Hydrogen Sulfide-Mediated Activation of O-Acetylserine (Thiol) Lyase and l/d-Cysteine Desulfhydrase Enhance Dehydration Tolerance in Eruca sativa Mill
Abstract
:1. Introduction
2. Results
2.1. Leaf Relative Water Content (LRWC), Rate of Water Loss, Electrolyte Leakage, and Thiobarbituric Acid Reactive Substances (TBARS)
2.2. Hydrogen Peroxide (H2O2) and Superoxide (O2•−) Content
2.3. Proline (Pro) and Glycine Betaine (GB) Content
2.4. Activities of Antioxidant Enzymes
2.5. Activity of O-Acetylserine (Thiol) Lyase (OAS-TL) Enzyme and Cysteine (Cys) Content
2.6. Activities of LCD and DCD Enzymes and H2S Content
2.7. Photosynthetic Pigments and Carbonic Anhydrase (CA) Activity
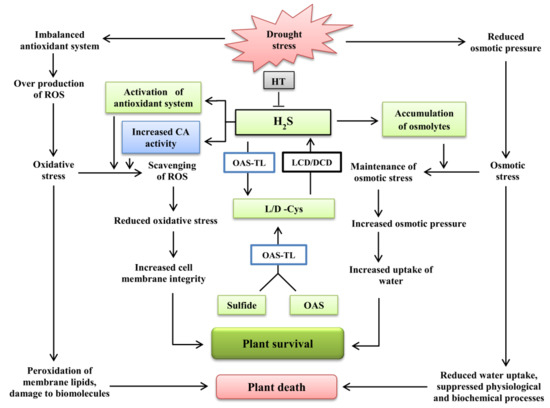
3. Discussion
4. Materials and Methods
4.1. Plant Culture and Treatments
4.2. Estimation of Leaf Relative Water Content (LRWC), Electrolyte Leakage, and Rate of Water Loss
4.3. Detection of Hydrogen Peroxide (H2O2) and Superoxide (O2•−) in Roots
4.4. Estimation of H2O2 and O2•− Content
4.5. Analysis of Lipid Peroxidation
4.6. Determination of Proline (Pro) and Glycine Betaine (GB) Content
4.7. Assay of Antioxidant Enzymes
4.8. Determination of OAS-TL Enzyme Activity and Cys Content
4.9. Measurement of LCD and DCD Enzyme Activities and H2S Content
4.10. Estimation of Photosynthetic Pigments and Carbonic Anhydrase (CA) Activity
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farooq, M.; Gogoi, N.; Barthakur, S.; Baroowa, B.; Bharadwaj, N.; Alghamdi, S.S.; Siddique, K.H.M. Drought stress in grain legumes during reproduction and grain filling. J. Agron. Crop Sci. 2017, 203, 81–102. [Google Scholar] [CrossRef]
- Salehi-Lisar, S.Y.; Bakhshayeshan-Agdam, H. Drought Stress in Plants: Causes, Consequences, and Tolerance. In Drought Stress Tolerance in Plants; Hossain, M., Wani, S., Bhattacharjee, S., Burritt, D., Tran, L.S., Eds.; Springer: Cham, Switzerland, 2016; Volume 1, pp. 1–16. [Google Scholar]
- FAO. The Impact of Disasters and Crises on Agriculture and Food Security; Food and Agriculture Organization of The United Nations: Rome, Italy, 2018. [Google Scholar]
- Siddiqui, M.H.; Al-Khaishany, M.Y.; Al-Qutami, M.A.; Al-Whaibi, M.H.; Grover, A.; Ali, H.M.; Al-Wahibi, M.S.; Bukhari, N.A. Response of different genotypes of faba bean plant to drought stress. Int. J. Mol. Sci. 2015, 15, 10214–10227. [Google Scholar] [CrossRef] [PubMed]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Dat, J.; Vandenabeele, S.; Vranová, E.; Van Montagu, M.; Inzé, D.; Van Breusegem, F. Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life Sci. 2000, 57, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef]
- Signorelli, S.; Corpas, F.J.; Borsania, O.; Barroso, J.B.; Monza, J. Water stress induces a differential and spatially distributed nitro-oxidative stress response in roots and leaves of Lotus japonicas. Plant Sci. 2013, 201–202, 137–146. [Google Scholar] [CrossRef]
- Signorelli, S.; Corpas, F.J.; Rodríguez-Ruiz, M.; Valderrama, R.; Barroso, J.B.; Borsani, O.; Monza, J. Drought stress triggers the accumulation of NO and SNOs in cortical cells of Lotus japonicus L. roots and the nitration of proteins with relevant metabolic function. Environ. Exp. Bot. 2018. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef]
- Parry, M.A.J.; Androlojc, P.J.; Khan, S.; Lea, P.J.; Keys, A.J. Rubisco activity: Effects of drought stress. Ann. Bot. 2002, 89, 833–839. [Google Scholar] [CrossRef]
- Bota, J.; Medrano, H.; Flexas, J. Is photosynthesis limited by decreased Rubisco acivity and RuBP content under progressive water stress? New Phytol. 2004, 162, 671–681. [Google Scholar] [CrossRef]
- Rahdari, P.; Hoseini, S.M. Drought stress: A review. Int. J. Agron. Plant Prod. 2012, 3, 443–446. [Google Scholar]
- Levitt, J. Responses of Plants to Environmental Stresses; Academic Press: New York, NY, USA, 1972. [Google Scholar]
- Morgan, J.M. Osmoregulation and water stress in higher plants. Annu. Rev. Plant Physiol. 1984, 35, 299–319. [Google Scholar] [CrossRef]
- Bartoli, C.G.; Simontacchi, M.; Tambussi, E.; Beltrano, J.; Montaldi, E.; Puntarulo, S. Drought and watering-dependent oxidative stress: Effect on antioxidant content in Triticum aestivum L. leaves. J. Exp. Bot. 1999, 50, 375–385. [Google Scholar] [CrossRef]
- Peñuelas, J.; Munné-Bosch, S.; Llusià, J.; Filella, I. Leaf reflectance and photo- and antioxidant protection in field-grown summer-stressed Phillyrea angustifolia. Optical signals of oxidative stress? New Phytol. 2004, 162, 115–124. [Google Scholar] [CrossRef]
- Wilson, L.G.; Bressan, R.A.; Filner, P. Light-dependent emission of hydrogen sulfide from plants. Plant Physiol. 1978, 61, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Rennenberg, H.; Huber, B.; Schroder, P.; Stahl, K.; Haunold, W.; Georgii, H.W.; Slovik, S.; Pfanz, H. Emission of volatile sulfur compounds from spruce trees. Plant Physiol. 1990, 92, 560–564. [Google Scholar] [CrossRef]
- Calderwood, A.; Kopriva, S. Hydrogen sulfide in plants: From dissipation of excess sulfur to signaling molecule. Nitric Oxide 2014, 41, 72–78. [Google Scholar] [CrossRef]
- Hancock, J.T.; Whiteman, M. Hydrogen sulfide and cell signaling: Team player or referee? Plant Physiol. Biochem. 2014, 78, 37–42. [Google Scholar] [CrossRef]
- Zhang, L.; Pei, Y.; Wang, H.; Jin, Z.; Liu, Z.; Qiao, Z.; Fang, H.; Zhang, Y. Hydrogen sulfde alleviates cadmiuminduced cell death through restraining ROS accumulation in roots of Brassica rapa L. ssp. pekinensis. Oxid. Med. Cell. Longev. 2015, 2015, 804603. [Google Scholar] [CrossRef]
- da-Silva, C.J.; Modolo, L.V. Hydrogen sulfide: A new endogenous player in an old mechanism of plant tolerance to high salinity. Acta Bot. Bras. 2017, 32, 150–160. [Google Scholar] [CrossRef]
- Khan, A.; Anwar, Y.; Hasan, M.M.; Iqbal, A.; Ali, M.; Alharby, H.F.; Hakeem, K.R.; Hasanuzzaman, M. Attenuation of drought stress in brassica seedlings with exogenous application of Ca2+ and H2O2. Plants 2017, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hu, S.L.; Zhang, Z.J.; Hua, L.Y.; Jiang, C.X.; Wei, Z.J.; Liu, J.; Wang, H.L.; Jiang, S.T. Hydrogen sulfide acts as a regulator of flower senescence in plants. Postharvest Biol. Technol. 2011, 60, 251–257. [Google Scholar] [CrossRef]
- Li, Z.G.; Gong, M.; Liu, P. Hydrogen sulfide is a mediator in H2O2-induced seed germination in Jatropha curcas. Acta Physiol. Plant. 2012, 34, 2207–2213. [Google Scholar] [CrossRef]
- Li, Z.G.; Ding, X.J.; Du, P.F. Hydrogen sulfide donor sodium hydrosulfide improved heat tolerance in maize and involvement of proline. J. Plant Physiol. 2013, 170, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Al-Whaibi, M.H.; Siddiqui, M.H.; Al-Munqadhi, B.M.A.; Sakran, A.M.; Ali, H.M.; Basalah, M.O. Influence of plant growth regulators on growth performance and photosynthetic pigments status of Eruca sativa Mill. J. Med. Plants Res. 2012, 6, 1948–1954. [Google Scholar]
- Lamy, E.; Schröder, J.; Paulus, S.; Brenk, P.; Stahl, T.; Mersch-Sundermann, V. Antigenotoxic properties of Eruca sativa (rocket plant), erucin and erysolin in human hepatoma (HepG2) cells towards benzo(a)pyrene and their mode of action. Food Chem. Toxicol. 2008, 46, 2415–2421. [Google Scholar] [CrossRef] [PubMed]
- Alqasoumi, S.; Al-Howiriny, T.A.; Al-Yahya, M.; Rafatullah, S. Gastroprotective effects of radish “Raphanus sativus” L. on experimental gastric ulcer models in rats. Farmacia 2008, 46, 204–214. [Google Scholar]
- Alqasoumi, S.; Al-Sohaibani, M.; Al-Howiriny, T.; Al-Yahya, M.; Rafatullah, S. Rocket “Eruca sativa”: A salad herb with potential gastric anti-ulcer activity. World J. Gastroenterol. 2009, 15, 1958–1965. [Google Scholar] [CrossRef]
- Miyazawa, M.; Maehara, T.; Kurose, K. Composition of the essential oil from the leaves of Eruca sativa. Flavour Fragr. J. 2002, 17, 187–190. [Google Scholar] [CrossRef]
- Chaves, M.M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.L.; Ricardo, C.P.P.; Osorio, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How plants cope with water stress in the field. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.H.; Khan, M.N.; Mohammad, F.; Khan, M.M.A. Role of nitrogen and gibberellin (GA3) in the regulation of enzyme activities and osmoprotectant accumulation in Brassica juncea L. under salt stress. J. Agron. Crop Sci. 2008, 194, 214–224. [Google Scholar] [CrossRef]
- Khan, M.N.; Siddiqui, M.H.; Mohammad, F.; Naeem, M.; Khan, M.M.A. Calcium chloride and gibberellic acid protect linseed (Linum usitatissimum L.) from NaCl stress by inducing antioxidative defence system and osmoprotectant accumulation. Acta Physiol. Plant. 2010, 32, 121–132. [Google Scholar] [CrossRef]
- Ma, D.; Ding, H.; Wang, C.; Qin, H.; Han, Q.; Hou, J.; Lu, H.; Xie, Y.; Guo, T. Alleviation of drought stress by hydrogen sulfide is partially related to the abscisic acid signaling pathway in wheat. PLoS ONE 2016, 11, e0163082. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.G.; Zhu, L.P. Hydrogen sulfide donor sodium hydrosulfide-induced accumulation of betaine is involved in the acquisition of heat tolerance in maize seedlings. Braz. J. Bot. 2015, 38, 31–38. [Google Scholar] [CrossRef]
- Luo, Z.; Li, D.; Du, R.; Mou, W. Hydrogen sulfide alleviates chilling injury of banana fruit by enhanced antioxidant system and proline content. Sci. Hortic. 2015, 183, 144–151. [Google Scholar] [CrossRef]
- Jin, Z.P.; Xue, S.W.; Luo, Y.N.; Tian, B.H.; Fang, H.H.; Li, H. Hydrogen sulfide interacting with abscisic acid in stomatal regulation responses to drought stress in Arabidopsis. Plant Physiol. Biochem. 2013, 62, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Chen, S.; Liu, D.; Liesche, J.; Shi, C.; Wang, J.; Ren, M.; Wang, X.; Yang, J.; Shi, W.; et al. Ethylene-induced hydrogen sulfide negatively regulates ethylene biosynthesis by persulfidation of ACO in tomato under osmotic stress. Front. Plant Sci. 2018, 9, 1517. [Google Scholar] [CrossRef] [PubMed]
- Olivella, C.; Vendrell, M.; Save, R. Abscisic acid and ethylene content in Gerbera jamesonii plants submitted to drought and rewatering. Biol. Plant. 1998, 41, 613–616. [Google Scholar] [CrossRef]
- García-Mata, C.; Lamattina, L. Hydrogen sulphide, a novel gasotransmitter involved in guard cell signalling. New Phytol. 2010, 188, 977–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Shang, Y.-T.; Wang, W.-H.; Chen, X.-Y.; He, E.-M.; Zheng, H.-L.; Shangguan, Z. Hydrogen sulfide-mediated polyamines and sugar changes are involved in hydrogen sulfide-induced drought tolerance in Spinacia oleracea seedlings. Front. Plant Sci. 2016, 7, 1173. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Singh, V.P.; Singh, S.; Kumar, J.; Prasad, S.M. Hydrogen sulfide alleviates toxic effects of arsenate in pea seedlings through up-regulation of the ascorbate-glutathione cycle: Possible involvement of nitric oxide. J. Plant Physiol. 2015, 181, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ye, T.; Chan, Z. Exogenous application of hydrogen sulfide donor sodium hydrosulfide enhanced multiple abiotic stress tolerance in bermudagrass (Cynodon dactylon (L). Pers.). Plant Physiol. Biochem. 2013, 71, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.N.; Wang, W.J.; Hou, L.X.; Liu, X. Hydrogen sulfide is involved in the chilling stress response in Vitis vinifera L. Acta Soc. Bot. Pol. 2013, 82, 295–302. [Google Scholar] [CrossRef]
- Matern, S.; Peskan-Berghoefer, T.; Gromes, R.; Kiesel, R.V.; Rausch, T. Imposed glutathione-mediated redox switch modulates the tobacco wound-induced protein kinase and salicylic acid-induced protein kinase activation state and impacts on defence against Pseudomonas syringae. J. Exp. Bot. 2015, 66, 1935–1950. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.; Bermúdez, M.A.; Romero, L.C.; Gotor, C.; García, I. Cysteine homeostasis plays an essential role in plant immunity. New Phytol. 2012, 193, 165–177. [Google Scholar] [CrossRef]
- Romero, L.C.; Aroca, M.A.; Laureano-Marín, A.M.; Moreno, I.; García, I.; Gotor, C. Cysteine and cysteine-related signaling pathways in Arabidopsis thaliana. Mol. Plant 2014, 7, 264–276. [Google Scholar] [CrossRef]
- Rennenberg, H.; Arabatzis, N.; Grundel, I. Cysteine desulphydrase activity in higher plants: Evidence for the action of L- and D-cysteine specific enzymes. Phytochemistry 1987, 26, 583–589. [Google Scholar] [CrossRef]
- Papenbrock, J.; Riemenschneider, A.; Kamp, A.; Schulz-Vogt, H.N.; Schmidt, A. Characterization of cysteine-degrading and H2S-releasing enzymes of higher plants—From the field to the test tube and back. Plant Biol. (Stuttg.) 2007, 9, 582–588. [Google Scholar] [CrossRef]
- Shi, H.; Ye, T.; Han, N.; Bian, H.; Liu, X.; Chan, Z. Hydrogen sulfide regulates abiotic stress tolerance and biotic stress resistance in Arabidopsis. J. Integr. Plant Biol. 2015, 57, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-Y.; Yu, H.-Y.; Kong, D.-S.; Yan, F.; Zhang, Y.-J. Effects of drought stress on growth and chlorophyll fluorescence of Lycium ruthenicum Murr. seedlings. Photosynthetica 2016, 54, 524–531. [Google Scholar] [CrossRef]
- Mafakheri, A.; Siosemardeh, A.; Bahramnejad, B.; Struik, P.C.; Sohrabi, Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010, 4, 580–585. [Google Scholar]
- Dalal, V.K.; Tripathy, B.C. Modulation of chlorophyll biosynthesis by water stress in rice seedlings during chloroplast biogenesis. Plant Cell Environ. 2012, 35, 1685–1703. [Google Scholar] [CrossRef]
- Ashraf, M.Y.; Azmi, A.R.; Khan, A.H.; Ala, S.A. Effect of water stress on total phenols, Peroxidase activity and chlorophyll content in wheat. Acta Physiol. Plant. 1994, 16, 185–191. [Google Scholar]
- Zhang, H.; Ye, Y.K.; Wang, S.H.; Luo, J.P.; Tang, J.; Ma, D.F. Hydrogen sulfide counteracts chlorophyll loss in sweet potato seedling leaves and alleviates oxidative damage against osmotic stress. Plant Growth Regul. 2009, 58, 243–250. [Google Scholar] [CrossRef]
- Chen, J.; Wu, F.-H.; Wang, W.-H.; Zheng, C.-J.; Lin, G.-H.; Dong, X.-J.; He, J.-X.; Pei, Z.-M.; Zheng, H.-L. Hydrogen sulphide enhances photosynthesis through promoting chloroplast biogenesis, photosynthetic enzyme expression, and thiol redox modification in Spinacia oleracea seedlings. J. Exp. Bot. 2011, 62, 4481–4493. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, F.-H.; Shang, Y.-T.; Wang, W.-H.; Hu, W.-J.; Simon, M.; Liu, X.; Shangguan, Z.-P.; Zheng, H.-L. Hydrogen sulphide improves adaptation of Zea mays seedlings to iron deficiency. J. Exp. Bot. 2015, 66, 6605–6622. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.M.; Xue, L.X.; Xie, H. A structurally novel salt regulated promoter of duplicated carbonic anhydrase gene 1 from Dunaliella salina. Mol. Biol. Rep. 2010, 37, 1143–1154. [Google Scholar] [CrossRef]
- Das, A.; Eldakak, M.; Paudel, B.; Kim, D.-W.; Hemmati, H.; Basu, C.; Rohila, J.S. Leaf proteome analysis reveals prospective drought and heat stress response mechanisms in soybean. BioMed Res. Int. 2016, 2016, 6021047. [Google Scholar] [CrossRef]
- Sun, W.H.; Wu, Y.Y.; Wen, X.Y.; Xiong, S.J.; He, H.G.; Wang, Y.; Lu, G.Q. Different mechanisms of photosynthetic response to drought stress in tomato and violet orychophragmus. Photosynthetica 2016, 5, 226–233. [Google Scholar] [CrossRef]
- Yamasaki, S.; Dillenburg, L.C. Measurements of leaf relative water content in Araucaria angustifolia. Rev. Bras. Fisiol. Veg. 1999, 11, 69–75. [Google Scholar]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. Changes in plant response to NaCl during development of rice (Oryza sativa L.) varieties differing in salinity resistance. J. Exp. Bot. 1995, 46, 1843–1852. [Google Scholar]
- Rodriguez-Serrano, M.; Romero-Puertas, M.C.; Pazmino, D.M.; Testillano, P.S.; Risueno, M.C.; delRio, L.A.; Sandalio, L.M. Cellular response of pea plants to cadmium toxicity: Cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol. 2009, 150, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Inhibition of nitrite formation from hydroxyl ammonium chloride, A simple assay for superoxide dismutase. Ann. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef]
- Cakmak, I.; Horst, J.H. Effects of aluminum on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Bates, L.S.; Walden, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1972, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Ann. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Upadhyaya, A.; Sankhla, D.; Davis, T.D.; Sankhla, N.; Smith, B.N. Effect of paclobutrazol on the activities of some enzymes of activated oxygen metabolism and lipid peroxidation in senescing soybean leaves. J. Plant Physiol. 1985, 121, 453–461. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Gaitonde, M.K. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem. J. 1967, 104, 627–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riemenschneider, A.; Riedel, K.; Hoefgen, R.; Papenbrock, J.; Hesse, H. Impact of reduced O-Acetylserine(thiol)lyase isoform contents on potato plant metabolism. Plant Physiol. 2005, 137, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A. Sulphur metabolism. D. Cysteine synthase. Methods Plant Biochem. 1990, 3, 349–354. [Google Scholar]
- Bloem, E.; Riemenschneider, A.; Volker, J.; Papenbrock, J.; Schmidt, A.; Salac, I.; Silvia, H.; Schnug, E. Sulphur supply and infection with Pyrenopeziza brassicae influence L-cysteine desulphydrase activity in Brassica napus L. J. Exp. Bot. 2004, 55, 2305–2312. [Google Scholar] [CrossRef] [PubMed]
- Riemenschneider, A.; Wegele, R.; Schmidt, A.; Papenbrock, I. Isolation and characterization of a D-cysteine desulfhydrase protein from Arabidopsis thaliana. FEBS J. 2005, 272, 1291–1304. [Google Scholar] [CrossRef]
- Nashef, A.S.; Osuga, D.T.; Feeney, R.E. Determination of hydrogen sulfide with 5,5b’-dithiobis-(2-nitrobenzoic acid), N-ethylmaleimide, and parachloromercuribenzoate. Anal. Biochem. 1977, 79, 394–405. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV–vis spectroscopy. In Current Protocols in Food Analytical Chemistry (CPFA); Wrolstad, R.E., Acree, T.E., An, H., Decker, E.A., Penner, M.H., Reid, D.S., Schwartz, S.J., Shoemaker, C.F., Sporns, P., Eds.; John Wiley and Sons: New York, NY, USA, 2011; pp. F4.3.1–F4.3.8. [Google Scholar]
- Dwivedi, R.S.; Randhawa, N.S. Evaluation of a rapid test for the hidden hunger of zinc in plants. Plant Soil 1974, 40, 445–451. [Google Scholar] [CrossRef]





| Treatments | Parameters | |||||
|---|---|---|---|---|---|---|
| Chl a (mg g−1 FW) | Chl b (mg g−1 FW) | Total Chl (mg g−1 FW) | Chl a/b | Total carotenoids (mg g−1 FW) | CA activity (μM CO2 kg−1 leaf FW s−1) | |
| Control | 1.84 ± 0.04 a | 0.96 ± 0.10 b | 2.80 ± 0.09 ab | 1.92 ± 0.29 cd | 3.68 ± 0.19 de | 276.40 ± 11.39 d |
| DS | 1.37 ± 0.06 d | 0.51 ± 0.04 d | 1.88 ± 0.07 d | 2.69 ± 0.11 b | 4.15 ± 0.06 b | 321.58 ± 3.96 bc |
| NaHS | 1.82 ± 0.06 ab | 1.07 ± 0.07 a | 2.89 ± 0.06 a | 1.70 ± 0.09 e | 3.82 ± 0.08 bc | 336.73 ± 4.96 b |
| NaHS + DS | 1.77 ± 0.03 ac | 0.85 ± 0.10 c | 2.62 ± 0.08 ac | 2.08 ± 0.24 c | 4.78 ± 0.11 a | 371.27 ± 7.14 a |
| HT + NaHS + DS | 1.25 ± 0.03 de | 0.25 ± 0.09 e | 1.50 ± 0.10 e | 5.00 ± 0.15 a | 3.76 ± 0.08 bd | 266.49 ± 15.22 de |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.N.; AlZuaibr, F.M.; Al-Huqail, A.A.; Siddiqui, M.H.; M. Ali, H.; Al-Muwayhi, M.A.; Al-Haque, H.N. Hydrogen Sulfide-Mediated Activation of O-Acetylserine (Thiol) Lyase and l/d-Cysteine Desulfhydrase Enhance Dehydration Tolerance in Eruca sativa Mill. Int. J. Mol. Sci. 2018, 19, 3981. https://doi.org/10.3390/ijms19123981
Khan MN, AlZuaibr FM, Al-Huqail AA, Siddiqui MH, M. Ali H, Al-Muwayhi MA, Al-Haque HN. Hydrogen Sulfide-Mediated Activation of O-Acetylserine (Thiol) Lyase and l/d-Cysteine Desulfhydrase Enhance Dehydration Tolerance in Eruca sativa Mill. International Journal of Molecular Sciences. 2018; 19(12):3981. https://doi.org/10.3390/ijms19123981
Chicago/Turabian StyleKhan, M. Nasir, Fahad M. AlZuaibr, Asma A. Al-Huqail, Manzer H. Siddiqui, Hayssam M. Ali, Mohammed A. Al-Muwayhi, and Hafiz N. Al-Haque. 2018. "Hydrogen Sulfide-Mediated Activation of O-Acetylserine (Thiol) Lyase and l/d-Cysteine Desulfhydrase Enhance Dehydration Tolerance in Eruca sativa Mill" International Journal of Molecular Sciences 19, no. 12: 3981. https://doi.org/10.3390/ijms19123981