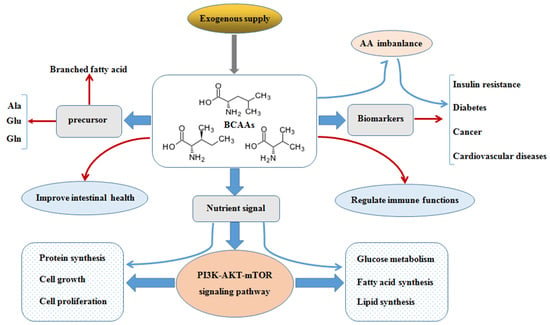
Branched Chain Amino Acids: Beyond Nutrition Metabolism
Abstract
:1. Introduction
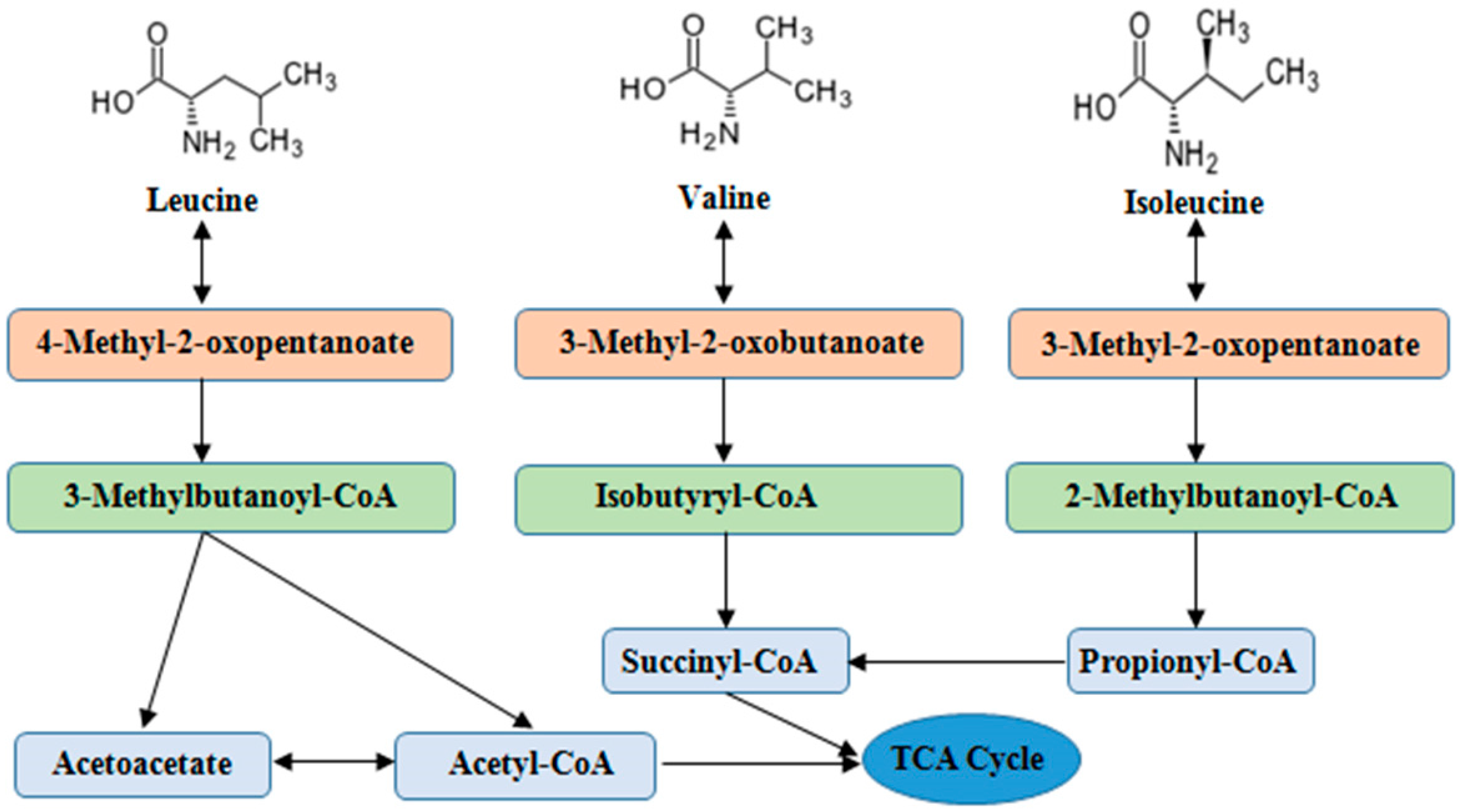
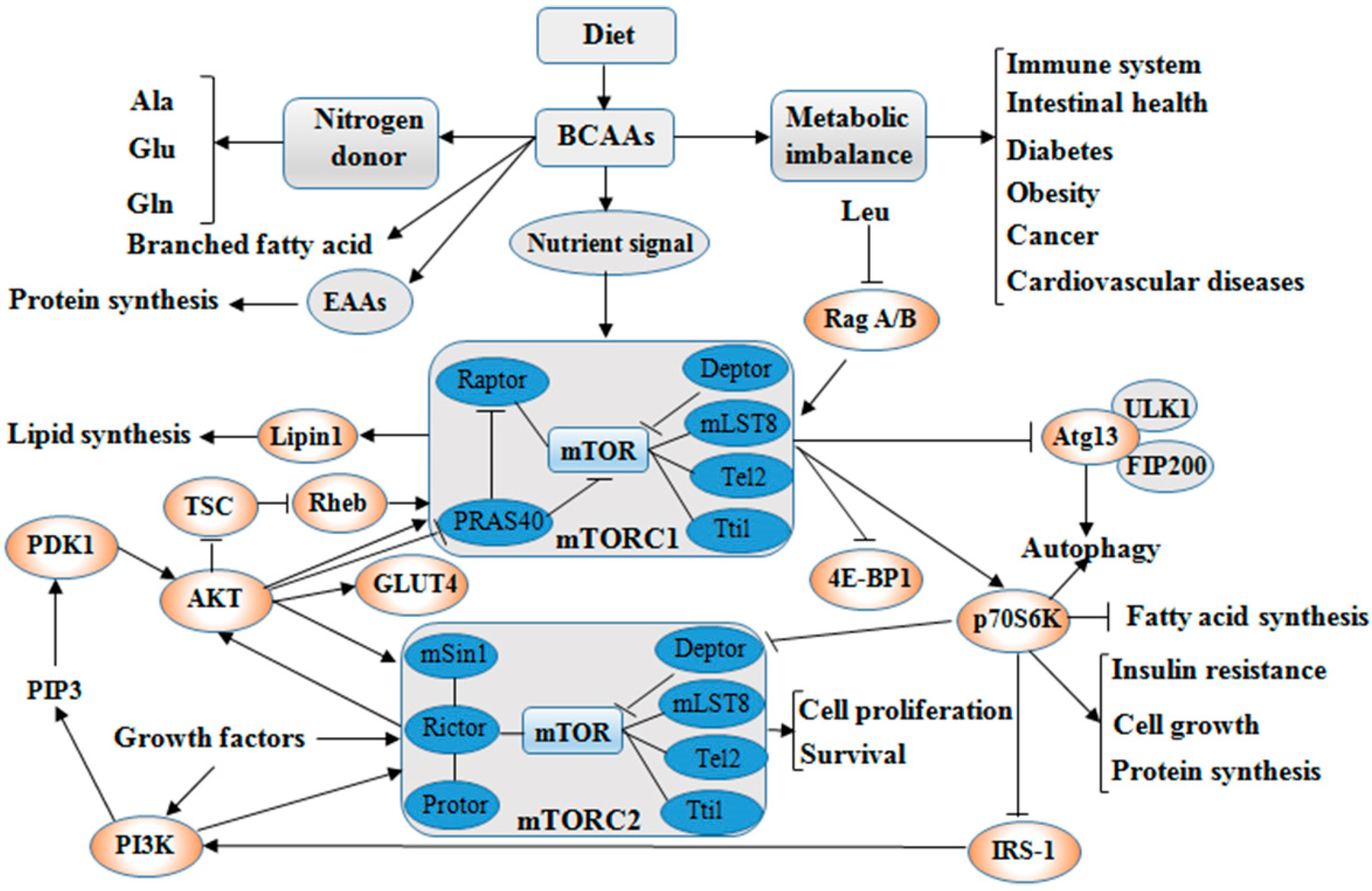
2. Catabolism and Balance of BCAAs
3. Roles of BCAAs in Nutrition Metabolism
3.1. Glucose and Lipid Metabolism
3.2. Protein Synthesis
4. Physiological Functions of BCAAs on Intestinal Health and Immunity
4.1. Intestinal Health
4.2. Immunity
5. BCAAs as Biomarkers in Diseases
5.1. Insulin Resistance (IR)
5.2. Type 2 Diabetic Mellitus (T2DM)
5.3. Cancer
5.4. Cardiovascular Diseases (CVDs)
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jewell, J.L.; Russell, R.C.; Guan, K.L. Amino acid signalling upstream of mTOR. Nat. Rev. Mol. Cell Biol. 2013, 14, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Jinzu, H.; Nagao, K.; Noguchi, Y.; Shimba, N.; Miyano, H.; Watanabe, T.; Iseki, K. Plasma amino acid profiles are associated with insulin, C-peptide and adiponectin levels in type 2 diabetic patients. Nutr. Diabetes 2014, 4, e133. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-canela, M.; Toledo, E.; Clish, C.B.; Hruby, A.; Liang, L.; Salas-Salvadó, J.; Razquin, C.; Corella, D.; Estruch, R.; Ros, E.; et al. Plasma branched-chain amino acids and incident cardiovascular disease in the predimed trial. Clin. Chem. 2016, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Batcha, B.C.; Hylanda, K.; Svetkey, L.P. Branch chain amino acids: Biomarkers of health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 86–89. [Google Scholar] [CrossRef] [PubMed]
- O’Donnella, J.S.; Massi, D.; Teng, M.W.L.; Mandala, M. PI3K-AKT-mTOR inhibition in cancer immunotherapy, redux. Semin. Cancer Biol. 2018, 48, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Dey, N.; De, P.; Leyland-Jones, B. PI3K-AKT-mTOR inhibitors in breast cancers: From tumor cell signaling to clinical trials. Pharmacol. Ther. 2017, 175, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Tato, I.; Bartrons, R.; Ventura, F.; Rosa, J.L. Amino acids activate mammalian target of rapamycin complex 2 (mTORC2) via PI3K/Akt signaling. J. Biol. Chem. 2011, 286, 6128–6142. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; López-Maside, L.; Donapetry-García, C.; Fernández-Fernández, C.; Sixto-Lea, C. Enzymes involved in branched-chain amino acid metabolism in humans. Amino Acids 2017, 49, 1005–1028. [Google Scholar] [CrossRef] [PubMed]
- Sperringer, J.E.; Addington, A.; Hutson, S.M. Branched-chain amino acids and brain metabolism. Neurochem. Res. 2017, 42, 1697–1709. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Li, L.; Rezaei, A.; Eslamfam, S.; Che, D.; Ma, X. Metabolites of dietary protein and peptides by intestinal microbes and their impacts on gut. Curr. Protein Pept. Sci. 2015, 16, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Boutry, C.; El-Kadi, S.W.; Suryawan, A.; Wheatley, S.M.; Orellana, R.A.; Kimball, S.R.; Nguyen, H.V.; Davis, T.A. Leucine pulses enhance skeletal muscle protein synthesis during continuous feeding in neonatal pigs. Am. J. Physiol. Endocrinol. Metab. 2013, 305, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zuo, F.; Zhao, S.; He, P.; Wei, H.; Xiang, Q.; Pang, J.; Peng, J. Dietary supplementation of branched-chain amino acids increases muscle net amino acid fluxes through elevating their substrate availability and intramuscular catabolism in young pigs. Br. J. Nutr. 2017, 117, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Bröer, S.; Bröer, A. Amino acid homeostasis and signalling in mammalian cells and organisms. Biochem. J. 2017, 474, 1935–1963. [Google Scholar] [CrossRef] [PubMed]
- Zhenyukh, O.; Civantos, E.; Ruiz-Ortega, M.; Sánchez, M.S.; Vázquez, C.; Peiró, C.; Egido, J.; Mas, S. High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mtorc1 activation. Free Radic. Biol. Med. 2017, 104, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.A.; Lashinger, L.M.; Rasmussen, A.J.; Hursting, S.D. Leucine supplementation differentially enhances pancreatic cancer growth in lean and overweight mice. Cancer Metab. 2014, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Neishabouri, S.H.; Hutson, S.M.; Davoodi, J. Chronic activation of mTOR complex 1 by branched chain amino acids and organ hypertrophy. Amino Acids 2015, 47, 1167–1182. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.; Oh, S.F.; Wada, S.; Rowe, G.C.; Liu, L.; Chan, M.C.; Rhee, J.; Hoshino, A.; Kim, B.; Ibrahim, A.; et al. A branched chain amino acid metabolite drives vascular transport of fat and causes insulin resistance. Nat. Med. 2016, 22, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Bar-Peled, L.; Sabatini, D.M. Regulation of mTORC1 by amino acids. Trends Cell Biol. 2014, 24, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Sonnet, D.S.; O’Leary, M.N.; Gutierrez, M.A.; Nguyen, S.M.; Mateen, S.; Hsu, Y.; Mitchell, K.P.; Lopez, A.J.; Vockley, J.; Kennedy, B.K.; et al. Metformin inhibits branched chain amino acid (BCAAs) derived ketoacidosis and promotes metabolic homeostasis in msud. Sci. Rep. 2016, 6, 28775. [Google Scholar] [CrossRef] [PubMed]
- Soomro, R.N.; Hu, R.; Qiao, Y.; El-Hack, M.E.A.; Abbasi, I.H.R.; Mohamed, M.A.E.; Alagawany, M.; Yang, X.; Yao, J.; Dhama, K. Effects of dietary protein sources and amino acid balance on growth performance, intestinal permeability and morphology in broiler chickens. Int. J. Pharm. 2017, 13, 378–387. [Google Scholar] [CrossRef]
- Nofal, M.; Zhang, K.; Han, S.; Rabinowitz, J.D. mTOR inhibition restores amino acid balance in cells dependent on catabolism of extracellular protein. Mol. Cell 2017, 67, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Wiltafsky, M.K.; Pfaffl, M.W.; Roth, F.X. The effects of branched-chain amino acid interactions on growth performance, blood metabolites, enzyme kinetics and transcriptomics in weaned pigs. Br. J. Nutr. 2010, 103, 964–976. [Google Scholar] [CrossRef] [PubMed]
- Zhen, H.; Kitaura, Y.; Kadota, Y.; Ishikawa, T.; Kondo, Y.; Xu, M.; Morishita, Y.; Ota, M.; Ito, T.; Shimomura, Y. mTORC1 is involved in the regulation of branched-chain amino acid catabolism in mouse heart. FEBS Open Bio 2016, 6, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Stipanuk, M.H. Leucine and protein synthesis: mTOR and beyond. Nutr. Rev. 2007, 65, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Sheriff, D.S.; Younis, M.Y.G.; Elshaari, F.A.; Mohamed, N.A.; Kuwaila, H.I.A.E.; Abdalla, S.A.S.; Elfaghi, R. A perspective on interaction between lipid and branched chain amino acids (BCAAs) in developing insulin resistance. Med. J. 2014, 1, 8–12. [Google Scholar]
- Herman, M.A.; She, P.; Peroni, O.D.; Lynch, C.J.; Kahn, B.B. Adipose tissue branched chain amino acid (BCAAs) metabolism modulates circulating BCAAs levels. J. Biol. Chem. 2010, 285, 11348–11356. [Google Scholar] [CrossRef] [PubMed]
- Cummings, N.E.; Williams, E.M.; Kasza, I.; Konon, E.N.; Schaid, M.D.; Schmidt, B.A.; Poudel, C.; Sherman, D.S.; Yu, D.; Arriola Apelo, S.I.; et al. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J. Physiol. 2017, 596, 623–645. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Muhamad, R.; Yan, G.; Yu, J.; Fan, Q.; Wang, Z.; Li, X.; Purnomoadi, A.; Achmadi, J.; Yan, X. Quantitative proteomics analysis reveals glutamine deprivation activates fatty acid β-oxidation pathway in HepG2 cells. Amino Acids 2016, 48, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, W.; Shao, J.; Wang, Y.; Zhou, M.; Sun, H. Branched-chain amino acid negatively regulates KLF15 expression via PI3K-AKT pathway. Front. Physiol. 2017, 8, 853. [Google Scholar] [CrossRef] [PubMed]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K pathway in human disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, S.; He, L.; Rong, Y.; Brier, L.W.; Sun, Q.; Liu, R.; Fan, W.; Chen, S.; Yue, Z.; et al. MTORC1-mediated NRBF2 phosphorylation functions as a switch for the class III PtdIns3K and autophagy. Autophagy 2017, 13, 592–607. [Google Scholar] [CrossRef] [PubMed]
- Doi, M.; Yamaoka, I.; Fukunaga, T.; Nakayama, M. Isoleucine, a potent plasma glucose-lowering amino acid, stimulates glucose uptake in C2C12, myotubes. Biochem. Biophys. Res. Commun. 2003, 312, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Doi, M.; Yamaoka, I.; Nakayama, M.; Mochizuki, S.; Sugahara, K.; Yoshizawa, F. Isoleucine, a blood glucose-lowering amino acid, increases glucose uptake in rat skeletal muscle in the absence of increases in AMP-activated protein kinase activity. J. Nutr. 2005, 135, 2103–2108. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, J.; Masaki, T.; Arakawa, M.; Seike, M.; Yoshimatsu, H. Isoleucine prevents the accumulation of tissue triglycerides and upregulates the expression of PPARα and uncoupling protein in diet-induced obese mice. J. Nutr. 2010, 140, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Meng, Q.; Zhang, Q.; Guo, F. Isoleucine or valine deprivation stimulates fat loss via increasing energy expenditure and regulating lipid metabolism in WAT. Amino Acids 2012, 43, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Grahame Hardie, D. AMP-activated protein kinase: A key regulator of energy balance with many roles in human disease. J. Intern. Med. 2014, 276, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Greene, E.; Li, W.; Kidd, M.T.; Dridi, S. Branched-chain amino acids modulate the expression of hepatic fatty acid metabolism-related genes in female broiler chickens. Mol. Nutr. Food Res. 2015, 59, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, F.; Wang, W.; Guo, Q.; Wen, C.; Yin, Y. Alteration of muscle fiber characteristics and the AMPK-SIRT1-PGC-1α axis in skeletal muscle of growing pigs fed low-protein diets with varying branched-chain amino acid ratios. Oncotarget 2017, 8, 107011–107021. [Google Scholar] [CrossRef] [PubMed]
- Ehling, S.; Reddy, T.M. Direct analysis of leucine and its metabolites β-hydroxy-β-methylbutyric acid, α-ketoisocaproic acid, and α-hydroxyisocaproic acid in human breast milk by liquid chromatography-mass spectrometry. J. Agric. Food Chem. 2015, 63, 7567–7573. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Zhou, M.; Mei, D.; Pan, B.; Liu, Y.; Jing, S.; Gu, X.; Huang, Y.; Li, G.; Wang, Y.; et al. Keto acid metabolites of branched-chain amino acids inhibit oxidative stress-induced necrosis and attenuate myocardial ischemia-reperfusion injury. J. Mol. Cell Cardiol. 2016, 101, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Hasselgren, P.O. Beta-Hydroxy-beta-methylbutyrate (HMB) and prevention of muscle wasting. Metabolism 2014, 63, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Medras, Z.J.H.; El-Sayed, N.M.; Zaitone, S.A.; Toraih, E.A.; Samie, M.M.; Moustafa, Y.M. Glutamine up-regulates pancreatic sodium-dependent neutral aminoacid transporter-2 and mitigates islets apoptosis in diabetic rats. Pharmacol. Rep. 2017, 70, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Han, M.; Li, D.; Hu, S.; Gilbreath, K.R.; Bazer, F.W.; Wu, G. L-Arginine promotes protein synthesis and cell growth in brown adipocyte precursor cells via the mTOR signal pathway. Amino Acids 2017, 49, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Hsieh, P.N.; Sweet, D.R.; Jain, M.K. Krüppel-like factor 15: Regulator of BCAAs metabolism and circadian protein rhythmicity. Pharmacol. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kimball, S.R.; Jefferson, L.S. New functions for amino acids: Effects on gene transcription and translation. Am. J. Clin. Nutr. 2006, 83, 500S–507S. [Google Scholar] [CrossRef] [PubMed]
- Columbus, D.A.; Fiorotto, M.L.; Davis, T.A. Leucine is a major regulator of muscle protein synthesis in neonates. Amino Acids 2015, 47, 259–270. [Google Scholar] [CrossRef] [PubMed]
- López, N.; Sánchez, J.; Palou, A.; Serra, F. Gender-associated impact of early leucine supplementation on adult predisposition to obesity in rats. Nutrients 2018, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.C.; Lang, C.H.; Crozier, S.J.; Anthony, T.G.; MacLean, D.A.; Kimball, S.R.; Jefferson, L.S. Contribution of insulin to the translational control of protein synthesis in skeletal muscle by leucine. Am. J. Physiol. 2002, 282, E1092–E1101. [Google Scholar] [CrossRef] [PubMed]
- Crozier, S.J.; Kimball, S.R.; Emmert, S.W.; Anthony, J.C.; Jefferson, L.S. Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J. Nutr. 2005, 135, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Churchward-Venne, T.A.; Breen, L.; Di Donato, D.M.; Hector, A.J.; Mitchell, C.J.; Moore, D.R.; Stellingwerff, T.; Breuille, D.; Offord, E.A.; Baker, S.K.; et al. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: A double-blind, randomized trial. Am. J. Clin. Nutr. 2014, 99, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Wyant, G.A.; Aburemaileh, M.; Wolfson, R.L.; Chen, W.W.; Freinkman, E.; Danai, L.V.; Heiden, M.G.V.; Sabatini, D.M. mTORC1 activator SLC38A9 is required to efflux essential amino acids from lysosomes and use protein as a nutrient. Cell 2017, 171, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.C.; Anthony, T.G.; Kimball, S.R.; Vary, T.C.; Jefferson, L.S. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J. Nutr. 2010, 130, 139–145. [Google Scholar] [CrossRef]
- Davis, T.A.; Fiorotto, M.L. Regulation of muscle growth in neonates. Curr. Opin. Nutr. Metab. Care 2009, 12, 78–85. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Eslamfam, S.; Ma, X.; Li, D. Autophagy and the nutritional signaling pathway. Front. Agric. Sci. Eng. 2016, 3, 222–230. [Google Scholar] [CrossRef]
- Jackman, S.R.; Witard, O.C.; Philp, A.; Wallis, G.A.; Baar, K.; Tipton, K.D. Branched-chain amino acid ingestion stimulates muscle myofibrillar protein synthesis following resistance exercise in humans. Front. Physiol. 2017, 8, 390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ren, M.; Zeng, X.; He, P.; Ma, X.; Qiao, S. Leucine stimulates ASCT2 amino acid transporter expression in porcine jejunal epithelial cell line (IPEC-J2) through PI3K/AKT/mTOR and ERK signaling pathways. Amino Acids 2014, 46, 2633–2642. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, R.L.; Chantranupong, L.; Saxton, R.A.; Shen, K.; Scaria, S.M.; Cantor, J.R.; Sabatini, D.M. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 2016, 351, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Han, J.M.; Jeong, S.J.; Park, M.C.; Kim, G.; Kwon, N.H.; Kim, H.K.; Ha, S.H.; Ryu, S.H.; Kim, S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell 2012, 149, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Huang, C.; Lian, K.; Wang, S.; Zhao, H.; Yan, F.; Zhang, X.; Zhang, J.; Xie, H.; An, R.; Tao, L. BCKA down-regulates mTORC2-Akt signal and enhances apoptosis susceptibility in cardiomyocytes. Biochem. Biophys. Res. Commun. 2016, 480, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J.; Hossain, T.; Limb, M.C.; Phillips, B.E.; Lund, J.; Williams, J.P.; Brook, M.S.; Cegielski, J.; Philp, A.; Ashcroft, S.; et al. Impact of the calcium form of β-hydroxy-β-methylbutyrate upon human skeletal muscle protein metabolism. Clin. Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Girón, M.D.; Vílchez, J.D.; Salto, R.; Manzano, M.; Sevillano, N.; Campos, N.; Argilés, J.M.; Rueda, R.; López-Pedrosa, J.M. Conversion of leucine to β-hydroxy-β-methylbutyrate by α-keto isocaproate dioxygenase is required for a potent stimulation of protein synthesis in l6 rat myotubes. J. Cachexia Sarcopenia Muscle 2016, 7, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, S.M.; El-Kadi, S.W.; Suryawa, A.; Boutry, C.; Orellana, R.A.; Nguyen, H.V.; Davis, S.R.; Davis, T.A. Protein synthesis in skeletal muscle of neonatal pigs is enhanced by administration of beta-hydroxy-beta-methylbutyrate. Am. J. Physiol. Endocrinol. Metab. 2014, 306, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Kao, M.; Columbus, D.A.; Suryawan, A.; Steinhoff-Wagner, J.; Hernandez-Garcia, A.; Nguyen, H.V.; Fiorotto, M.L.; Davis, T.A. Enteral β-hydroxy-β-methylbutyrate supplementation increases protein synthesis in skeletal muscle of neonatal pigs. Am. J. Physiol. Endocrinol. Metab. 2016, 310, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Gerlinger-Romero, F.; Guimarães-Ferreira, L.; Yonamine, C.Y.; Salgueiro, R.B.; Nunes, M.T. Effects of beta-hydroxy-beta-methylbutyrate (HMB) on the expression of ubiquitin ligases, protein synthesis pathways and contractile function in extensor digitorum longus (DEL) of fed and fasting rats. J. Physiol. Sci. 2017, 68, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Aversa, Z.; Bonetto, A.; Costelli, P.; Minero, V.G.; Penna, F.; Baccino, F.M.; Lucia, S.; Rossi Faelli, F.; Muscaritoli, M. β-hydroxy-β-methylbutyrate (HMB) attenuates muscle and body weight loss in experimental cancer cachexia. Int. J. Oncol. 2011, 38, 713–720. [Google Scholar] [PubMed]
- Van Zanten, A.R. Glutamine and antioxidants: Status of their use in critical illness. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Garrett, W.S.; Gordon, J.I.; Glimcher, L.H. Homeostasis and inflammation in the intestine. Cell 2010, 140, 859–870. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Han, M.; Farrar, S.; Ma, X. Impacts and regulation of dietary nutrients on gut microbiome and immunity. Protein Pept. Lett. 2017, 24, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Mcgaha, T.L.; Huang, L.; Lemos, H.; Metz, R.; Mautino, M.; Prendergast, G.C.; Mellor, A.L. Amino acid catabolism: A pivotal regulator of innate and adaptive immunity. Immunol. Rev. 2012, 249, 135–157. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Zhang, S.H.; Zeng, X.F.; Liu, H.; Qiao, S.Y. Branched-chain amino acids are beneficial to maintain growth performance and intestinal immune-related function in weaned piglets fed protein restricted diet. Asian-australas. J. Anim. Sci. 2015, 28, 1742–1750. [Google Scholar]
- Ren, M.; Zhang, S.; Liu, X.; Li, S.; Mao, X.; Zeng, X.; Qiao, S. Different lipopolysaccharide branched-chain amino acids modulate porcine intestinal endogenous β-defensin expression through the Sirt1/ERK/90RSK pathway. J. Agric. Food Chem. 2016, 64, 337–3379. [Google Scholar]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A double-edged sword for health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.D.; Deng, Y.P.; Liu, Y.; Qu, B.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.; Wu, P.; Zhang, Y.; Zhou, X.; Feng, L. Dietary leucine regulates the intestinal immune status, immune-related signalling molecules and tight junction transcript abundance in grass carp (Ctenopharyngodon idella). Aquaculture 2015, 444, 134–142. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Tian, Y.; Huang, C.; Li, D.; Zhong, Q.; Ma, X. Interaction between microbes and host intestinal health: Modulation by dietary nutrients and gut-brain-endocrine-immune axis. Curr. Protein Pept. Sci. 2015, 16, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Tian, Y.; Wu, Y.; Ma, X. Contributions of the interaction between dietary protein and gut microbiota to intestinal health. Curr. Protein Pept. Sci. 2017, 18, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Feng, L.; Liu, Y.; Jiang, W.; Wu, P.; Jiang, J.; Zhang, Y.; Zhou, X. Effect of dietary isoleucine on the immunity, antioxidant status, tight junctions and microflora in the intestine of juvenile jian carp (Cyprinus carpio, var. jian). Fish Shellfish Immunol. 2014, 41, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Branched-chain amino acid and immunity. J. Nutr. 2006, 136, 288S–293S. [Google Scholar] [CrossRef] [PubMed]
- De Simone, R.; Vissicchio, F.; Mingarelli, C.; De Nuccio, C.; Visentin, S.; Ajmone-Cat, M.A.; Minghetti, L. Branched-chain amino acids influence the immune properties of microglial cells and their responsiveness to pro-inflammatory signals. Biochim. Biophys. Acta 2013, 1832, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Guo, P.; Zhang, J.; He, T.; Kim, S.W.; Zhang, G.; Ma, X. Nutrients mediate intestinal bacteria-mucosal immune crosstalk. Front. Immunol. 2018, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, I. Impairment of innate immune responses in cirrhotic patients and treatment by branched-chain amino acids. World J. Gastroenterol. 2014, 20, 7298–7305. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Santiago, C.; Rivas-Santiago, B.; León, D.; Castañeda-Delgado, J.; Hernández, P.R. Induction of β-efensins by L-isoleucine as novel immunotherapy in experimental murine tuberculosis. Clin. Exp. Immunol. 2011, 164, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Bassit, R.A.; Sawada, L.A.; Bacurau, R.F.; Navarro, F.; Martins, E.; Santos, R.V.; Caperuto, E.C.; Rogeri, P.; Rosa, L.F.C. Branched-chain amino acid supplementation and the immune response of long-distance athletes. Nutrition 2002, 18, 376–379. [Google Scholar] [CrossRef]
- Kakazu, E.; Kanno, N.; Ueno, Y.; Shimosegawa, T. Extracellular branched-chain amino acids, especially valine, regulate maturation and function of monocyte-derived dendritic cells. J. Immunol. 2007, 179, 7137–7146. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, Y.; Jiang, J.; Wu, P.; Jiang, W.; Li, S.; Tang, L.; Kuang, S.; Feng, L.; Zhou, X. Effects of dietary isoleucine on the immune response, antioxidant status and gene expression in the head kidney of juvenile jian carp (Cyprinus carpio var. jian). Fish Shellfish Immunol. 2013, 35, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Wu, L.; Li, G.; Tao, S.; Sheng, Z.; Meng, Q.; Li, F.; Yu, L.; Li, L. Effects of enteral nutrition with parenteral glutamine supplementation on the immunological function in septic rats. Br. J. Nutr. 2015, 113, 1712–1722. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Gao, Q.; Dong, S.; Lan, Y.; Ye, Z.; Wen, B. Regulation of dietary glutamine on the growth, intestinal function, immunity and antioxidant capacity of sea cucumber Apostichopus japonicus (selenka). Fish Shellfish Immunol. 2016, 50, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Mai, K.; Xu, W.; Liufu, Z.; Zhang, Y.; Peng, M.; Chen, J.; Ai, Q. Effects of dietary arginine and glutamine on growth performance, nonspecific immunity, and disease resistance in relation to arginine catabolism in juvenile turbot (Scophthalmus maximus L.). Aquaculture 2017, 468, 246–254. [Google Scholar] [CrossRef]
- Guillet, C.; Delcourt, I.; Rance, M.; Giraudet, C.; Walrand, S.; Bedu, M.; Duche, P.; Boirie, Y.J. Changes in basal and insulin and amino acid response of whole body and skeletal muscle proteins in obese men. J. Clin. Endocrinol. Metab. 2009, 94, 3044–3050. [Google Scholar] [CrossRef] [PubMed]
- Asghari, G.; Farhadnejad, H.; Teymoori, F.; Mirmiran, P.; Tohidi, M.; Azizi, F. High dietary intakes of branched-hain amino acids is associated with increased risk of insulin resistance in adults. J. Diabetes 2017. [Google Scholar] [CrossRef]
- Allam-Ndoul, B.; Guénard, F.; Garneau, V.; Barbier, O.; Pérusse, L.; Vohl, M. Associations between branched chain amino acid levels, obesity and cardiometabolic complications. Integr. Obes. Diabetes 2015, 1, 157–162. [Google Scholar] [CrossRef]
- McCormack, S.E.; Shaham, O.; McCarthy, M.A.; Deik, A.A.; Wang, T.J.; Gerszten, R.E.; Clish, C.B.; Mootha, V.K.; Grinspoon, S.K.; Fleischman, A. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr. Obes. 2013, 8, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Gannon, N.P.; Schnuck, J.K.; Vaughan, R.A. BCAA metabolism and insulin sensitivity-dysregulated by metabolic status? Mol. Nutr. Food Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, R.; Goto, A.; Budhathoki, S.; Yamaji, T.; Yamamoto, H.; Kato, Y.; Iwasaki, M.; Tsugane, S. Association between plasma concentrations of branched-chain amino acids and adipokines in Japanese adults without diabetes. Sci. Rep. 2018, 8, 1043. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Nakamura, K.; Wada, K.; Tsuji, M.; Tamai, Y.; Kawachi, T. Branched-chain amino acid intake and the risk of diabetes in a Japanese community: the Takayama study. Am. J. Epidemiol. 2013, 178, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Y.; Qi, Q.; Hruby, A.; Manson, J.E.; Willett, W.C.; Wolpin, B.M.; Hu, F.B.; Qi, L. Cumulative consumption of branched-chain amino acids and incidence of type 2 diabetes. Int. J. Epidemiol. 2016, 45, 1482–1492. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Tavintharan, S.; Sum, C.F.; Woon, K.; Lim, S.C.; Ong, C.N. Metabolic signature shift in type 2 diabetes mellitus revealed by mass spectrometry-based metabolomics. J. Clin. Endocrinol. Metab. 2013, 98, E1060–E1065. [Google Scholar] [CrossRef] [PubMed]
- Maida, A.; Chan, J.; Sjøberg, K.A.; Zota, A.; Schmoll, D.; Kiens, B.; Herzig, S.; Rose, A.J. Repletion of branched chain amino acids reverses mtorc1 signaling but not improved metabolism during dietary protein dilution. Mol. Metab. 2017, 6, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Elshorbagy, A.; Jernerén, F.; Basta, F.; Basta, C.; Turner, C.; Khaled, M.; Refsum, H. Amino acid changes during transition to a vegan diet supplemented with fish in healthy humans. Eur. J. Nutr. 2017, 56, 1953–1962. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Mardinoglu, A.; Gogg, S.; Lotta, L.A.; Stančákováe, A.; Nerstedt, A.; Boren, J.; Blüher, M.; Ferrannini, E.; Langenberg, C.; Wareham, N.J.; et al. Elevated plasma levels of 3-Hydroxyisobutyric acid are associated with incident Type 2 diabetes. EBioMedicine 2018, 27, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Morgensztern, D.; Mcleod, H.L. PI3K/AKT/mTOR pathway as a target for cancer therapy. Anticancer Drugs 2005, 16, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.M.; Koreckij, T.D.; Corey, E. Targeted therapy for advanced prostate cancer: inhibition of the PI3K/AKT/mTOR pathway. Curr. Cancer Drug Targets 2009, 9, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Dibble, C.C.; Cantley, L.C. Regulation of mtorc1 by pi3k signaling. Trends Cell Biol. 2015, 25, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Reina-Campos, M.; Moscat, J.; Diaz-Meco, M. Metabolism shapes the tumor microenvironment. Curr. Opin. Cell Biol. 2017, 48, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Deberardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef] [PubMed]
- Ananieva, E.A.; Wilkinson, A.C. Branched-chain amino acid metabolism in cancer. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Han, J. Branched-chain amino acid transaminase 1 (BCAT1) promotes the growth of breast cancer cells through improving mTOR-mediated mitochondrial biogenesis and function. Biochem. Biophys. Res. Commun. 2017, 486, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.H.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Crosslin, D.R.; Haynes, C.; Dungan, J.; Newby, L.K.; Hauser, E.R.; Ginsburg, G.S.; et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ. Cardiovasc. Genet. 2010, 3, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Olson, K.C.; Gao, C.; Prosdocimo, D.A.; Zhou, M.; Wang, Z.; Jeyaraj, D.; Youn, J.; Ren, S.; Liu, Y.; et al. Catabolic defect of branched-chain amino acids promotes heart failure. Circulation 2016, 133, 2038–2049. [Google Scholar] [CrossRef] [PubMed]
- Mangge, H.; Zelzer, S.; Prüller, F.; Schnedl, W.J.; Weghuber, D.; Enko, D.; Bergsten, P.; Haybaeck, J.; Meinitzer, A. Branched-chain amino acids are associated with cardiometabolic risk profiles found already in lean, overweight and obese young. J. Nutr. Biochem. 2016, 32, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Gilstrap, L.G.; Wang, T.J. Biomarkers and cardiovascular risk assessment for primary prevention: An update. Clin. Chem. 2012, 58, 72–82. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, C.; He, T.; Zhang, W.; Zhang, G.; Ma, X. Branched Chain Amino Acids: Beyond Nutrition Metabolism. Int. J. Mol. Sci. 2018, 19, 954. https://doi.org/10.3390/ijms19040954
Nie C, He T, Zhang W, Zhang G, Ma X. Branched Chain Amino Acids: Beyond Nutrition Metabolism. International Journal of Molecular Sciences. 2018; 19(4):954. https://doi.org/10.3390/ijms19040954
Chicago/Turabian StyleNie, Cunxi, Ting He, Wenju Zhang, Guolong Zhang, and Xi Ma. 2018. "Branched Chain Amino Acids: Beyond Nutrition Metabolism" International Journal of Molecular Sciences 19, no. 4: 954. https://doi.org/10.3390/ijms19040954