MF and 20 who received JACI-BMAC were retrospectively evaluated. Both treatments showed significant pre- to postoperative improvements in all FAOS subscale. MF showed a significant improvement in VAS. Average osteochondral lesion diameter was significantly larger in JACI-BMAC group compared to MF group. (Osteochondral Lesions of the Talus) | |
| Bone | Hernigou et al., 2002 [65] | Cell separator (Cobe 2991) 400× g/5 min | CFU-F = 25 × 103 cells | 7 years | (+) 116 patients (189 hips) injected with BMAC after core decompression with a small trocar. Total hip replacement was needed in 34 hips (22 patients) among 189 hips treated. Patients with a higher number of progenitor cells transplanted had better outcomes (ONFH) |
| Hernigou et al., 2005 [66] | Cell separator (Cobe 2991) 1200× g/5 min | CFU-F = 5.1 × 104 cells Progenitors = 5.49 × 104 (53 patients) 1.93 × 104 (7 patients) | 4 months | (+) Bone union was obtained in 53 of the 60 patients that received the higher number of progenitor cells. The BMAC efficacy is related to the number of progenitors in the graft. (Nonunions) | |
| Tabatabaee et al., 2015 [67] | Bone marrow was filtered and washed. Then was centrifuged for 400g/5–10 min | NC = 4.76 × 103 cells | 24 months | (+) 28 hips were randomized in 2 groups of core decompression with and without BMAC. The mean WOMAC and VAS scores in all patients improved significantly (p < 0.001). MRI showed a significant improvement in group treated with BMAC (p = 0.046) and significant worsening in the non-treated group (p < 0.001). (ONFH) | |
| Hauzeur et al., 2018 [68] | Spectra cell separator (777,006,300; Cobe) | NC = 3.46 × 109 cells CFU-F = 3.46 × 106 NC | 24 months | (=) Double blind RCT study comparing two groups: core decompression plus saline injection or core decompression plus BMAC implantation. Both groups included 19 patients (23 hips). No differences were observed between groups for THR requirements, clinical evaluation and radiological evolution. In both groups, 15/23 hips needed THR. (ONFH) |
| Tissue | Study | Biomaterial | Formulation | Preparation (PRP/BMAC) | Follow-up | Findings |
|---|---|---|---|---|---|---|
| Bone | Dallari et al., 2007 [103] | Bone Chip | Scaffold | PRP/BMAC | 1 year | (=) No significant difference between PRP/BMAC groups and empty lyophilized bone controls as all patients reported relieved knee pain and full range of motion. |
| Sauerbier et al., 2010 [106] | Bovine Bone Mineral | Particles | BMAC | 4 months | (=) New bone formation was 19.9% but not significantly different from the synthetic polysaccharide isolation method control. | |
| Jager et al., 2011 [104] | Collagen | Sponge | BMAC | 1 year | (+) Radiography showed significant bone remodeling in all groups, but healing was longer when compared to BMAC/hydroxyapatite controls. | |
| Yassibag-Berkman et al., 2007 [107] | β-TCP | Slurry | PRP | 1 year | (=) No significant difference between clinical parameters in PRP and control groups | |
| Attia et al., 2010 [108] | β-TCP | Slurry | PRP | 1 year | (+) Significant reduction of probing depth and increase in clinical attachment gain (p < 0.01) | |
| Saini et al., 2011 [109] | β-TCP | Slurry | PRP | 9 months | (+) Significant decrease in pocket depth and increase in clinical attachment (p < 0.05) in β TCP/PRP compared to β TCP alone | |
| Özdemir et al., 2012 [110] | β-TCP | Slurry | PRP | 6 months | (=) All 6 parameters evaluating clinical outcome were not significant between PRP and control groups (p < 0.05) | |
| Okuda et al., 2005 [111] | HAp | Scaffold | PRP | 1 year | (+/−) There were significant differences in gingival index, bleeding on probing, probing depth and clinical attachment level, in PRP groups compared baseline (p < 0.001), and significant differences in PRP versus control groups in probing depth, clinical attachment gain, and vertical attachment (p < 0.05) No significant difference in defect change between PRP scaffolds versus saline scaffolds, however, positive significant gain was seen compared to baseline measurements (p < 0.01) | |
| Vaishnavi et al., 2011 [112] | HAp | Scaffold | PRP | 1 year | (=) Radiographic evaluation showed bone regeneration in all groups (scaffold, PRP, scaffold with PRP) except the negative control | |
| Menezes et al., 2012 [113] | HAp | Scaffold | PRP | 4 years | (+) Significant differences in defect fill were seen in PRP/hydroxyapatite treated group compared to hydroxyapatite/saline control (p < 0.001) | |
| Kutkut et al., 2012 [114] | MGCSH | Scaffold | PRP | 3 months | (+) Radiographic evaluation confirmed more dense bone in MGCSH and PRP groups compared to empty MGCSH, and histomorphic analysis showed a statistically significant difference between these groups (p < 0.05) | |
| Cartilage | Siclari et al., 2012, 2014 [81,115,116] | PLGA-HA | Matrix | PRP | 1–5 years | (+) Histology showed homogenous repair tissues and good integration of repair tissues to the subchondral bone and adjacent cartilage and immunohistochemistry showed signs of hyaline like cartilage formation. At 2 years, hyaline-to hyaline cartilage repair tissue that was rich with a chondrocyte morphology, proteoglycans, and type-II collagen. At 4 years, MRI confirmed good defect and volume filling in 20 of 21 patients and received high MOCART scores. |
| Siclari et al., 2018 [117] | PLGA-HA | Matrix | PRP | 2 years | (+) AOFAS rating increased significantly (p < 0.01). ROM increased significantly (p < 0.01). | |
| Enea et al., 2013 [118] | PLGA-HA | Matrix | BMAC | 2 years | (+) MRIs showed all patients had complete defect and volume filling and resurfacing of articular cartilage to previous cartilage level. Some bone marrow edema and subchondral irregularities were observed—as well subchondral irregularities. | |
| Giannini et al., 2009 [119] | HA | Membrane | BMAC | 2 years | (+) MRI done 12 months postoperatively showed tissue regeneration in all 48 patients. Integration with the healthy cartilage was complete, and transition zones were smooth in all patients. Immunohistologic results confirmed new cartilaginous tissues with varied hyaline cartilage tissue remodeling. | |
| Buda et al., 2010 [120] | HA | Scaffold | BMAC | 2 years | (+) Post-treatment IKDC and KOOS scores were significantly higher than pre-treatment (p < 0.0005). MRIs taken at 1 and 2 years after treatment shown subchondral bone and cartilage regeneration. Histological analysis showed a proteoglycan rich matrix and collagen II throughout the regenerated tissue. | |
| Gobbi et al., 2016 [121] | HA | Scaffold | BMAC | 5 years | (+) All HA-BMAC scaffold treated patients maintained classification as normal or nearly normal by IKDC, KOOS, Tegner, and Lysholm. No quality of repair studies were done in this study. | |
| Gobbi et al., 2017 [122] | HA | Scaffold | BMAC | 4 years | (+) KOOS, IDKC, Tegner, and VAS scores were significantly improved in both the over and under 45 year-old groups. MRI determined 80% defect filling in the over 45 group and 71% defect filling in the under 45 group, and histology on 3 and 2 patients from these groups, respectively, showed good tissue repair with varying amounts of hyaline-like tissue. | |
| Dhollander et al., 2011 [123] | Collagen | Scaffold | PRP | 2 years | (+) VAS, Tegner, Kujala patellofemoral, and KOOS scores showed improvement. MRI data showed incomplete filling in 3/5 patients, hypertrophy in 2/5 patients. Complete integration with adjacent was observed in all patients, but the surface of the repair tissue was irregular in all patients during 1 year and 2 year post-operative scans. MOCART scores remained stable during the 2 year period. | |
| Enea et al., 2015 [124] | Collagen | Membrane | BMAC | 2 years | (+/−) Significant (p < 0.05) IKDC subjective score improvement, Lysholm score, VAS, and activity level pre vs. post-operative, but no change in Tegner score. MRI scans taken between 6–9mo after surgery showed reconstitution of original cartilage levels, bone marrow edema and/or subchondral; irregularities for all cases. Histologically, only 1/5 had hyaline-like matrix, and that matrix did not exhibit cell arrangements of normal articular cartilage. | |
| Gigante et al., 2012 [82] | Collagen | Membrane | BMAC | 2 year | (+) Full weight bearing in 6 weeks, jogging at 6 months, continuously asymptomatic at 24 months. MRI scan at 12 months showed good defect filling with tissue signal signals similar to that or surrounding tissue without signs of bone marrow edema. | |
| Gigante et al., 2011 [125] | Collagen | Scaffold | BMAC | 1 year | (+/−) All 5 patients self-reported themselves as asymptomatic. Mean histological scores of 59.8(SD 14.5). 1 had hyaline-like cartilage,3 had hyaline/fibrocartilage, and 1 had fibrocartilage formation. Columnar structures of normal articular cartilage were not observed in any case. | |
| Giannini et al., 2013 [126] | Collagen | Scaffold | BMAC | 4 years | (+/−) AOFAS score improved significantly (p < 0.0005) at 24mo, but decreased significantly between 24mo and 36mo (p < 0.001), and 24mo to 48mo (p < 0.005). MRI T2 mapping analysis showed that regenerated tissue has similar values to that of hyaline cartilage −9/20 had complete defect filling and 13/20 had tissue integration at the border zone. However, a majority of patients also had damaged subchondral lamina, disrupted subchondral bone, and subchondral edema. | |
| Gobbi et al., 2011 [127] | Collagen | Matrix | BMAC | 2 years | (+) Visual analog scale (VAS), IKDC, KOOS, Lysholm, Marx, SF-36, and Tegner scores all showed significant improvement after the final follow-up of 15 patients (p < 0.005). MRI T2 and histology showed hyaline like cartilage formation and complete defect filling of 12 of 15 patients with no signs of hypertrophy. Integration with adjacent cartilage was complete in 14 of those same patients. | |
| Krych et al., 2016 [105] | PLGA | Scaffold | PRP/BMAC | 1 year | (+) No subjective clinical outcome measures were included in 11 control patients, 23 PRP treated patients, and 12 BMAC treated patients. PRP and BMAC patients both had significantly (p < 0.002, p < 0.03) better fill than the control and was more hyaline like as determined my MRI T2 mapping. | |
| Skowronski et al. 2013 [128] | Collagen | Membrane | BMAC | 5 years | (+) An improvement was observed in 52 out of 54 patients in all scales (KOOS, Lysholm, VAS, KOOs pain) after comparison between Preoperative and 12 months post-operatively. No differences were observed between 12 months and 5 years after surgery. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, F.S.M.; Shams, S.; Silva, E.A.; Stilhano, R.S. PRP and BMAC for Musculoskeletal Conditions via Biomaterial Carriers. Int. J. Mol. Sci. 2019, 20, 5328. https://doi.org/10.3390/ijms20215328
Yamaguchi FSM, Shams S, Silva EA, Stilhano RS. PRP and BMAC for Musculoskeletal Conditions via Biomaterial Carriers. International Journal of Molecular Sciences. 2019; 20(21):5328. https://doi.org/10.3390/ijms20215328
Chicago/Turabian StyleYamaguchi, Fabio S. M., Shahin Shams, Eduardo A. Silva, and Roberta S. Stilhano. 2019. "PRP and BMAC for Musculoskeletal Conditions via Biomaterial Carriers" International Journal of Molecular Sciences 20, no. 21: 5328. https://doi.org/10.3390/ijms20215328