Vitamin D: Effect on Haematopoiesis and Immune System and Clinical Applications
Abstract
:1. Vitamin D: Metabolism and Mechanisms of Action
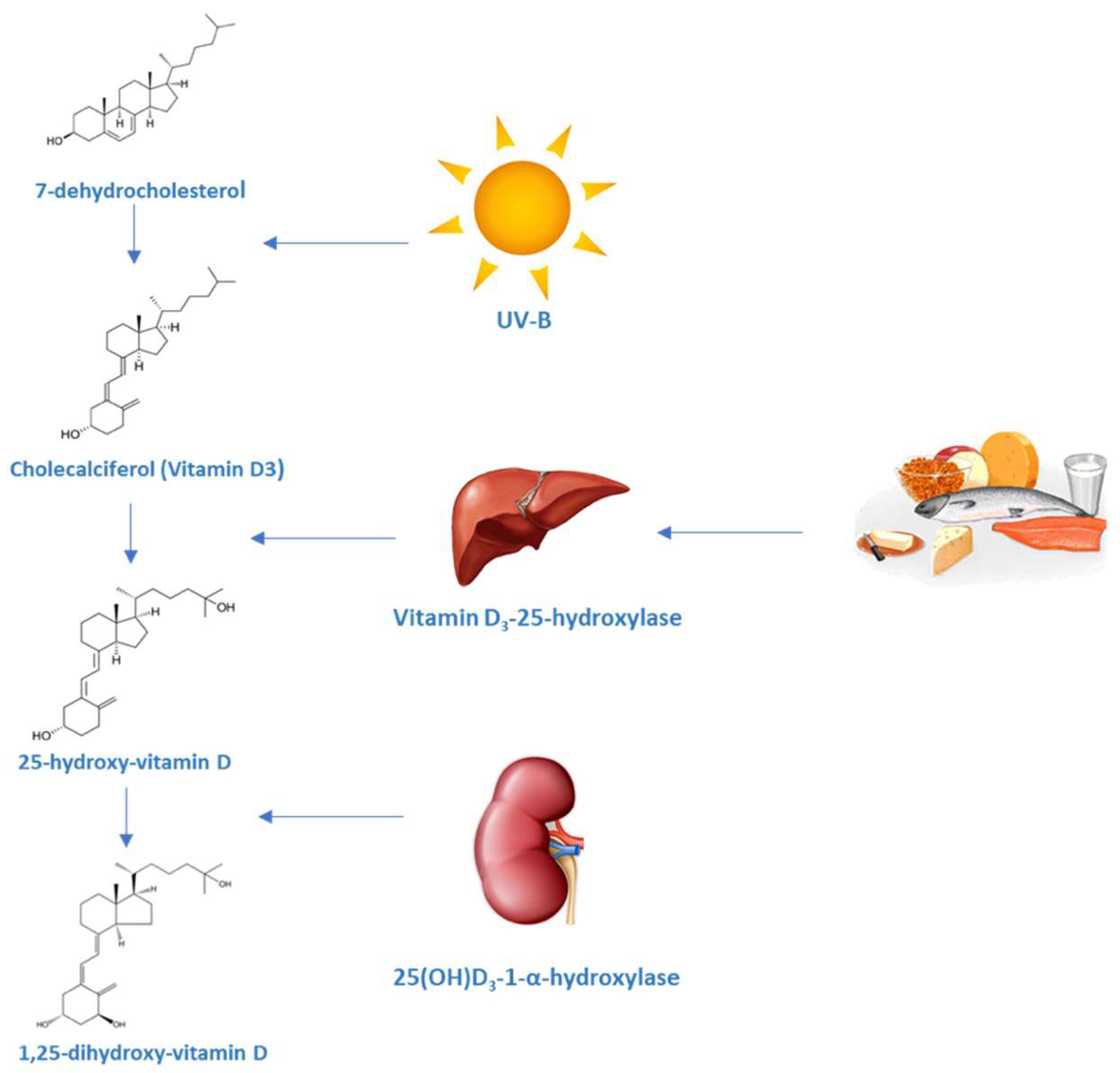
1.1. Production and Metabolism of Vitamin D
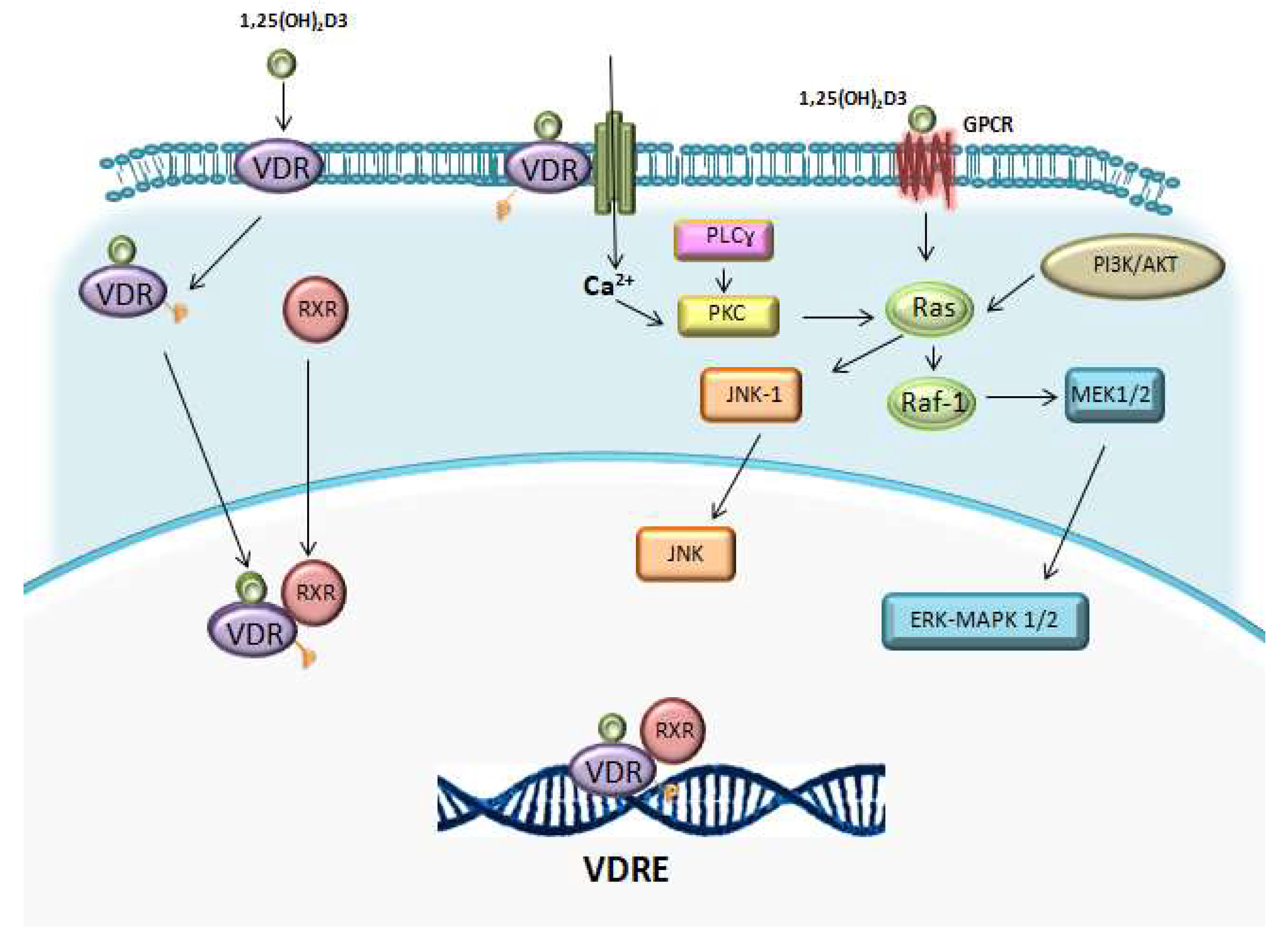
1.2. Vitamin D Receptor (VDR)
2. Effect of Vitamin D on the Immune System
2.1. Vitamin D and Innate Immunity
2.1.1. Macrophages, Vitamin D and Cathelicidin
2.1.2. Dendritic Cells and Antigenic Presentation
2.2. Vitamin D and Adaptive Immunity
2.2.1. Vitamin D and T Lymphocytes
2.2.2. Vitamin D and B Lymphocytes
2.3. Genetic Fingerprint of Vitamin D in the Immune System
2.4. Vitamin D Levels and Immune Function
3. Vitamin D in Haematopoiesis and Hematopoietic Stem Cells
4. Clinical Applications
4.1. Use of Vitamin D in the Treatment of Hematologic Malignancies
4.2. Vitamin D as a Modulator of the Immune Response in Allogeneic Transplantation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| vit D | Vitamin D |
| 25(OH)D | Calcidiol, 25 hydroxycholecalciferol or 25-hidroxivitamin D |
| 1,25(OH)2D3 | 1,25-Dihydroxyvitamin D3 |
| DBP | Vitamin D-binding protein |
| CYP2R1 | Vitamin D 25-hydroxylase or cytochrome P450 2R1 |
| CYP27B1 | 1-α-Hydroxylase (1α-hydroxylase) |
| CYP24A1 | Cytochrome P450 family 24 subfamily A member 1 |
| FGF-23 | Fibroblast growth factor 23 |
| PTH | Parathyroid hormone |
| TNFα | Tumour necrosis factor α |
| IFNγ | Interferon gamma |
| UV | Ultra violet |
| VDR | Vitamin D receptor |
| RXR | Retinoid-X-receptor |
| VDRE | Vitamin D responsive elements |
| HAT | Histone acetyl transferases |
| NCoR | Nuclear receptor corepressor |
| SMRT NFAT | Retinoid or thyroid-hormone receptors Nuclear factor of activated T-cells |
| TSH | Thyroid-stimulating hormone |
| TRH | Thyrotropin-releasing hormone |
| TLR | Toll like receptor |
| NF-κB | Nuclear factor α B |
| DC | Dendritic cells |
| M-DCs | Myeloid dendritic cells |
| P-DCs | Plasmacytoid dendritic cells |
| MR | Manose receptors |
| CAMP | Cathelicidin |
| DEFB4 | β defensin 2 |
| CCR10 | C-C chemokine receptor type 10 |
| NOD2 | Nucleotide-binding oligomerization domain-containing protein 2 |
| SINE | Introduction of a nuclear element |
| PAMPs | Molecular patterns associated with pathogen surveillance |
| PRRs | Pattern recognition receptors |
| MDR | Multidrug resistance |
| HAMP | Hepcidin antimicrobial peptide |
| CFU-GM | Granulocyte macrophage colony forming cell |
| RAR | Retinoic acid receptor |
| RXR | Retinoid X receptor |
| SRC | Steroid receptor coactivator |
| DRIP | Vitamin D receptor interacting protein |
| PKC | Protein kinase C |
| MAPK | Mitogen activated kinase |
| iNKT | Invariant natural killer T cells |
| HSPC | Hematopoietic stem progenitor cell |
| HSC | Hematopoiectic stem cell |
| MDS | Myelodysplastic syndromes |
| AML | Acute myeloblastic leukaemias |
| ATRA | All-trans retinoic acid |
| APL | Acute promyelocytic leukaemia |
| EFS | Event free survival |
| AZA | 5-Azacytidine |
| HR | Hazard ratio |
| CI | Confidence interval |
| OS | Overall survival |
| HSCT | Hematopoietic stem cell transplantation |
| CMV | Cytomegalovirus |
| cGVHD | Chronic graft-versus-host disease |
| aGVHD | Acute graft-versus-host disease |
References
- Aranow, C. Vitamin D and the immune system. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and novel actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Richtand, N.M.; McNeill, S.C.; Holick, S.A.; Frommer, J.E.; Henley, J.W.; Potts, J.T.J. Isolation and identification of previtamin D3 from the skin of rats exposed to ultraviolet irradiation. Biochemistry 1979, 18, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; McLaughlin, J.A.; Clark, M.B.; Holick, S.A.; Potts, J.T.J.; Anderson, R.R.; Blank, I.H.; Parrish, J.A.; Elias, P. Photosynthesis of previtamin D3 in human and the physiologic consequences. Science 1980, 210, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Bell, N.H.; Greene, A.; Epstein, S.; Oexmann, M.J.; Shaw, S.; Shary, J. Evidence for alteration of the vitamin D endocrine system in blacks. J. Clin. Investig. 1985, 76, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Rachez, C.; Gamble, M.; Chang, C.P.; Atkins, G.B.; Lazar, M.A.; Freedman, L.P. The DRIP complex and SRC-1/p160 coactivators share similar nuclear receptor binding determinants but constitute functionally distinct complexes. Mol. Cell Biol. 2000, 20, 2718–2726. [Google Scholar] [CrossRef] [PubMed]
- Seth-Vollenweider, T.; Joshi, S.; Dhawan, P.; Sif, S.; Christakos, S. Novel mechanism of negative regulation of 1,25-dihydroxyvitamin D3-induced 25-hydroxyvitamin D3 24-hydroxylase (Cyp24a1) transcription: Epigenetic modification involving cross-talk between protein-arginine methyltransferase 5 and the SWI/SNF complex. J. Biol. Chem. 2014, 289, 33958–33970. [Google Scholar] [CrossRef] [PubMed]
- Perissi, V.; Jepsen, K.; Glass, C.K.; Rosenfeld, M.G. Deconstructing repression: Evolving models of co-repressor action. Nat. Rev. Genet. 2010, 11, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Horlein, A.J.; Naar, A.M.; Heinzel, T.; Torchia, J.; Gloss, B.; Kurokawa, R.; Ryan, A.; Kamei, Y.; Söderström, M.; Glass, C.K.; et al. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature 1995, 377, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Whitfield, G.K.; Kaneko, I.; Haussler, C.A.; Hsieh, D.; Hsieh, J.C.; Jurutka, P.W. Molecular mechanisms of vitamin D action. Calcif. Tissue Int. 2013, 92, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Van Etten, E.; Verlinden, L.; Giulietti, A.; Ramos-Lopez, E.; Branisteanu, D.D.; Ferreira, G.B.; Overbergh, L.; Verstuyf, A.; Bouillon, R.; Roep, B.O.; et al. The vitamin D receptor gene FokI polymorphism: Functional impact on the immune system. Eur. J. Immunol. 2007, 37, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.S.; Eccleshall, T.R.; Gross, C.; Dawson-Hughes, B.; Feldman, D. The vitamin D receptor start codon polymorphism (FokI) and bone mineral density in premenopausal American black and white women. J. Bone Miner. Res. 1997, 12, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. Vitamin D and the immune system: New perspectives on an old theme. Endocrinol. Metab. Clin. N. Am. 2010, 39, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Eisman, J.A.; Martin, T.J.; MacIntyre, I.; Moseley, J.M. 1,25-dihydroxyvitamin-D-receptor in breast cancer cells. Lancet 1979, 314, 1335–1336. [Google Scholar] [CrossRef]
- Makishima, M.; Lu, T.T.; Xie, W.; Whitfield, G.K.; Domoto, H.; Evans, R.M.; Haussler, M.R.; Mangelsdorf, D.J. Vitamin D receptor as an intestinal bile acid sensor. Science 2002, 296, 1313–1316. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; Van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef] [PubMed]
- Sooy, K.; Schermerhorn, T.; Noda, M.; Surana, M.; Rhoten, W.B.; Meyer, M.; Fleischer, N.; Sharp, G.W.; Christakos, S. Calbindin-D(28k) controls [Ca(2+)](i) and insulin release. Evidence obtained from calbindin-d(28k) knockout mice and β cell lines. J. Biol. Chem. 1999, 274, 34343–34349. [Google Scholar] [CrossRef] [PubMed]
- Tornquist, K. Pretreatment with 1,25-dihydroxycholecalciferol enhances thyrotropin-releasing hormoneand inositol 1,4,5-trisphosphate-induced release of sequestered Ca2+ in permeabilized GH4C1 pituitary cells. Endocrinology 1992, 131, 1677–1681. [Google Scholar] [CrossRef] [PubMed]
- Herscovitch, K.; Dauletbaev, N.; Lands, L.C. Vitamin D as an anti-microbial and anti-inflammatory therapy for Cystic Fibrosis. Paediatr. Respir. Rev. 2013, 15, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Selles, J.; Boland, R. Rapid stimulation of calcium uptake and protein phosphorylation in isolated cardiac muscle by 1,25-dihydroxyvitamin D3. Mol. Cell Endocrinol. 1991, 77, 67–73. [Google Scholar] [CrossRef]
- Girgis, C.M.; Clifton-Bligh, R.J.; Hamrick, M.W.; Holick, M.F.; Gunton, J.E. The roles of vitamin D in skeletal muscle: Form, function, and metabolism. Endocr. Rev. 2013, 34, 33–83. [Google Scholar] [CrossRef] [PubMed]
- Boland, R. Role of vitamin D in skeletal muscle function. Endocr. Rev. 1986, 7, 434–448. [Google Scholar] [CrossRef] [PubMed]
- Abe, E.; Miyaura, C.; Tanaka, H.; Shiina, Y.; Kuribayashi, T.; Suda, S.; Nishii, Y.; DeLuca, H.F.; Suda, T. 1α,25-dihydroxyvitamin D3 promotes fusion of mouse alveolar macrophages both by a direct mechanism and by a spleen cell-mediated indirect mechanism. Proc. Natl. Acad. Sci. USA 1983, 80, 5583–5587. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, A.K.; Amento, E.P.; Serog, B.; Glimcher, L.H. 1,25-Dihydroxyvitamin D3 inhibits antigen-induced T cell activation. J. Immunol. 1984, 133, 1748–1754. [Google Scholar] [PubMed]
- Adams, J.S.; Gacad, M.A. Characterization of 1α-hydroxylation of vitamin D3 sterols by cultured alveolar macrophages from patients with sarcoidosis. J. Exp. Med. 1985, 161, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Sharma, O.P.; Gacad, M.A.; Singer, F.R. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J. Clin. Investig. 1983, 72, 1856–1860. [Google Scholar] [CrossRef] [PubMed]
- Barbour, G.L.; Coburn, J.W.; Slatopolsky, E.; Norman, A.W.; Horst, R.L. Hypercalcemia in an anephric patient with sarcoidosis: Evidence for extrarenal generation of 1,25-dihydroxyvitamin D. N. Engl. J. Med. 1981, 305, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. Vitamin D and the immune system. J. Endocrinol. 1992, 132, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M.; Burke, F.; Evans, K.N.; Lammas, D.A.; Sansom, D.M.; Liu, P.; Modlin, R.L.; Adams, J.S. Extra-renal 25-hydroxyvitamin D3-1α-hydroxylase in human health and disease. J. Steroid Biochem. Mol. Biol. 2007, 103, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Abe, E.; Miyaura, C.; Sakagami, H.; Takeda, M.; Konno, K.; Yamazaki, T.; Yoshiki, S.; Suda, T. Differentiation of mouse myeloid leukemia cells induced by 1α,25-dihydroxyvitamin D3. Proc. Natl. Acad. Sci. USA 1981, 78, 4990–4994. [Google Scholar] [CrossRef] [PubMed]
- Koeffler, H.P.; Amatruda, T.; Ikekawa, N.; Kobayashi, Y.; DeLuca, H.F. Induction of macrophage differentiation of human normal and leukemic myeloid stem cells by 1,25-dihydroxyvitamin D3 and its fluorinated analogues. Cancer Res. 1984, 44, 5624–5628. [Google Scholar] [PubMed]
- Tanaka, H.; Abe, E.; Miyaura, C.; Shiina, Y.; Suda, T. 1α,25-dihydroxyvitamin D3 induces differentiation of human promyelocytic leukemia cells (HL-60) into monocyte-macrophages, but not into granulocytes. Biochem. Biophys. Res. Commun. 1983, 117, 86–92. [Google Scholar] [CrossRef]
- Koeffler, H.P.; Reichel, H.; Bishop, J.E.; Norman, A.W. Gamma-Interferon stimulates production of 1,25-dihydroxyvitamin D3 by normal human macrophages. Biochem. Biophys. Res. Commun. 1985, 127, 596–603. [Google Scholar] [CrossRef]
- Kreutz, M.; Andreesen, R.; Krause, S.W.; Szabo, A.; Ritz, E.; Reichel, H. 1,25-dihydroxyvitamin D3 production and vitamin D3 receptor expression are developmentally regulated during differentiation of human monocytes into macrophages. Blood 1993, 82, 1300–1307. [Google Scholar] [PubMed]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F.; Borregaard, N.; Koeffler, H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005, 19, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004, 173, 2909–2912. [Google Scholar] [CrossRef] [PubMed]
- Krutzik, S.R.; Hewison, M.; Liu, P.T.; Robles, J.A.; Stenger, S.; Adams, J.S.; Modlin, R.L. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J. Immunol. 2008, 181, 7115–7120. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Kaplan, A.T.; Low, J.; Low, J.; Nguyen, L.; Liu, G.Y.; Equils, O.; Hewison, M. Vitamin D induces innate antibacterial responses in human trophoblasts via an intracrine pathway. Biol. Reprod. 2009, 80, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Nguyen, L.; Wu, S.; Encinas, C.; Adams, J.S.; Hewison, M. Alternative splicing of vitamin D-24-hydroxylase: A novel mechanism for the regulation of extrarenal 1,25-dihydroxyvitamin D synthesis. J. Biol. Chem. 2005, 280, 20604. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, K.; Wessner, B.; Laggner, U.; Ploder, M.; Tamandl, D.; Friedl, J.; Zügel, U.; Steinmeyer, A.; Pollak, A.; Roth, E.; et al. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur. J. Immunol. 2006, 36, 361. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J. IPC: Professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 2005, 23, 275–306. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Trinchieri, G.; Liu, Y.J. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 2004, 5, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Mahnke, K.; Johfnson, T.S.; Ring, S.; Enk, A.H. Tolerogenic dendritic cells and regulatory T cells: A two-way relationship. J. Dermatol. Sci. 2007, 46, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Brennan, A.; Katz, D.R.; Nunn, J.D.; Barker, S.; Hewison, M.; Fraher, L.J.; O’Riordan, J.L. Dendritic cells from human tissues express receptors for the immunoregulatory vitamin D3 metabolite, dihydroxycholecalciferol. Immunology 1987, 61, 457–461. [Google Scholar] [PubMed]
- Dam, T.N.; Moller, B.; Hindkjaer, J.; Kragballe, K. The vitamin D3 analog calcipotriol suppresses the number and antigen-presenting function of Langerhans cells in normal human skin. J. Investig. Dermatol. Symp. Proc. 1996, 1, 72–77. [Google Scholar] [PubMed]
- Gauzzi, M.C.; Purificato, C.; Donato, K.; Jin, Y.; Wang, L.; Daniel, K.C.; Maghazachi, A.A.; Belardelli, F.; Adorini, L.; Gessani, S. Suppressive effect of 1α,25-dihydroxyvitamin D3 on type I IFN-mediated monocyte differentiation into dendritic cells: Impairment of functional activities and chemotaxis. J. Immunol. 2005, 174, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Penna, G.; Adorini, L. 1α,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J. Immunol. 2000, 164, 2405–2411. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.D.; Lutz, W.H.; Phan, V.A.; Bachman, L.A.; McKean, D.J.; Kumar, R. Potent inhibition of dendritic cell differentiation and maturation by vitamin D analogs. Biochem. Biophys. Res. Commun. 2000, 270, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Gregori, S.; Casorati, M.; Amuchastegui, S.; Smiroldo, S.; Davalli, A.M.; Adorini, L. Regulatory T cells induced by 1α,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J. Immunol. 2001, 167, 1945–1953. [Google Scholar] [CrossRef] [PubMed]
- Barrat, F.J.; Cua, D.J.; Boonstra, A.; Richards, D.F.; Crain, C.; Savelkoul, H.F.; de Waal-Malefyt, R.; Coffman, R.L.; Hawrylowicz, C.M.; O’Garra, A. In vitro generation of IL-10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by Th1- and Th2-inducing cytokines. J. Exp. Med. 2002, 195, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M.; Hawiger, D.; Nussenzweig, M.C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 2003, 21, 685–711. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.M.; Archer, D.C.; Beck, L.; Spiegelberg, H.L. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: Preferential inhibition of Th1 functions. J. Nutr. 1995, 125, 1704S–1708S. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, A.; Barrat, F.J.; Crain, C.; Heath, V.L.; Savelkoul, H.F.; O’Garra, A. 1α,25-Dihydroxyvitamin D3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J. Immunol. 2001, 167, 4974–4980. [Google Scholar] [CrossRef] [PubMed]
- Overbergh, L.; Decallonne, B.; Waer, M.; Rutgeerts, O.; Valckx, D.; Casteels, K.M.; Laureys, J.; Bouillon, R.; Mathieu, C. 1α,25-dihydroxyvitamin D3 induces an autoantigenspecific T-helper 1/T-helper 2 immune shift in NOD mice immunized with GAD65 (p524–543). Diabetes 2000, 49, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- O’Kelly, J.; Hisatake, J.; Hisatake, Y.; Bishop, J.; Norman, A.; Koeffler, H.P. Normal myelopoiesis but abnormal T lymphocyte responses in vitamin D receptor knockout mice. J. Clin. Investig. 2002, 109, 1091–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregori, S.; Giarratana, N.; Smiroldo, S.; Uskokovic, M.; Adorini, L. A 1α,25-dihydroxyvitamin D (3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes 2002, 51, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.T.; Hatton, R.D.; Mangan, P.R.; Harrington, L.E. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 2007, 25, 821–852. [Google Scholar] [CrossRef] [PubMed]
- Daniel, C.; Sartory, N.A.; Zahn, N.; Radeke, H.H.; Stein, J.M. Immune modulatory treatment of TNBS colitis with calcitriol is associated with a change of a Th1/Th17 to a Th2 and regulatory T cell profile. J. Pharmacol. Exp. Ther. 2008, 324, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Nguyen, L.; Chun, R.F.; Lagishetty, V.; Ren, S.; Wu, S.; Hollis, B.; DeLuca, H.F.; Adams, J.S.; Hewison, M. Altered endocrine and autocrine metabolism of vitamin D in amouse model of gastrointestinal inflammation. Endocrinology 2008, 149, 4799–4808. [Google Scholar] [CrossRef] [PubMed]
- Adorini, L.; Penna, G.; Giarratana, N.; Roncari, A.; Amuchastegui, S.; Daniel, K.C.; Uskokovic, M. Dendritic cells as key targets for immunomodulation by Vitamin D receptor ligands. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Bachman, L.A.; Kumar, R.; Griffin, M.D. Generation of antigen-specific, interleukin-10-producing T cells using dendritic cell stimulation and steroid hormone conditioning. Transpl. Immunol. 2003, 11, 323–333. [Google Scholar] [CrossRef]
- Provvedini, D.M.; Manolagas, S.C. 1α,25-dihydroxyvitamin D3 receptor distribution and effects in subpopulations of normal human T lymphocytes. J. Clin. Endocrinol. Metab. 1989, 68, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Urry, Z.; Xystrakis, E.; Richards, D.F.; McDonald, J.; Sattar, Z.; Cousins, D.J.; Corrigan, C.J.; Hickman, E.; Brown, Z.; Hawrylowicz, C.M. Ligation of TLR9 induced on human IL-10-secreting Tregs by 1α,25-dihydroxyvitamin D3 abrogates regulatory function. J. Clin. Investig. 2009, 119, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Nunn, J.D.; Katz, D.R.; Barker, S.; Fraher, L.J.; Hewison, M.; Hendy, G.N.; O’Riordan, J.L. Regulation of human tonsillar T-cell proliferation by the active metabolite of vitamin D3. Immunology 1986, 59, 479–484. [Google Scholar] [PubMed]
- Vanham, G.; Ceuppens, J.L.; Bouillon, R. T lymphocytes and their CD4 subset are direct targets for the inhibitory effect of calcitriol. Cell Immunol. 1989, 124, 320–333. [Google Scholar] [CrossRef]
- Veldman, C.M.; Cantorna, M.T.; DeLuca, H.F. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch. Biochem. Biophys. 2000, 374, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Willheim, M.; Thien, R.; Schrattbauer, K.; Bajna, E.; Holub, M.; Gruber, R.; Baier, K.; Pietschmann, P.; Reinisch, W.; Scheiner, O.; et al. Regulatory effects of 1α,25-dihydroxyvitamin D3 on the cytokine production of human peripheral blood lymphocytes. J. Clin. Endocrinol. Metab. 1999, 84, 3739–3744. [Google Scholar] [CrossRef] [PubMed]
- Iho, S.; Iwamoto, K.; Kura, F.; Okuno, Y.; Takahashi, T.; Hoshino, T. Mechanism in 1,25(OH)2D3-induced suppression of helper/suppressor function of CD4/CD8 cells to immunoglobulin production in B cells. Cell Immunol. 1990, 127, 12–25. [Google Scholar] [CrossRef]
- Meehan, T.F.; DeLuca, H.F. CD8(+) T cells are not necessary for 1α,25-dihydroxyvitamin D(3) to suppress experimental autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci. USA 2002, 99, 5557–5560. [Google Scholar] [CrossRef] [PubMed]
- Topilski, I.; Flaishon, L.; Naveh, Y.; Harmelin, A.; Levo, Y.; Shachar, I. The anti-inflammatory effects of 1,25-dihydroxyvitamin D3 on Th2 cells in vivo are due in part to the control of integrin-mediated T lymphocyte homing. Eur. J. Immunol. 2004, 34, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Sigmundsdottir, H.; Pan, J.; Debes, G.F.; Alt, C.; Habtezion, A.; Soler, D.; Butcher, E.C. DCs metabolize sunlight-induced vitamin D3 to “program” T cell attraction to the epidermal chemokine CCL27. Nat. Immunol. 2007, 8, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Bruce, D.; Froicu, M.; Weaver, V.; Cantorna, M.T. Failure of T cell homing, reduced CD4/CD8αα intraepithelial lymphocytes, and inflammation in the gut of vitamin D receptor KO mice. Proc. Natl. Acad. Sci. USA 2008, 105, 20834–20839. [Google Scholar] [CrossRef] [PubMed]
- Rolf, L.; Muris, H.A.; Hupperts, R.; Damoiseaux, J. Vitamin D effects on B cell function in autoimmunity. Ann. N.Y. Acad. Sci. 2014, 1317, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Baumgarth, N. The double life of a B-1 cell: Selfreactivity selects for protective effector functions. Nat. Rev. Immunol. 2011, 11, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, M.; Damoiseaux, J.; Duijvestijn, A.; Tervaert, J.W. The therapeutic potential of targeting B cells and anti-oxLDL antibodies in atherosclerosis. Autoimmun. Rev. 2009, 9, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Kalampokis, I.; Yoshizaki, A.; Tedder, T.F. IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res. Ther. 2013, 15, S1. [Google Scholar] [CrossRef] [PubMed]
- Mauri, C.; Bosma, A. Immune regulatory function of B cells. Annu. Rev. Immunol. 2012, 30, 221–241. [Google Scholar] [CrossRef] [PubMed]
- Fillatreau, S.; Sweenie, C.H.; McGeachy, M.J.; Gray, D.; Anderton, S.M. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 2002, 3, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Mauri, C.; Gray, D.; Mushtaq, N.; Londei, M. Prevention of arthritis by interleukin 10-producing B cells. J. Exp. Med. 2003, 197, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.B.; Flach, C.F.; Czerkinsky, C.; Holmgren, J. B lymphocytes promote expansion of regulatory T cells in oral tolerance: Powerful induction by antigen coupled to cholera toxin B subunit. J. Immunol. 2008, 181, 8278–8287. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.A.; Noreña, L.Y.; Flores-Borja, F.; Rawlings, D.J.; Isenberg, D.A.; Ehrenstein, M.R.; Mauri, C. CD19(+) CD24(hi) CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity 2010, 32, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Duddy, M.; Niino, M.; Adatia, F.; Hebert, S.; Freedman, M.; Atkins, H.; Kim, H.J.; Bar-Or, A. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J. Immunol. 2007, 178, 6092–6099. [Google Scholar] [CrossRef] [PubMed]
- Knippenberg, S.; Peelen, E.; Smolders, J.; Thewissen, M.; Menheere, P.; Tervaert, J.W.; Hupperts, R.; Damoiseaux, J. Reduction in IL-10 producing B cells (B reg) in multiple sclerosis is accompanied by a reduced naive/memory B reg ratio during a relapse but not in remission. J. Neuroimmunol. 2011, 239, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Ladha, S.S.; Yang, L.; Liu, Q.; Shi, S.X.; Su, N.; Bomprezzi, R.; Shi, F.D. Interleukin-10 producing-B cells and their association with responsiveness to rituximab in myasthenia gravis. Muscle Nerve 2014, 49, 487–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Chen, S.; Lipsky, P.E. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007, 179, 1634–1647. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.W.; Kouttab, N.; Ford, D.; Maizel, A.L. Vitamin D-mediated gene regulation in phenotypically defined human B cell subpopulations. Endocrinology 2000, 141, 3225–3234. [Google Scholar] [CrossRef] [PubMed]
- Heine, G.; Niesner, U.; Chang, H.D.; Steinmeyer, A.; Zügel, U.; Zuberbier, T.; Radbruch, A.; Worm, M. 1,25-dihydroxyvitamin D(3) promotes IL-10 production in human B cells. Eur. J. Immunol. 2000, 38, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
- Lugar, P.L.; Love, C.; Grammer, A.C.; Dave, S.S.; Lipsky, P.E. Molecular characterization of circulating plasma cells in patients with active systemic lupus erythematosus. PLoS ONE 2012, 7, e44362. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.M.; Adams, J.S.; Sakai, R.; Jordan, S.C. 1α,25-dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. J. Clin. Investig. 1984, 74, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Vayuvegula, B.; Gupta, S. 1,25-Dihydroxyvitamin D3-mediated inhibition of human B cell differentiation. Clin. Exp. Immunol. 1987, 69, 639–646. [Google Scholar] [PubMed]
- Provedini, D.M.; Tsoukas, C.D.; Deftos, L.J.; Manolagas, S.C. 1α,25- Dihydroxyvitamin D3-binding macromolecules in human B lymphocytes: Effects on immunoglobulin production. J. Immunol. 1986, 136, 2734–2740. [Google Scholar]
- Iho, S.; Takahashi, T.; Kura, F.; Sugiyama, H.; Hoshino, T. The effect of 1,25-dihydroxyvitamin D3 on in vitro immunoglobulin production in human B cells. J. Immunol. 1986, 136, 4427–4431. [Google Scholar] [PubMed]
- Hartmann, B.; Heine, G.; Babina, M.; Steinmeyer, A.; Zügel, U.; Radbruch, A.; Worm, M. Targeting the vitamin D receptor inhibits the B cell-dependent allergic immune response. Allergy 2011, 66, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Heine, G.; Anton, K.; Henz, B.M.; Worm, M. 1α,25-dihydroxyvitamin D3 inhibits anti-CD40 plus IL-4-mediated IgE production in vitro. Eur. J. Immunol. 2002, 32, 3395–3404. [Google Scholar] [CrossRef]
- Nashold, F.E.; Hoag, K.A.; Goverman, J.; Hayes, C.E. Rag-1-dependent cells are necessary for 1,25-dihydroxyvitamin D(3) prevention of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2001, 119, 16–29. [Google Scholar] [CrossRef]
- Spach, K.M.; Nashold, F.E.; Dittel, B.N.; Hayes, C.E. IL-10 signaling is essential for 1,25 dihydroxyvitamin D3-mediated inhibition of experimental autoimmune encephalomyelitis. J. Immunol. 2006, 177, 6030–6037. [Google Scholar] [CrossRef] [PubMed]
- Chun, R.F.; Liu, P.T.; Modlin, R.L.; Adams, J.S.; Hewison, M. Impact of vitamin D on immune function: Lessons learned from genome-wide analysis. Front. Phisiol. 2014, 5, 151. [Google Scholar] [CrossRef] [PubMed]
- Kallas, M.; Green, F.; Hewison, M.; White, C.; Kline, G. Rare causes of calcitriol-mediated hypercalcemia: A case report and literature review. J. Clin. Endocrinol. Metab. 2010, 95, 3111–3117. [Google Scholar] [CrossRef] [PubMed]
- Reichel, H.; Koeffler, H.P.; Norman, A.W. Regulation of 25-hydroxyvitamin D3 metabolism in a human promyelocytic leukemia cell line (HL60):1,25-dihydroxyvitamin D3 stimulates the synthesis of 24, 25-dihydroxyvitamin D3. Arch. Biochem. Biophys. 1986, 251, 222–231. [Google Scholar] [CrossRef]
- Akutsu, N.; Lin, R.; Bastien, Y.; Bestawros, A.; Enepekides, D.J.; Black, M.J.; White, J.H. Regulation of gene expression by 1α,25-dihydroxyvitamin D3 and its analog EB1089 undergrowth-inhibitory conditions in squamous carcinoma cells. Mol. Endocrinol. 2001, 15, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Nagai, Y.; Sladek, R.; Bastien, Y.; Ho, J.; Petrecca, K.; Sotiropoulou, G.; Diamandis, E.P.; Hudson, T.J.; White, J.H. Expression profiling in squamous carcinoma cells reveals pleiotropic effects of vitaminD3 analog EB1089 signaling on cell proliferation, differentiation, and immune system regulation. Mol. Endocrinol. 2002, 16, 1243–1256. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Tavera-Mendoza, L.E.; Laperriere, D.; Libby, E.; MacLeod, N.B.; Nagai, Y.; Bourdeau, V.; Konstorum, A.; Lallemant, B.; Zhang, R.; et al. Large-scale in silico and microarray-based identification of direct1,25-dihydroxyvitamin D3 target genes. Mol. Endocrinol. 2005, 19, 2685–2695. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.T.; Schenk, M.; Walker, V.P.; Dempsey, P.W.; Kanchanapoomi, M.; Wheelwright, M.; Vazirnia, A.; Zhang, X.; Steinmeyer, A.; Zügel, U.; et al. Convergence of IL-1 β and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS ONE 2009, 4, e5810. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Dabbas, B.; Laperriere, D.; Bitton, A.J.; Soualhine, H.; Tavera-Mendoza, L.E.; Dionne, S.; Servant, M.J.; Bitton, A.; Seidman, E.G.; et al. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin β2 innate immune pathway defective in Crohn disease. J. Biol. Chem. 2010, 285, 2227–2231. [Google Scholar] [CrossRef] [PubMed]
- Moresco, E.M.; LaVine, D.; Beutler, B. Toll-like receptors. Curr. Biol. 2011, 21, R488–R493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacchetta, J.Z.J.; Sea, J.L.; Sea, J.L.; Chun, R.F.; Lisse, T.S.; Zavala, K.; Nayak, A.; Wesseling-Perry, K.; Westerman, M.; Hollis, B.W.; et al. Suppression of iron-regulatory hepcidin by vitamin D. J. Am. Soc. Nephrol. 2014, 25, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Iron in innate immunity: Starve the invaders. Curr. Opin. Immunol. 2009, 21, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Drakesmith, H.; Prentice, A.M. Hepcidin and the iron-infection axis. Science 2012, 338, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, B.; Kwon, E.; Choi, S.J.; Lee, Y.H.; Song, G.G.; Sohn, J.; Ji, J.D. Regulation of TREM-1 expression by 1,25-dihydroxyvitamin D3 in human monocytes/macrophages. Immunol. Lett. 2013, 154, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, J.; Mondal, K.; Ehrnsperger, A.; Andreesen, R.; Kreutz, M. Regulation of 25-hydroxyvitamin D3-1α-hydroxylase and production of 1 α,25-dihydroxyvitamin D3 by human dendritic cells. Blood 2003, 102, 3314–3316. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M.; Freeman, L.; Hughes, S.V.; Evans, K.N.; Bland, R.; Eliopoulos, A.G.; Kilby, M.D.; Moss, P.A.; Chakraverty, R. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J. Immunol. 2003, 170, 5382–5390. [Google Scholar] [CrossRef] [PubMed]
- Ooi, J.H.; McDaniel, K.L.; Weaver, V.; Cantorna, M.T. Murine CD8+T cells but not macrophages express the vitamin D1α-hydroxylase. J. Nutr. Biochem. 2014, 25, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, R.J.; Llewelyn, M.; Toossi, Z.; Patel, P.; Pasvol, G.; Lalvani, A.; Wright, D.; Latif, M.; Davidson, R.N. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: A case-control study. Lancet 2000, 355, 618–621. [Google Scholar] [CrossRef]
- Ustianowski, A.; Shaffer, R.; Collin, S.; Wilkinson, R.J.; Davidson, R.N. Prevalence and associations of vitamin D deficiency in foreign-born persons with tuberculosis in London. J. Infect. 2005, 50, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Williams, A.J.; Anderson, S.T. Vitamin D deficiency and insufficiency in children with tuberculosis. Pediatr. Infect. Dis. J. 2008, 27, 941–942. [Google Scholar] [CrossRef] [PubMed]
- Wejse, C.; Gomes, V.F.; Rabna, P.; Gustafson, P.; Aaby, P.; Lisse, I.M.; Andersen, P.L.; Glerup, H.; Sodemann, M. Vitamin D as supplementary treatment for tuberculosis: A double-blind, randomized, placebo-controlled trial. Am. J. Respir. Crit. Care Med. 2009, 179, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Timms, P.M.; Bothamley, G.H.; Hanifa, Y.; Islam, K.; Claxton, A.P.; Packe, G.E.; Moore-Gillon, J.C.; Darmalingam, M.; Davidson, R.N.; et al. High-dose vitamin D (3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: A double-blind randomised controlled trial. Lancet 2011, 377, 242–250. [Google Scholar] [CrossRef]
- Coussens, A.K.; Wilkinson, R.J.; Hanifa, Y.; Nikolayevskyy, V.; Elkington, P.T.; Islam, K.; Timms, P.M.; Venton, T.R.; Bothamley, G.H.; Packe, G.E.; et al. Vitamin D accelerates resolution of inflammatory responses during tuberculosis treatment. Proc. Natl. Acad. Sci. USA. 2012, 109, 15449–15454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeng, L.; Yamshchikov, A.V.; Judd, S.E.; Blumberg, H.M.; Martin, G.S.; Ziegler, T.R.; Tangpricha, V. Alterations in vitamin D status and antimicrobial peptide levels in patients in the intensive care unit with sepsis. J. Transl. Med. 2009, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Cannell, J.J.; Vieth, R.; Umhau, J.C.; Holick, M.F.; Grant, W.B.; Madronich, S.; Garland, C.F.; Giovannucci, E. Epidemic influenza and vitamin D. Epidemiol. Infect. 2006, 134, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Hossein-nezhad, A.; Spira, A.; Holick, M.F. Influence of vitamin D status and vitamin D3 supplementation on genome wide expression of white blood cells: A randomized double-blind clinical trial. PLoS ONE 2013, 8, e58725. [Google Scholar] [CrossRef] [PubMed]
- Lagishetty, V.; Misharin, A.V.; Liu, N.Q.; Lisse, T.S.; Chun, R.F.; Ouyang, Y.; McLachlan, S.M.; Adams, J.S.; Hewison, M. Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology 2010, 151, 2423–2432. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Stappenbeck, T.S.; Hong, C.V.; Gordon, J.I. Angiogenins: A new class of microbicidal proteins involved in innate immunity. Nat. Immunol. 2003, 4, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Packey, C.D.; Sartor, R.B. Commensal bacteria, traditional and opportunistic pathogens, dysbiosis and bacterial killing in inflammatory bowel diseases. Curr. Opin. Infect. Dis. 2009, 22, 292–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauridsen, A.L.; Vestergaard, P.; Hermann, A.P.; Brot, C.; Heickendorff, L.; Mosekilde, L.; Nexo, E. Plasma concentrations of 25-hydroxy-vitamin D and 1,25-dihydroxy-vitamin D are related to the phenotype of Gc (vitamin D-binding protein): Across-sectional study on 595 early postmenopausal women. Calcif. Tissue Int. 2005, 77, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, A.L.; Vestergaard, P.; Nexo, E. Mean serum concentration of vitamin D-binding protein (Gc globulin) is related to the Gc phenotype in women. Clin. Chem. 2001, 47, 753–756. [Google Scholar] [PubMed]
- Wang, T.J.; Zhang, F.; Richards, J.B.; Kestenbaum, B.; Van Meurs, J.B.; Berry, D.; Kiel, D.P.; Streeten, E.A.; Ohlsson, C.; Koller, D.L.; et al. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet 2010, 376, 180–188. [Google Scholar] [CrossRef]
- Chun, R.F.; Lauridsen, A.L.; Suon, L.; Zella, L.A.; Pike, J.W.; Modlin, R.L.; Martineau, A.R.; Wilkinson, R.J.; Adams, J.; Hewison, M. Vitamin D-binding protein directs monocyte responses to 25-hydroxy-and 1,25-dihydroxy vitamin D. J. Clin. Endocrinol. Metab. 2010, 95, 3368–3376. [Google Scholar] [CrossRef] [PubMed]
- Chun, R.F.; Peercy, B.E.; Adams, J.S.; Hewison, M. Vitamin D binding protein and monocyte response to 25-hydroxy vitamin D and 1,25-dihydroxyvitamin D: Analysis by mathematical modeling. PLoS ONE 2012, 7, e30773. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Mirandola, L.; Pandey, A.; Nguyen, D.D.; Jenkins, M.R.; Turcel, M.; Cobos, E.; Chiriva-Internati, M. Application of vitamin D and derivatives in hematological malignancies. Cancer Lett. 2012, 319, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Miyaura, C.; Abe, E.; Kuribayashia, T.; Tanakaa, H.; Konno, K.; Nishii, Y.; Suda, T. 1α,25-dihydroxyvitamin D3 induced differentiation of human myeloid leukemia cells. Biochem. Biophys. Res. Commun. 1981, 105, 937–943. [Google Scholar] [CrossRef]
- Choudhuri, U.; Adams, J.A.; Byrom, N.; McCarthy, D.M.; Barrett, J. 1,25-Dihydroxyvitamin D3 induces normal mononuclear blood cells to differentiate in the direction of monocyte-macrophages. Haematologia (Budap) 1990, 23, 9–19. [Google Scholar] [PubMed]
- Takahashi, T.; Suzuki, A.; Ichiba, S.; Okuno, Y.; Sugiyama, H.; Imura, H.; Nakamura, K.; Iho, S.; Hoshino, T. Effect of 1,25(OH)2D3 on normal human CFU-GM: Target cells of the agent in the suppression of colony formation and induction of macrophage colonies. Int. J. Hematol. 1991, 54, 57–63. [Google Scholar] [PubMed]
- Hall, A.C.; Juckett, M.B. The Role of vitamin D in hematologic disease and stem cell transplantation. Nutrients 2013, 5, 2206–2221. [Google Scholar] [CrossRef] [PubMed]
- Bunce, C.M.; Brown, G.; Hewison, M. Vitamin D and hematopoiesis. Trends Endocrinol. Metab. 1997, 8, 245–251. [Google Scholar] [CrossRef]
- Grande, A.; Montanari, M.; Tagliafico, E.; Manfredini, R.; Zanocco Marani, T.; Siena, M.; Tenedini, E.; Gallinelli, A.; Ferrari, S. Physiological levels of 1α,25 dihydroxyvitamin D3 induce the monocytic commitment of CD34+ hematopoietic progenitors. J. Leukoc. Biol. 2002, 71, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.D. Transcriptional control of granulocyte and monocyte development. Oncogene 2007, 26, 6816–6828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labrecque, J.; Allan, D.; Chambon, P.; Iscove, N.N.; Lohnes, D.; Hoang, T. Impaired granulocytic differentiation in vitro in hematopoietic cells lacking retinoic acid receptors α1 and gamma. Blood 1998, 92, 607–615. [Google Scholar] [PubMed]
- Studzinski, G.P.; Harrison, J.S.; Wang, X.; Sarkar, S.; Kalia, V.; Danilenko, M. Vitamin D control of hematopoietic cell differentiation and leukemia. J. Cell Biochem. 2015, 116, 1500–1512. [Google Scholar] [CrossRef] [PubMed]
- Hughes, P.J.; Marcinkowska, E.; Gocek, E.; Studzinski, G.P.; Brown, G. Vitamin D3-driven signals for myeloid cell differentiation-implications for differentiation therapy. Leuk. Res. 2010, 34, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, S.; Saleem, A.; Emoto, Y.; Stone, R.; Rapp, U.; Kufe, D. Activation of raf-1 and mitogen-activated protein kinases during monocytic differentiation of human myeloid leukemia cells. J. Biol. Chem. 1994, 269, 872–878. [Google Scholar] [PubMed]
- Okazaki, T.; Bielawska, A.; Domae, N.; Bell, R.M.; Hannun, Y.A. Characteristics and partial purification of a novel cytosolic, magnesium-independent, neutral sphingomyelinase activated in the early signal transduction of 1α,25-dihydroxyvitamin D3-induced HL-60 cell differentiation. J. Biol. Chem. 1994, 269, 4070–4077. [Google Scholar] [PubMed]
- Okazaki, T.; Bielawska, A.; Bell, R.M.; Hannun, Y.A. Role of ceramide as a lipid mediator of 1α,25-dihydroxyvitamin D3-induced HL-60 cell differentiation. J. Biol. Chem. 1990, 265, 15823–15831. [Google Scholar] [PubMed]
- Hmama, Z.; Nandan, D.; Sly, L.; Knutson, K.L.; Herrera-Velit, P.; Reiner, N.E. 1α,25-dihydroxyvitamin D3–induced myeloid cell differentiation is regulated by a vitamin D receptor–phosphatidylinositol 3-kinase signaling complex. J. Exp. Med. 1999, 190, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Studzinski, G.P. AKT pathway is activated by 1, 25-dihydroxyvitamin D3 and participates in its anti-apoptotic effect and cell cycle control in differentiating HL60 cells. Cell Cycle 2006, 5, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Cantorna, M.T. The vitamin D receptor is required for iNKT cell development. Proc. Natl. Acad. Sci. USA 2008, 105, 5207–5212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weeres, M.A.; Robien, K.; Ahn, Y.O.; Neulen, M.L.; Bergerson, R.; Miller, J.S.; Verneris, M.R. The effects of 1,25-dihydroxyvitamin D3 on in vitro human NK cell development from hematopoietic stem cells. J. Immunol. 2014, 193, 3456–3462. [Google Scholar] [CrossRef] [PubMed]
- Jeanson, N.T.; Scadden, D.T. Vitamin D receptor deletion leads to increased hematopoietic stem and progenitor cells residing in the spleen. Blood 2010, 116, 4126–4129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortes, M.; Chen, M.J.; Stachura, D.L.; Liu, S.Y.; Kwan, W.; Wright, F.; Vo, L.T.; Theodore, L.N.; Esain, V.; Frost, I.M.; et al. Developmental Vitamin D availability impacts hematopoietic stem cell production. Cell Rep. 2016, 17, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Cortes, M.; Liu, S.Y.; Kwan, W.; Alexa, K.; Goessling, W.; North, T.E. Accumulation of the vitamin D precursor cholecalciferol antagonizes hedgehog signaling to impair hemogenic endothelium formation. Stem Cell Rep. 2015, 5, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Muto, A.; Kizaki, M.; Yamato, K.; Kawai, Y.; Kamata-Matsushita, M.; Ueno, H.; Ohguchi, M.; Nishihara, T.; Koeffler, H.P.; Ikeda, Y. 1,25-dihydroxyvitamin D3 induces differentiation of a retinoic acid–resistant acute promyelocytic leukemia cell line (UF-1) associated with expression of p21 WAF1/CIP1 and p27 KIP1. Blood 1999, 93, 2225–2233. [Google Scholar] [PubMed]
- Bastie, J.N.; Balitrand, N.; Guidez, F.; Guillemot, I.; Larghero, J.; Calabresse, C.; Chomienne, C.; Delva, L. 1α,25-dihydroxyvitamin D3 transrepresses retinoic acid transcriptional activity via vitamin D receptor in myeloid cells. Mol. Endocrinol. 2004, 18, 2685–2699. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Studzinski, G.P. Retinoblastoma protein and CCAAT/enhancer-binding protein β are required for 1,25-dihydroxyvitamin D3-induced monocytic differentiation of HL60 cells. Cancer Res. 2004, 64, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Gemelli, C.; Orlandi, C.; Zanocco Marani, T.; Martello, A.; Vignudelli, T.; Ferrari, F.; Montanari, M.; Parenti, S.; Testa, A.; Grande, A.; et al. The vitamin D3/Hox-A10 pathway supports MafB function during the monocyte differentiation of human CD34+ hemopoietic progenitors. J. Immunol. 2008, 181, 5660–5672. [Google Scholar] [CrossRef] [PubMed]
- Koeffler, H.P.; Hirji, K.; Itri, L. 1,25-dihydroxyvitamin D3: In vivo and in vitro effects on human preleukemic and leukemic cells. Cancer Treat. Rep. 1985, 69, 1399–1407. [Google Scholar] [PubMed]
- Callens, C.; Coulon, S.; Naudin, J.; Radford-Weiss, I.; Boissel, N.; Raffoux, E.; Wang, P.H.; Agarwal, S.; Tamouza, H.; Paubelle, E.; et al. Targeting iron homeostasis induces cellular differentiation and synergizes with differentiating agents in acute myeloid leukemia. J. Exp. Med. 2010, 207, 731–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molnar, I.; Stark, N.; Lovato, J.; Powell, B.L.; Cruz, J.; Hurd, D.D.; Mathieu, J.S.; Chen, T.C.; Holick, M.F.; Cambra, S.; et al. Treatment of low-risk myelodysplastic syndromes with high-dose daily oral cholecalciferol (2000–4000 IU vitamin D(3)). Leukemia 2007, 21, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Motomura, S.; Kanamori, H.; Maruta, A.; Kodama, F.; Ohkubo, T. The effect of 1-hydroxyvitamin D3 for prolongation of leukemic transformation-free survival in myelodysplastic syndromes. Am. J. Hematol. 1991, 38, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Siitonen, T.; Timonen, T.; Juvonen, E.; Terävä, V.; Kutila, A.; Honkanen, T.; Mikkola, M.; Hallman, H.; Kauppila, M.; Nyländen, P.; et al. Valproic acid combined with 13-cis retinoic acid and 1,25-dihydroxyvitamin D3 in the treatment of patients with myelodysplastic syndromes. Haematologica 2007, 92, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, D.; Darbesio, A.; Giai, V.; Genuardi, M.; Dellacasa, C.M.; Sorasio, R.; Bertini, M.; Boccadoro, M. Efficacy of a combination of human recombinant erythropoietin + 13-cis-retinoic acid and dihydroxylated vitamin D3 to improve moderate to severe anaemia in low/intermediate risk myelodysplastic syndromes. Br. J. Haematol. 2009, 144, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Slapak, C.A.; Desforges, J.F.; Fogaren, T.; Miller, K.B. Treatment of acute myeloid leukemia in the elderly with low-dose cytarabine, hydroxyurea, and calcitriol. Am. J. Hematol. 1992, 41, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.S.; Bershadskiy, A. Clinical experience using vitamin D and analogs in the treatment of myelodysplasia and acute myeloid leukemia: A review of the literature. Leuk. Res. Treat. 2012, 2012, 125814. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, D.; Campa, E.; Dellacasa, C.; Campana, S.; Foli, C.; Boccadoro, M. Differentiating agents + low-dose chemotherapy in the management of old/poor prognosis patients with acute myeloid leukemia or myelodysplastic syndrome. Haematologica 2004, 89, 619–620. [Google Scholar] [PubMed]
- Hellstrom, E.; Robert, K.H.; Hellström, E.; Robèrt, K.H.; Samuelsson, J.; Lindemalm, C.; Grimfors, G.; Kimby, E.; Oberg, G.; Winqvist, I.; et al. Treatment of myelodysplastic syndromes with retinoic acid and 1α-hydroxy-vitamin D3 in combination with low-dose ara-c is not superior to ara-c alone. Results from a randomized study. The scandinavian myelodysplasia group (SMG). Eur. J. Haematol. 1990, 45, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Radujkovic, A.; Schnitzler, P.; Ho, A.D.; Dreger, P.; Luft, T. Low serum vitamin D levels are associated with shorter survival after first-line azacitidine treatment in patients with myelodysplastic syndrome and secondary oligoblastic acute myeloid leukemia. Clin. Nutr. 2017, 36, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Muindi, J.R.; Tan, W.; Hu, Q.; Wang, D.; Liu, S.; Wilding, G.E.; Ford, L.A.; Sait, S.N.; Block, A.W.; et al. Low 25(OH) vitamin D3 levels are associated with adverse outcome in newly diagnosed, intensively treated adult acute myeloid leukemia. Cancer 2014, 120, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Pardanani, A.; Drake, M.T.; Finke, C.; Lasho, T.L.; Rozell, S.A.; Jimma, T.; Tefferi, A. Vitamin D insufficiency in myeloproliferative neoplasms and myelodysplastic syndromes: Clinical correlates and prognostic studies. Am. J. Hematol. 2011, 86, 1013–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, W.H.; Seol, J.G.; Kim, E.S.; Binderup, L.; Koeffler, H.P.; Kim, B.K.; Lee, Y.Y. The induction of apoptosis by a combined 1,25(OH)2D3 analog, EB1089 and TGF-β1 in NCI-H929 multiple myeloma cells. Int. J. Oncol. 2002, 20, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Park, W.H.; Seol, J.G.; Kim, E.S.; Jung, C.W.; Lee, C.C.; Binderup, L.; Koeffler, H.P.; Kim, B.K.; Lee, Y.Y. Cell cycle arrest induced by the vitamin D(3) analog EB1089 in NCI-H929 myeloma cells is associated with induction of the cyclin-dependent kinase inhibitor p27. Exp. Cell Res. 2000, 254, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Puthier, D.; Bataille, R.; Barillé, S.; Mellerin, M.P.; Harousseau, J.L.; Ponzio, A.; Robillard, N.; Wijdenes, J.; Amiot, M. Myeloma cell growth arrest, apoptosis, and interleukin-6 receptor modulation induced by EB1089, a vitamin D3 derivative, alone or in association with dexamethasone. Blood 1996, 88, 4659–4666. [Google Scholar] [PubMed]
- Luong, Q.T.; Koeffler, H.P. Vitamin D compounds in leukemia. J. Steroid Biochem. Mol. Biol. 2005, 97, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.L.; Salles, G.; Goldman, B.; Fisher, R.I.; Brice, P.; Press, O.; Casasnovas, O.; Maloney, D.G.; Soubeyran, P.; Rimsza, L.; et al. Low serum vitamin D levels are associated with inferior survival in follicular lymphoma: A prospective evaluation in swog and lysa studies. J. Clin. Oncol. 2015, 33, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Tracy, S.I.; Maurer, M.J.; Witzig, T.E.; Drake, M.T.; Ansell, S.M.; Nowakowski, G.S.; Thompson, C.A.; Inwards, D.J.; Johnston, P.B.; Micallef, I.N.; et al. Vitamin D insufficiency is associated with an increased risk of early clinical failure in follicular lymphoma. Blood Cancer J. 2017, 7, e595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohaus, S.; Tisi, M.C.; Bellesi, S.; Maiolo, E.; Alma, E.; Tartaglia, G.; Corrente, F.; Cuccaro, A.; D’Alo’, F.; Basile, U.; et al. Vitamin D deficiency and supplementation in patients with aggressive B-cell lymphomas treated with immunochemotherapy. Cancer Med. 2018, 7, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Shin, D.Y.; Kim, J.H.; Bae, J.Y.; Lee, K.H.; See, C.J.; Kim, N.; Park, E.K.; Ra, E.K.; Lee, J.E.; et al. Impact of vitamin D receptor gene polymorphisms on clinical outcomes of HLA-matched sibling hematopoietic stem cell transplantation. Clin. Transplant. 2012, 26, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Bogunia-Kubik, K.; Middleton, P.; Norden, J.; Dickinson, A.; Lange, A. Association of vitamin D receptor polymorphisms with the outcome of allogeneic haematopoietic stem cell transplantation. Int. J. Immunogenet. 2008, 35, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Middleton, P.G.; Cullup, H.; Dickinson, A.M.; Norden, J.; Jackson, G.H.; Taylor, P.R.; Cavet, J. Vitamin D receptor gene polymorphism associates with graft-versus-host disease and survival in HLA-matched sibling allogeneic bone marrow transplantation. Bone Marrow Transplant. 2002, 30, 223–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glotzbecker, B.; Ho, V.T.; Aldridge, J.; Kim, H.T.; Horowitz, G.; Ritz, J.; Soiffer, R.; Avigan, D.; Rosenblatt, J. Low levels of 25-hydroxyvitamin D before allogeneic hematopoietic SCT correlate with the development of chronic GVHD. Bone Marrow Transplant. 2013, 48, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Von Bahr, L.; Blennow, O.; Alm, J.; Björklund, A.; Malmberg, K.J.; Mougiakakos, D.; Le Blanc, A.; Oefner, P.J.; Labopin, M.; Ljungman, P.; et al. Increased incidence of chronic GvHD and CMV disease in patients with vitamin D deficiency before allogeneic stem cell transplantation. Bone Marrow Transplant. 2015, 50, 1217–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansson, M.E.; Norlin, A.C.; Omazic, B.; Wikström, A.C.; Bergman, P.; Winiarski, J.; Remberger, M.; Sundin, M. Vitamin D levels affect outcome in pediatric hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2014, 20, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Velázquez, T.; Montero, I.; Sánchez-Guijo, F.; Parody, R.; Saldaña, R.; Valcarcel, D.; López-Godino, O.; Ferra, I.; Coll, C.; Cuesta, M.; et al. Immunomodulatory effect of vitamin D after allogeneic stem cell transplantation: Results of a prospective multicenter clinical trial. Clin. Cancer Res. 2016, 22, 5673–5681. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medrano, M.; Carrillo-Cruz, E.; Montero, I.; Perez-Simon, J.A. Vitamin D: Effect on Haematopoiesis and Immune System and Clinical Applications. Int. J. Mol. Sci. 2018, 19, 2663. https://doi.org/10.3390/ijms19092663
Medrano M, Carrillo-Cruz E, Montero I, Perez-Simon JA. Vitamin D: Effect on Haematopoiesis and Immune System and Clinical Applications. International Journal of Molecular Sciences. 2018; 19(9):2663. https://doi.org/10.3390/ijms19092663
Chicago/Turabian StyleMedrano, Mayte, Estrella Carrillo-Cruz, Isabel Montero, and Jose A Perez-Simon. 2018. "Vitamin D: Effect on Haematopoiesis and Immune System and Clinical Applications" International Journal of Molecular Sciences 19, no. 9: 2663. https://doi.org/10.3390/ijms19092663





