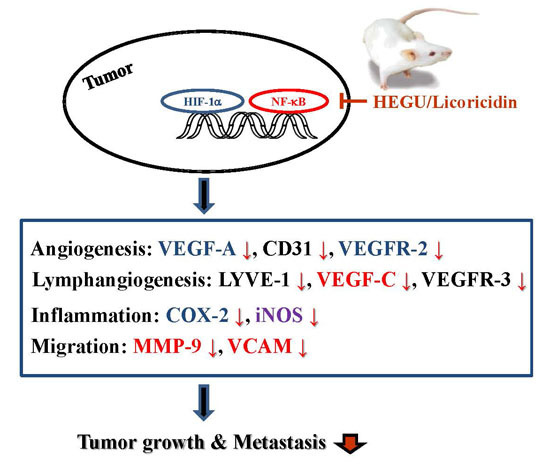
Licoricidin, an Active Compound in the Hexane/Ethanol Extract of Glycyrrhiza uralensis, Inhibits Lung Metastasis of 4T1 Murine Mammary Carcinoma Cells
Abstract
:1. Introduction
2. Results
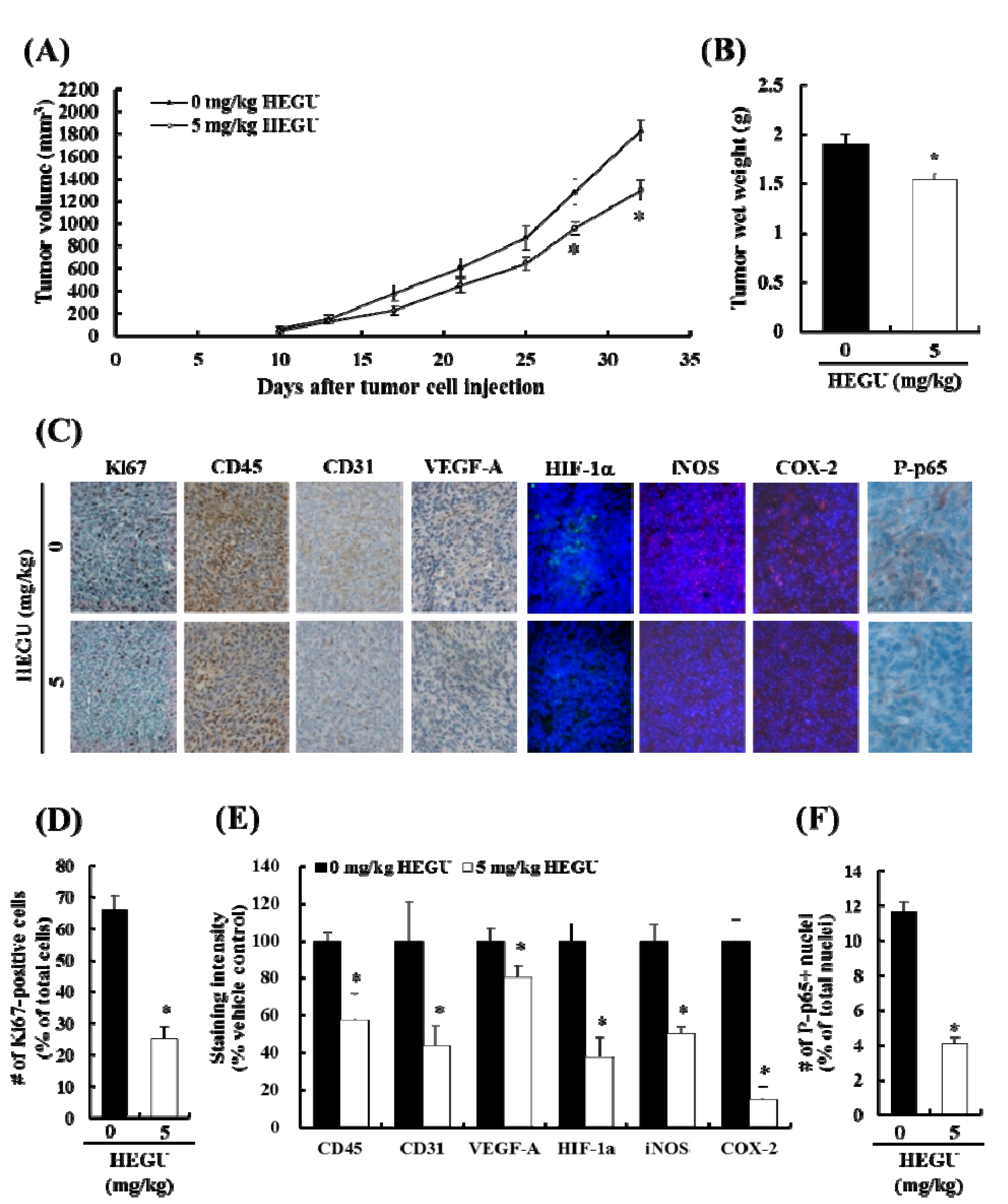
2.1. Addition of Glycyrrhiza uralensis (HEGU) to Drinking Water Inhibits Solid Tumor Growth and Decreases Cell Proliferation and the Expression of Proteins Related to Angiogenesis and Inflammation in 4T1 Mammary Tumor Tissues in BALB/c Mice
2.2. HEGU in Drinking Water Inhibits Lung Metastasis in the 4T1 Mouse Tumor Model
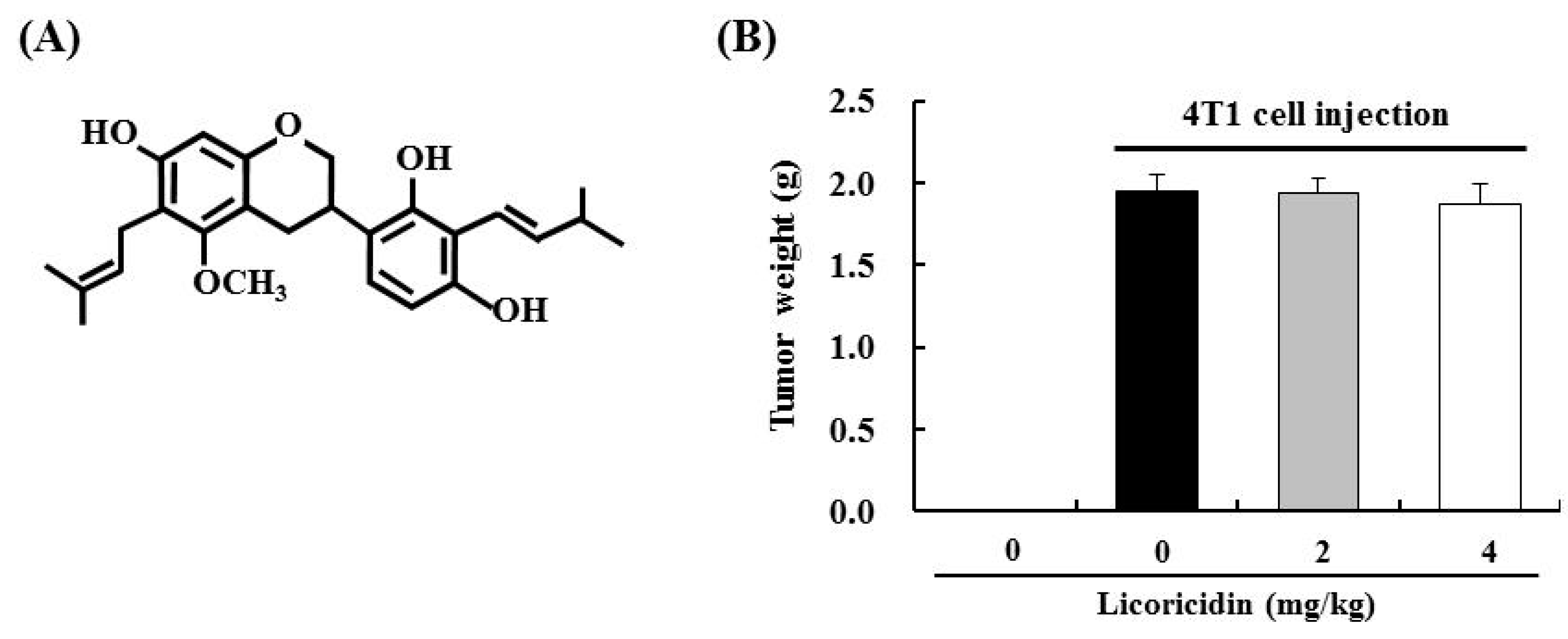
2.3. Licoricidin Inhibits Lung Metastasis in the 4T1 Mouse Tumor Model
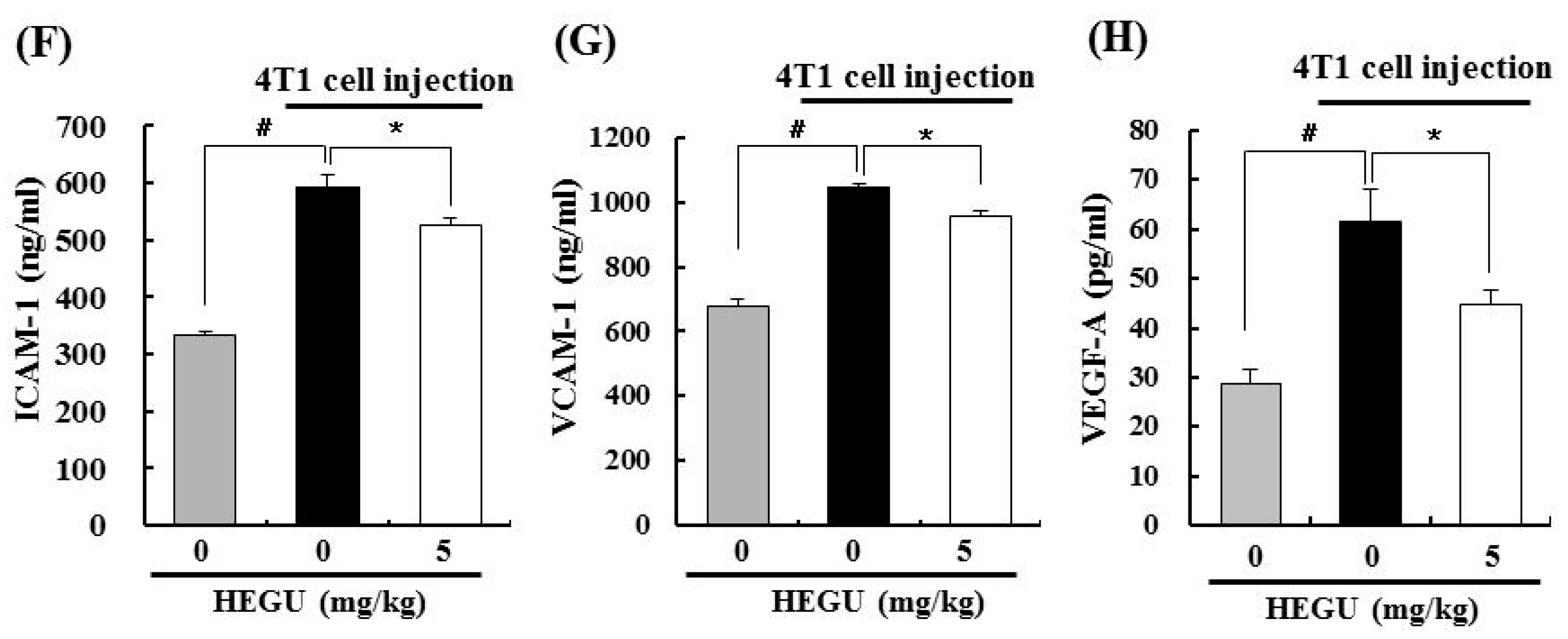
2.4. Licoricidin Decreases the Infiltration of Macrophages, Especially M2 Cells, and the Expression of Proteins Related to Inflammation, Angiogenesis, and Lymphangiogenesis in 4T1 Mammary Tumor Tissues in BALB/c Mice
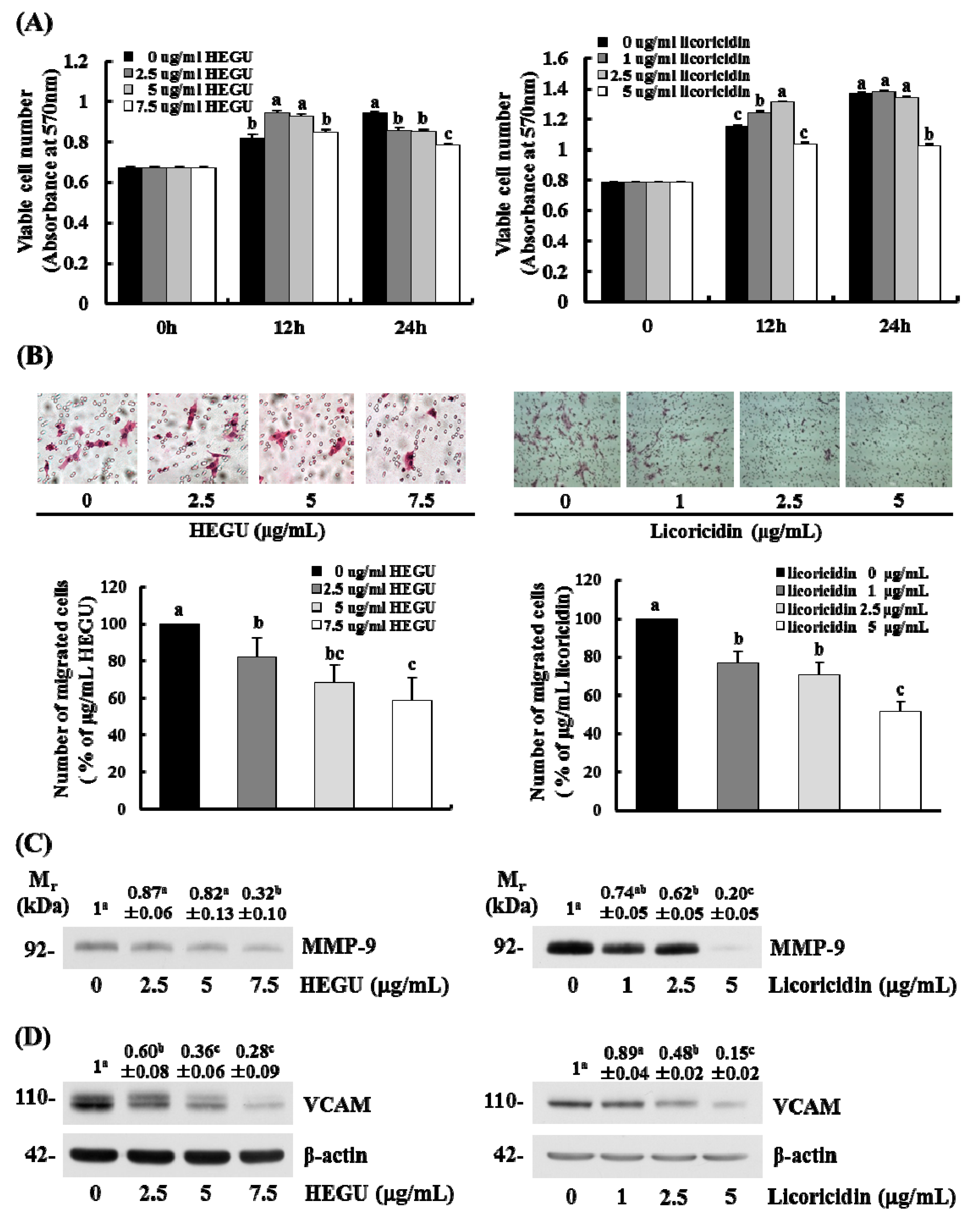
2.5. HEGU and Licoricidin Inhibit the Migration of 4T1 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Line
4.3. Animal Study Design
4.4. Immunohistochemical (IHC) and IFstaining
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Western Blot Analysis
4.7. Transwell Migration Assay
4.8. Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| HEGU | Hexane/ethanol extract of Glycyrrhiza uralensis |
| CD31 | Platelet endothelial cell adhesion molecule-1 |
| VEGF-A | Vascular endothelial growth factor-A |
| LYVE-1 | Lymphatic vessel endothelial receptor-1 |
| HIF-1α | Hypoxia-inducible factor-1α |
| iNOS | Inducible nitric oxide synthase |
| COX-2 | cyclooxygenase-2 |
| MMP-9 | Matrix metalloproteinase-9 |
| TIMP-1 | Tissue inhibitor of metalloproteinase-1 |
| ICAM | Intercellular adhesion molecule |
| VCAM | Vascular cell adhesion molecule |
| IHC | Immunohistochemical |
| IF | Immunofluorescence |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| VEGF-R | Vascular endothelial growth factor receptor |
| CDK4 | Cyclin-dependent kinase 4 |
References
- DeSantis, C.; Ma, J.; Bryan, L.; Jemal, A. Breast cancer statistics, 2013. CA: Cancer J. Clin. 2014, 64, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Solomayer, E.F.; Diel, I.J.; Meyberg, G.C.; Gollan, C.; Bastert, G. Metastatic breast cancer: Clinical course, prognosis and therapy related to the first site of metastasis. Breast Cancer Res. Treat. 2000, 59, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Thirugnanam, S.; Xu, L.; Ramaswamy, K.; Gnanasekar, M. Glycyrrhizin induces apoptosis in prostate cancer cell lines DU-145 and LNCaP. Oncol. Rep. 2008, 20, 1387–1392. [Google Scholar] [PubMed]
- Suzuki, F.; Schmitt, D.A.; Utsunomiya, T.; Pollard, R.B. Stimulation of host resistance against tumors by glycyrrhizin, an active component of licorice roots. In Vivo 1992, 6, 589–596. [Google Scholar] [PubMed]
- Celik, M.M.; Karakus, A.; Zeren, C.; Demir, M.; Bayarogullari, H.; Duru, M.; Al, M. Licorice induced hypokalemia, edema, and thrombocytopenia. Hum. Exp. Toxicol. 2012, 31, 1295–1298. [Google Scholar] [CrossRef] [PubMed]
- Olukoga, A.; Donaldson, D. Liquorice and its health implications. J. R. Soc. Promot. Health 2000, 120, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Seon, M.R.; Lim, S.S.; Kim, J.S.; Chun, H.S.; Park, J.H. Hexane/ethanol extract of Glycyrrhiza uralensis licorice suppresses doxorubicin-induced apoptosis in H9c2 rat cardiac myoblasts. Exp. Biol. Med. (Maywood) 2008, 233, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Seon, M.R.; Lim, S.S.; Choi, H.J.; Park, S.Y.; Cho, H.J.; Kim, J.K.; Kim, J.; Kwon, D.Y.; Park, J.H. Isoangustone A present in hexane/ethanol extract of Glycyrrhiza uralensis induces apoptosis in DU145 human prostate cancer cells via the activation of DR4 and intrinsic apoptosis pathway. Mol. Nutr. Food Res. 2010, 54, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Seon, M.R.; Park, S.Y.; Kwon, S.J.; Lim, S.S.; Choi, H.J.; Park, H.; Lim do, Y.; Kim, J.S.; Lee, C.H.; Kim, J.; et al. Hexane/ethanol extract of Glycyrrhiza uralensis and its active compound isoangustone a induce G1 cycle arrest in DU145 human prostate and 4T1 murine mammary cancer cells. J. Nutr. Biochem. 2012, 23, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lim, S.S.; Kim, J.K.; Kang, I.J.; Kim, J.S.; Lee, C.; Kim, J.; Park, J.H. Hexane-Ethanol extract of Glycyrrhiza uralensis containing licoricidin inhibits the metastatic capacity of DU145 human prostate cancer cells. Br. J. Nutr. 2010, 104, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- La, V.D.; Tanabe, S.; Bergeron, C.; Gafner, S.; Grenier, D. Modulation of matrix metalloproteinase and cytokine production by licorice isolates licoricidin and licorisoflavan A: Potential therapeutic approach for periodontitis. J. Periodontol. 2011, 82, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Gafner, S.; Bergeron, C.; Villinski, J.R.; Godejohann, M.; Kessler, P.; Cardellina, J.H.; Ferreira, D.; Feghali, K.; Grenier, D. Isoflavonoids and coumarins from Glycyrrhiza uralensis: Antibacterial activity against oral pathogens and conversion of isoflavans into isoflavan-quinones during purification. J. Nat. Prod. 2011, 74, 2514–2519. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; Christofori, G. Rebuilding cancer metastasis in the mouse. Mol. Oncol. 2013, 7, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.F.; Groom, A.C.; MacDonald, I.C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2002, 2, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Seyfried, T.N.; Huysentruyt, L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef]
- Stockmann, C.; Schadendorf, D.; Klose, R.; Helfrich, I. The impact of the immune system on tumor: Angiogenesis and vascular remodeling. Front. Oncol. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.A.; Kang, Y. The metastasis-promoting roles of tumor-associated immune cells. J. Mol. Med. 2013, 91, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Ruffell, B.; DeNardo, D.G.; Affara, N.I.; Coussens, L.M. Lymphocytes in cancer development: Polarization towards pro-tumor immunity. Cytokine Growth Factor Rev. 2010, 21, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Komohara, Y.; Fujiwara, Y.; Ohnishi, K.; Takeya, M. Tumor-Associated macrophages: Potential therapeutic targets for anti-cancer therapy. Adv. Drug Deliv. Rev. 2015, 99 Pt B, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Fang, M.; Alroy, J.; Sahagian, G.G. Imagable 4T1 model for the study of late stage breast cancer. BMC Cancer 2008, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Sauter, B.V.; Martinet, O.; Zhang, W.J.; Mandeli, J.; Woo, S.L. Adenovirus-Mediated gene transfer of endostatin in vivo results in high level of transgene expression and inhibition of tumor growth and metastases. Proc. Natl. Acad. Sci. USA 2000, 97, 4802–4807. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P. The role of hypoxia-induced factors in tumor progression. Oncologist 2004, 9 (Suppl. 5), 10–17. [Google Scholar] [CrossRef] [PubMed]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell. Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Carmeliet, P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005, 69 (Suppl. 3), 4–10. [Google Scholar] [CrossRef] [PubMed]
- Baluk, P.; McDonald, D.M. Markers for microscopic imaging of lymphangiogenesis and angiogenesis. Ann. N. Y. Acad. Sci. 2008, 1131, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.A.; Giaccia, A.J. Hypoxia, gene expression, and metastasis. Cancer Metastasis Rev. 2007, 26, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Johnson, R.S. Hypoxia: A key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007, 26, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Shen, S.; Verma, I.M. NF-κB, an active player in human cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, C.; Giannoudis, A.; Lewis, C.E. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood 2004, 104, 2224–2234. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Jung, J.I.; Lim do, Y.; Kwon, G.T.; Her, S.; Park, J.H. Bone marrow-derived, alternatively activated macrophages enhance solid tumor growth and lung metastasis of mammary carcinoma cells in a BALB/c mouse orthotopic model. Breast Cancer Res. 2012, 14, R81. [Google Scholar] [CrossRef] [PubMed]
- Deryugina, E.I.; Quigley, J.P. Tumor angiogenesis: MMP-Mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix Biol. J. Int. Soc. Matrix Biol. 2015, 44–46, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.P. Cell adhesion molecules in the development and progression of malignant melanoma. Cancer Metastasis Rev. 1999, 18, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Kim, W.K.; Kim, E.J.; Jung, K.C.; Park, S.; Lee, H.S.; Tyner, A.L.; Park, J.H. Conjugated linoleic acid inhibits cell proliferation and ErbB3 signaling in HT-29 human colon cell line. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G996–G1005. [Google Scholar] [CrossRef] [PubMed]
- Kwon, G.T.; Cho, H.J.; Chung, W.Y.; Park, K.K.; Moon, A.; Park, J.H. Isoliquiritigenin inhibits migration and invasion of prostate cancer cells: Possible mediation by decreased JNK/AP-1 signaling. J. Nutr. Biochem. 2009, 20, 663–676. [Google Scholar] [CrossRef] [PubMed]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.Y.; Kwon, S.J.; Lim, S.S.; Kim, J.-K.; Lee, K.W.; Park, J.H.Y. Licoricidin, an Active Compound in the Hexane/Ethanol Extract of Glycyrrhiza uralensis, Inhibits Lung Metastasis of 4T1 Murine Mammary Carcinoma Cells. Int. J. Mol. Sci. 2016, 17, 934. https://doi.org/10.3390/ijms17060934
Park SY, Kwon SJ, Lim SS, Kim J-K, Lee KW, Park JHY. Licoricidin, an Active Compound in the Hexane/Ethanol Extract of Glycyrrhiza uralensis, Inhibits Lung Metastasis of 4T1 Murine Mammary Carcinoma Cells. International Journal of Molecular Sciences. 2016; 17(6):934. https://doi.org/10.3390/ijms17060934
Chicago/Turabian StylePark, So Young, Soo Jin Kwon, Soon Sung Lim, Jin-Kyu Kim, Ki Won Lee, and Jung Han Yoon Park. 2016. "Licoricidin, an Active Compound in the Hexane/Ethanol Extract of Glycyrrhiza uralensis, Inhibits Lung Metastasis of 4T1 Murine Mammary Carcinoma Cells" International Journal of Molecular Sciences 17, no. 6: 934. https://doi.org/10.3390/ijms17060934