Modulating Composition and Metabolic Activity of the Gut Microbiota in IBD Patients
Abstract
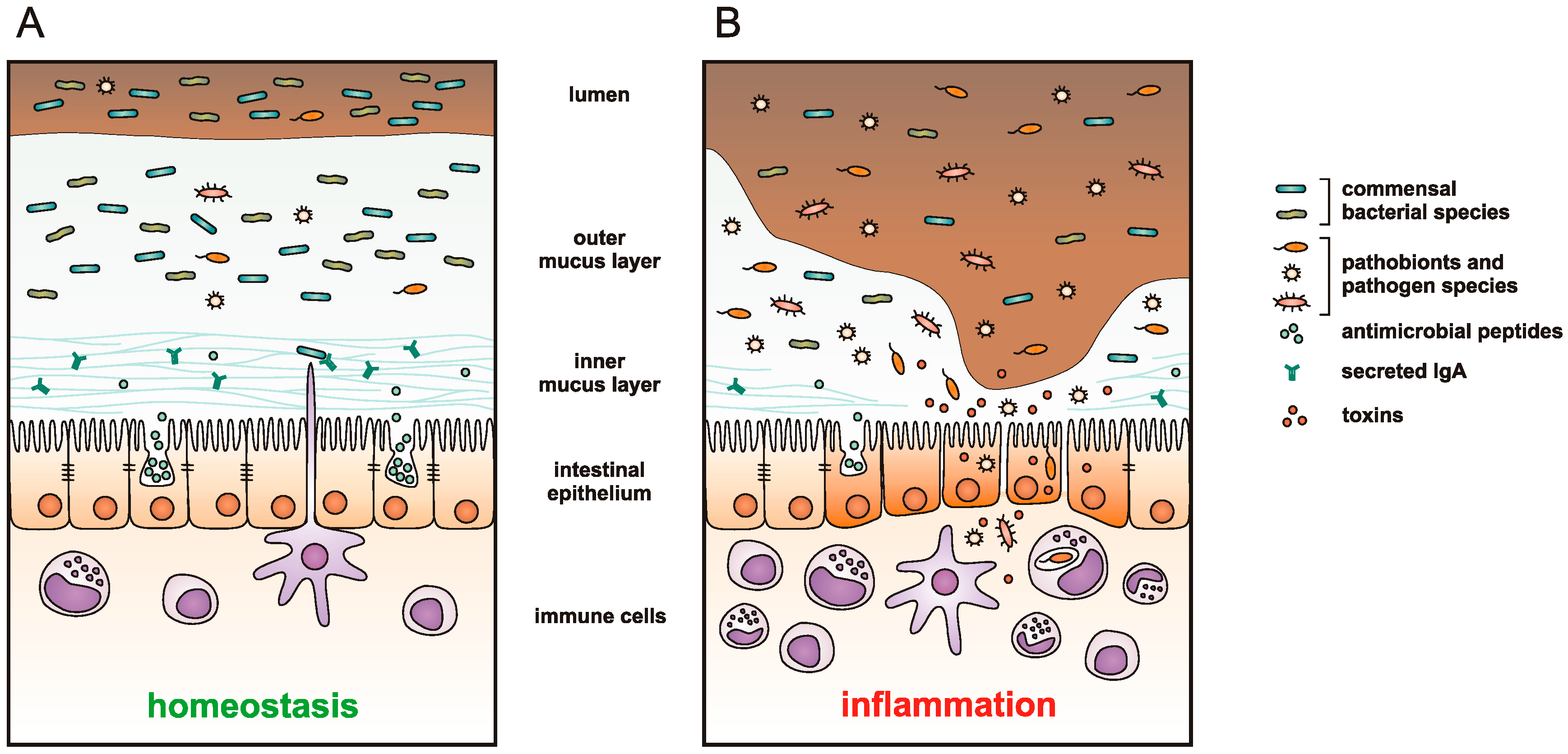
:1. Introduction
2. Nutritional Patterns and Dietary Interventions
3. Supplementation with Probiotics, Prebiotics and Synbiotics
4. Use of Antibiotics
5. Fecal Microbiota Transplantation
6. Investigational Approaches
7. Conclusions and Future Perspectives
Acknowledgments
Conflicts of Interest
Abbreviations
| IBD | inflammatory bowel disease |
| CD | Crohn’s disease |
| UC | ulcerative colitis |
| FMT | fecal microbiota transplantation |
| SCFA | short-chain fatty acids |
| CRP | C-reactive protein |
| EEN | exclusive enteral nutrition |
| PEN | partial enteral nutrition |
| SVD | semi-vegetarian diet |
| SCD | specific carbohydrate diet |
| low-FODMAP | low-fermentable oligosaccharide, disaccharide, monosaccharide, and polyol diet |
| IBD-AID | IBD-anti-inflammatory diet |
| ECCO | European Crohn's and Colitis Organisation |
| GBF | germinated barley foodstuff |
| CDI | Clostridium difficile infection |
| ESCMID | European Society of Clinical Microbiology and Infectious Diseases |
References
- Sekirov, I.; Russell, S.L.; Antunes, L.C.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 2006, 124, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Evans, R.M. Microbiology: Wealth management in the gut. Nature 2013, 500, 538–539. [Google Scholar] [CrossRef] [PubMed]
- Ottman, N.; Smidt, H.; de Vos, W.M.; Belzer, C. The function of our microbiota: Who is out there and what do they do? Front. Cell. Infect. Microbiol. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Manichanh, C.; Borruel, N.; Casellas, F.; Guarner, F. The gut microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Nagalingam, N.A.; Lynch, S.V. Role of the microbiota in inflammatory bowel diseases. Inflamm. Bowel Dis. 2012, 18, 968–984. [Google Scholar] [CrossRef] [PubMed]
- Manichanh, C.; Rigottier-Gois, L.; Bonnaud, E.; Gloux, K.; Pelletier, E.; Frangeul, L.; Nalin, R.; Jarrin, C.; Chardon, P.; Marteau, P.; et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 2006, 55, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B. Microbial influences in inflammatory bowel diseases. Gastroenterology 2008, 134, 577–594. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, H.; Kataoka, K.; Ishikawa, H.; Ikata, K.; Arimochi, H.; Iwasaki, T.; Ohnishi, Y.; Kuwahara, T.; Yasutomo, K. Reduced diversity and imbalance of fecal microbiota in patients with ulcerative colitis. Dig. Dis. Sci. 2012, 57, 2955–2964. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, J.; Kudo, T.; Sakata, S.; Benno, Y.; Sugiyama, T. Diversity of mucosa-associated microbiota in active and inactive ulcerative colitis. Scand. J. Gastroenterol. 2009, 44, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Ott, S.J.; Musfeldt, M.; Wenderoth, D.F.; Hampe, J.; Brant, O.; Folsch, U.R.; Timmis, K.N.; Schreiber, S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut 2004, 53, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.; Xu, B.; Wang, X.; Zhang, Y.; Wang, H.; Kong, X.; Zhu, H.; Wu, K. The biodiversity and composition of the dominant fecal microbiota in patients with inflammatory bowel disease. Diagn. Microbiol. Infect. Dis. 2013, 75, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Thibault, R.; Blachier, F.; Darcy-Vrillon, B.; De, C.P.; Bourreille, A.; Segain, J.P. Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: A transport deficiency. Inflamm. Bowel Dis. 2010, 16, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Kanai, T. The gut microbiota and inflammatory bowel disease. Semin. Immunopathol. 2015, 37, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Albenberg, L.G.; Lewis, J.D.; Wu, G.D. Food and the gut microbiota in inflammatory bowel diseases: A critical connection. Curr. Opin. Gastroenterol. 2012, 28, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [PubMed]
- Hou, J.K.; Abraham, B.; El-Serag, H. Dietary intake and risk of developing inflammatory bowel disease: A systematic review of the literature. Am. J. Gastroenterol. 2011, 106, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Albenberg, L.; Compher, C.; Baldassano, R.; Piccoli, D.; Lewis, J.D.; Wu, G.D. Diet in the pathogenesis and treatment of inflammatory bowel diseases. Gastroenterology 2015, 148, 1087–1106. [Google Scholar] [CrossRef] [PubMed]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; Cavalieri, D.; Di, P.M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.W.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.; Brown, D.; Stares, M.D.; Scott, P.; Bergerat, A.; et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011, 5, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, L.; Relman, D.A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. USA 2011, 108, 4554–4561. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cobas, A.E.; Gosalbes, M.J.; Friedrichs, A.; Knecht, H.; Artacho, A.; Eismann, K.; Otto, W.; Rojo, D.; Bargiela, R.; von, B.M.; et al. Gut microbiota disturbance during antibiotic therapy: A multi-omic approach. Gut 2013, 62, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Materna, A.C.; Friedman, J.; Campos-Baptista, M.I.; Blackburn, M.C.; Perrotta, A.; Erdman, S.E.; Alm, E.J. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 2014, 15, R89. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [PubMed]
- Marlow, G.; Ellett, S.; Ferguson, I.R.; Zhu, S.; Karunasinghe, N.; Jesuthasan, A.C.; Han, D.Y.; Fraser, A.G.; Ferguson, L.R. Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn’s disease patients. Hum. Genom. 2013, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Fidalgo, S.; Sanchez, D.I.; Cardeno, A.; Alarcon, D.L.L. Influence of extra virgin olive oil diet enriched with hydroxytyrosol in a chronic DSS colitis model. Eur. J. Nutr. 2012, 51, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Biasi, F.; Deiana, M.; Guina, T.; Gamba, P.; Leonarduzzi, G.; Poli, G. Wine consumption and intestinal redox homeostasis. Redox. Biol. 2014, 2, 795–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kansal, S.; Wagner, J.; Kirkwood, C.D.; Catto-Smith, A.G. Enteral nutrition in Crohn’s disease: An underused therapy. Gastroenterol. Res. Pract. 2013, 2013, 482108. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, O.; Cordischi, L.; Cirulli, M.; Paganelli, M.; Labalestra, V.; Uccini, S.; Russo, P.M.; Cucchiara, S. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: A randomized controlled open-label trial. Clin. Gastroenterol. Hepatol. 2006, 4, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Dziechciarz, P.; Horvath, A.; Shamir, R.; Szajewska, H. Meta-analysis: Enteral nutrition in active Crohn’s disease in children. Aliment. Pharmacol. Ther. 2007, 26, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Heuschkel, R.B.; Menache, C.C.; Megerian, J.T.; Baird, A.E. Enteral nutrition and corticosteroids in the treatment of acute Crohn’s disease in children. J. Pediatr. Gastroenterol. Nutr. 2000, 31, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Grover, Z.; Muir, R.; Lewindon, P. Exclusive enteral nutrition induces early clinical, mucosal and transmural remission in paediatric Crohn’s disease. J. Gastroenterol. 2014, 49, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Day, A.S.; Whitten, K.E.; Lemberg, D.A.; Clarkson, C.; Vitug-Sales, M.; Jackson, R.; Bohane, T.D. Exclusive enteral feeding as primary therapy for Crohn’s disease in Australian children and adolescents: A feasible and effective approach. J. Gastroenterol. Hepatol. 2006, 21, 1609–1614. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.D.; Wadhera, V.; Leach, S.T.; Woodhead, H.J.; Lemberg, D.A.; Mendoza-Cruz, A.C.; Day, A.S. Vitamin D deficiency in children with inflammatory bowel disease. Dig. Dis. Sci. 2011, 56, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Whitten, K.E.; Leach, S.T.; Bohane, T.D.; Woodhead, H.J.; Day, A.S. Effect of exclusive enteral nutrition on bone turnover in children with Crohn’s disease. J. Gastroenterol. 2010, 45, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Afzal, N.A.; van Der Zaag-Loonen, H.J.; Arnaud-Battandier, F.; Davies, S.; Murch, S.; Derkx, B.; Heuschkel, R.; Fell, J.M. Improvement in quality of life of children with acute Crohn’s disease does not parallel mucosal healing after treatment with exclusive enteral nutrition. Aliment. Pharmacol. Ther. 2004, 20, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Day, A.S.; Whitten, K.E.; Sidler, M.; Lemberg, D.A. Systematic review: Nutritional therapy in paediatric Crohn’s disease. Aliment. Pharmacol. Ther. 2008, 27, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Shiraki, M.; Nakahigashi, M.; Umegae, S.; Matsumoto, K. Enteral nutrition to suppress postoperative Crohn’s disease recurrence: A five-year prospective cohort study. Int. J. Colorectal. Dis. 2013, 28, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Nakahigashi, M.; Umegae, S.; Kitagawa, T.; Matsumoto, K. Impact of long-term enteral nutrition on clinical and endoscopic recurrence after resection for Crohn’s disease: A prospective, non-randomized, parallel, controlled study. Aliment. Pharmacol. Ther. 2007, 25, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Wall, C.L.; Day, A.S.; Gearry, R.B. Use of exclusive enteral nutrition in adults with Crohn’s disease: A review. World J. Gastroenterol. 2013, 19, 7652–7660. [Google Scholar] [CrossRef] [PubMed]
- Zachos, M.; Tondeur, M.; Griffiths, A.M. Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane. Database Syst. Rev. 2007, CD000542. [Google Scholar] [CrossRef] [Green Version]
- Lionetti, P.; Callegari, M.L.; Ferrari, S.; Cavicchi, M.C.; Pozzi, E.; de, M.M.; Morelli, L. Enteral nutrition and microflora in pediatric Crohn’s disease. JPEN J. Parenter. Enteral Nutr. 2005, 29, S173–S175. [Google Scholar] [CrossRef] [PubMed]
- Tjellstrom, B.; Hogberg, L.; Stenhammar, L.; Magnusson, K.E.; Midtvedt, T.; Norin, E.; Sundqvist, T. Effect of exclusive enteral nutrition on gut microflora function in children with Crohn’s disease. Scand. J. Gastroenterol. 2012, 47, 1454–1459. [Google Scholar] [CrossRef] [PubMed]
- Gerasimidis, K.; Bertz, M.; Hanske, L.; Junick, J.; Biskou, O.; Aguilera, M.; Garrick, V.; Russell, R.K.; Blaut, M.; McGrogan, P.; et al. Decline in presumptively protective gut bacterial species and metabolites are paradoxically associated with disease improvement in pediatric Crohn’s disease during enteral nutrition. Inflamm. Bowel Dis. 2014, 20, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Palm, N.W.; de Zoete, M.R.; Cullen, T.W.; Barry, N.A.; Stefanowski, J.; Hao, L.; Degnan, P.H.; Hu, J.; Peter, I.; Zhang, W.; et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 2014, 158, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O.; Day, A.S.; Leach, S.T.; Lemberg, D.A.; Nielsen, S.; Mitchell, H.M. Effect of exclusive enteral nutrition on the microbiota of children with newly diagnosed Crohn’s disease. Clin. Transl. Gastroenterol. 2015, 6, e71. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Rampertab, S.D.; Mullin, G.E. Existing dietary guidelines for Crohn’s disease and ulcerative colitis. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Chiba, M.; Abe, T.; Tsuda, H.; Sugawara, T.; Tsuda, S.; Tozawa, H.; Fujiwara, K.; Imai, H. Lifestyle-related disease in Crohn’s disease: Relapse prevention by a semi-vegetarian diet. World J. Gastroenterol. 2010, 16, 2484–2495. [Google Scholar] [CrossRef] [PubMed]
- Haas, S.V.; Haas, M.P. The treatment of celiac disease with the specific carbohydrate diet; report on 191 additional cases. Am. J. Gastroenterol. 1955, 23, 344–360. [Google Scholar] [PubMed]
- Gottschall, E. Breaking the Vicious Cycle: Intestinal Health through Diet; Kirkton Press: Baltimore, ON, Canada, 2012. [Google Scholar]
- Suskind, D.L.; Wahbeh, G.; Gregory, N.; Vendettuoli, H.; Christie, D. Nutritional therapy in pediatric Crohn disease: The specific carbohydrate diet. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.A.; Gold, B.D.; Oliva, S.; Lewis, J.; Stallworth, A.; Koch, B.; Eshee, L.; Mason, D. Clinical and mucosal improvement with specific carbohydrate diet in pediatric Crohn disease. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.R.; Shepherd, S.J. Personal view: Food for thought—Western lifestyle and susceptibility to Crohn’s disease. The FODMAP hypothesis. Aliment. Pharmacol. Ther. 2005, 21, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Croagh, C.; Shepherd, S.J.; Berryman, M.; Muir, J.G.; Gibson, P.R. Pilot study on the effect of reducing dietary FODMAP intake on bowel function in patients without a colon. Inflamm. Bowel. Dis. 2007, 13, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Gearry, R.B.; Irving, P.M.; Barrett, J.S.; Nathan, D.M.; Shepherd, S.J.; Gibson, P.R. Reduction of dietary poorly absorbed short-chain carbohydrates (FODMAPs) improves abdominal symptoms in patients with inflammatory bowel disease—A pilot study. J. Crohns Colitis 2009, 3, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.K.; Lee, D.; Lewis, J. Diet and inflammatory bowel disease: Review of patient-targeted recommendations. Clin. Gastroenterol. Hepatol. 2014, 12, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Olendzki, B.C.; Silverstein, T.D.; Persuitte, G.M.; Ma, Y.; Baldwin, K.R.; Cave, D. An anti-inflammatory diet as treatment for inflammatory bowel disease: A case series report. Nutr. J. 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, N.; Kumar, D. Food-specific IgG4-guided exclusion diets improve symptoms in Crohn’s disease: A pilot study. Colorectal Dis. 2011, 13, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Bentz, S.; Hausmann, M.; Piberger, H.; Kellermeier, S.; Paul, S.; Held, L.; Falk, W.; Obermeier, F.; Fried, M.; Scholmerich, J.; et al. Clinical relevance of IgG antibodies against food antigens in Crohn’s disease: A double-blind cross-over diet intervention study. Digestion 2010, 81, 252–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, C.K.; Gibson, P.R.; Shepherd, S.J. Design of clinical trials evaluating dietary interventions in patients with functional gastrointestinal disorders. Am. J. Gastroenterol. 2013, 108, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeir, J.; De, V.M. Probiotics, prebiotics, and synbiotics—Approaching a definition. Am. J. Clin. Nutr. 2001, 73, 361S–364S. [Google Scholar] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Cary, V.A.; Boullata, J. What is the evidence for the use of probiotics in the treatment of inflammatory bowel disease? J. Clin. Nurs. 2010, 19, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Henker, J.; Muller, S.; Laass, M.W.; Schreiner, A.; Schulze, J. Probiotic Escherichia coli Nissle 1917 (EcN) for successful remission maintenance of ulcerative colitis in children and adolescents: An open-label pilot study. Z. Gastroenterol. 2008, 46, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Kruis, W.; Schutz, E.; Fric, P.; Fixa, B.; Judmaier, G.; Stolte, M. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment. Pharmacol. Ther. 1997, 11, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Zocco, M.A.; dal Verme, L.Z.; Cremonini, F.; Piscaglia, A.C.; Nista, E.C.; Candelli, M.; Novi, M.; Rigante, D.; Cazzato, I.A.; Ojetti, V.; et al. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment. Pharmacol. Ther. 2006, 23, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Bousvaros, A.; Guandalini, S.; Baldassano, R.N.; Botelho, C.; Evans, J.; Ferry, G.D.; Goldin, B.; Hartigan, L.; Kugathasan, S.; Levy, J.; et al. A randomized, double-blind trial of Lactobacillus GG versus placebo in addition to standard maintenance therapy for children with Crohn’s disease. Inflamm. Bowel Dis. 2005, 11, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.; Timmer, A.; Herfarth, H.H.; Sartor, R.B.; Vanderhoof, J.A.; Rath, H.C. Lactobacillus GG in inducing and maintaining remission of Crohn’s disease. BMC Gastroenterol. 2004, 4, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliva, S.; Di, N.G.; Ferrari, F.; Mallardo, S.; Rossi, P.; Patrizi, G.; Cucchiara, S.; Stronati, L. Randomised clinical trial: The effectiveness of Lactobacillus reuteri ATCC 55730 rectal enema in children with active distal ulcerative colitis. Aliment. Pharmacol. Ther. 2012, 35, 327–334. [Google Scholar] [PubMed]
- Kato, K.; Mizuno, S.; Umesaki, Y.; Ishii, Y.; Sugitani, M.; Imaoka, A.; Otsuka, M.; Hasunuma, O.; Kurihara, R.; Iwasaki, A.; et al. Randomized placebo-controlled trial assessing the effect of bifidobacteria-fermented milk on active ulcerative colitis. Aliment. Pharmacol. Ther. 2004, 20, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Guslandi, M. Saccharomyces boulardii plus rifaximin in mesalamine-intolerant ulcerative colitis. J. Clin. Gastroenterol. 2010, 44, 385. [Google Scholar] [CrossRef] [PubMed]
- Venturi, A.; Gionchetti, P.; Rizzello, F.; Johansson, R.; Zucconi, E.; Brigidi, P.; Matteuzzi, D.; Campieri, M. Impact on the composition of the faecal flora by a new probiotic preparation: Preliminary data on maintenance treatment of patients with ulcerative colitis. Aliment. Pharmacol. Ther. 1999, 13, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Midha, V.; Makharia, G.K.; Ahuja, V.; Singal, D.; Goswami, P.; Tandon, R.K. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin. Gastroenterol. Hepatol. 2009, 7, 1202–1209. [Google Scholar]
- Tursi, A.; Brandimarte, G.; Papa, A.; Giglio, A.; Elisei, W.; Giorgetti, G.M.; Forti, G.; Morini, S.; Hassan, C.; Pistoia, M.A.; et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: A double-blind, randomized, placebo-controlled study. Am. J. Gastroenterol. 2010, 105, 2218–2227. [Google Scholar] [PubMed]
- Mardini, H.E.; Grigorian, A.Y. Probiotic mix VSL#3 is effective adjunctive therapy for mild to moderately active ulcerative colitis: A meta-analysis. Inflamm. Bowel Dis. 2014, 20, 1562–1567. [Google Scholar] [PubMed]
- Gionchetti, P.; Rizzello, F.; Helwig, U.; Venturi, A.; Lammers, K.M.; Brigidi, P.; Vitali, B.; Poggioli, G.; Miglioli, M.; Campieri, M. Prophylaxis of pouchitis onset with probiotic therapy: A double-blind, placebo-controlled trial. Gastroenterology 2003, 124, 1202–1209. [Google Scholar] [CrossRef]
- Shen, J.; Zuo, Z.X.; Mao, A.P. Effect of probiotics on inducing remission and maintaining therapy in ulcerative colitis, Crohn’s disease, and pouchitis: Meta-analysis of randomized controlled trials. Inflamm. Bowel Dis. 2014, 20, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Biancone, L.; Michetti, P.; Travis, S.; Escher, J.C.; Moser, G.; Forbes, A.; Hoffmann, J.C.; Dignass, A.; Gionchetti, P.; Jantschek, G.; et al. European evidence-based Consensus on the management of ulcerative colitis: Special situations. J. Crohns Colitis 2008, 2, 63–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braat, H.; Rottiers, P.; Hommes, D.W.; Huyghebaert, N.; Remaut, E.; Remon, J.P.; van Deventer, S.J.; Neirynck, S.; Peppelenbosch, M.P.; Steidler, L. A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn’s disease. Clin. Gastroenterol. Hepatol. 2006, 4, 754–759. [Google Scholar] [CrossRef] [PubMed]
- De Moreno de LeBlanc, A.; del Carmen, S.; Chatel, J.M.; Miyoshi, A.; Azevedo, V.; Langella, P.; Bermdez-Humarín, L.G.; LeBlanc, J.G. Current review of genetically modified lactic acid bacteria for the prevention and treatment of colitis using murine models. Gastroenterol. Res. Pract. 2015, 2015, 146972. [Google Scholar] [CrossRef] [PubMed]
- Del Carmen, S.; de Moreno de LeBlanc, A.; Martin, R.; Chain, F.; Langella, P.; Bermdez-Humaín, L.G.; LeBlanc, J.G. Genetically Engineered Immunomodulatory Streptococcus thermophilus Strains Producing Antioxidant Enzymes Exhibit Enhanced Anti-Inflammatory Activities. Appl. Environm. Microbiol. 2014, 80, 869–877. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; del Carmen, S.; Miyoshi, A.; Azevedo, V.; Sesma, F.; Langella, P.; Bermdez-Humarín, L.G.; Watterlot, L.; Perdigon, G.; de Moreno de LeBlanc, A. Use of superoxide dismutase and catalase producing lactic acid bacteria in TNBS induced Crohn’s disease in mice. J. Biotechnol. 2011, 151, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Floch, M.H.; Walker, W.A.; Madsen, K.; Sanders, M.E.; Macfarlane, G.T.; Flint, H.J.; Dieleman, L.A.; Ringel, Y.; Guandalini, S.; Kelly, C.P.; et al. Recommendations for probiotic use-2011 update. J. Clin. Gastroenterol. 2011, 45, S168–S171. [Google Scholar] [CrossRef] [PubMed]
- Dignass, A.; Lindsay, J.O.; Sturm, A.; Windsor, A.; Colombel, J.F.; Allez, M.; d’Haens, G.; d’Hoore, A.; Mantzaris, G.; Novacek, G.; et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: Current management. J. Crohns Colitis 2012, 6, 991–1030. [Google Scholar] [CrossRef] [PubMed]
- Meijer, B.J.; Dieleman, L.A. Probiotics in the treatment of human inflammatory bowel diseases: Update 2011. J. Clin. Gastroenterol. 2011, 45, S139–S144. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M. Prebiotics: The concept revisited. J. Nutr. 2007, 137, 830S–837S. [Google Scholar] [PubMed]
- Damaskos, D.; Kolios, G. Probiotics and prebiotics in inflammatory bowel disease: Microflora “on the scope”. Br. J. Clin. Pharmacol. 2008, 65, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Kanauchi, O.; Matsumoto, Y.; Matsumura, M.; Fukuoka, M.; Bamba, T. The beneficial effects of microflora, especially obligate anaerobes, and their products on the colonic environment in inflammatory bowel disease. Curr. Pharm. Des. 2005, 11, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Kanauchi, O.; Mitsuyama, K.; Homma, T.; Takahama, K.; Fujiyama, Y.; Andoh, A.; Araki, Y.; Suga, T.; Hibi, T.; Naganuma, M.; et al. Treatment of ulcerative colitis patients by long-term administration of germinated barley foodstuff: Multi-center open trial. Int. J. Mol. Med. 2003, 12, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Hanai, H.; Kanauchi, O.; Mitsuyama, K.; Andoh, A.; Takeuchi, K.; Takayuki, I.; Araki, Y.; Fujiyama, Y.; Toyonaga, A.; Sata, M.; et al. Germinated barley foodstuff prolongs remission in patients with ulcerative colitis. Int. J. Mol. Med. 2004, 13, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Faghfoori, Z.; Navai, L.; Shakerhosseini, R.; Somi, M.H.; Nikniaz, Z.; Norouzi, M.F. Effects of an oral supplementation of germinated barley foodstuff on serum tumour necrosis factor-α, interleukin-6 and -8 in patients with ulcerative colitis. Ann. Clin. Biochem. 2011, 48, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Faghfoori, Z.; Shakerhosseini, R.; Navai, L.; Somi, M.H.; Nikniaz, Z.; Abadi, A. Effects of an oral supplementation of germinated barley foodstuff on serum CRP level and clinical signs in patients with ulcerative colitis. Health Promot. Perspect. 2014, 4, 116–121. [Google Scholar] [PubMed]
- Lindsay, J.O.; Whelan, K.; Stagg, A.J.; Gobin, P.; Al-Hassi, H.O.; Rayment, N.; Kamm, M.A.; Knight, S.C.; Forbes, A. Clinical, microbiological, and immunological effects of fructo-oligosaccharide in patients with Crohn’s disease. Gut 2006, 55, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, J.L.; Hedin, C.R.H.; Koutsoumpas, A.; Ng, S.C.; McCarthy, N.E.; Hart, A.L.; Kamm, M.A.; Sanderson, J.D.; Knight, S.C.; Forbes, A.; et al. Randomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn’s disease. Gut 2011, 60, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Casellas, F.; Borruel, N.; Torrejon, A.; Varela, E.; Antolin, M.; Guarner, F.; Malagelada, J.R. Oral oligofructose-enriched inulin supplementation in acute ulcerative colitis is well tolerated and associated with lowered faecal calprotectin. Aliment. Pharmacol. Ther. 2007, 25, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Welters, C.F.; Heineman, E.; Thunnissen, F.B.; van den Bogaard, A.E.; Soeters, P.B.; Baeten, C.G. Effect of dietary inulin supplementation on inflammation of pouch mucosa in patients with an ileal pouch-anal anastomosis. Dis. Colon Rectum. 2002, 45, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Bengmark, S. Pre-, pro- and synbiotics. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Furrie, E.; Macfarlane, S.; Kennedy, A.; Cummings, J.H.; Walsh, S.V.; O’neil, D.A.; Macfarlane, G.T. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: A randomised controlled pilot trial. Gut 2005, 54, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, S.; Gudis, K.; Mitsui, K.; Seo, T.; Yonezawa, M.; Tanaka, S.; Tatsuguchi, A.; Sakamoto, C. A randomized controlled trial on the efficacy of synbiotic versus probiotic or prebiotic treatment to improve the quality of life in patients with ulcerative colitis. Nutrition 2009, 25, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Matsumoto, S.; Ohashi, Y.; Imaoka, A.; Setoyama, H.; Umesaki, Y.; Tanaka, R.; Otani, T. Beneficial effects of probiotic bifidobacterium and galacto-oligosaccharide in patients with ulcerative colitis: A randomized controlled study. Digestion 2011, 84, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: Antibiotics, probiotics, and prebiotics. Gastroenterology 2004, 126, 1620–1633. [Google Scholar] [CrossRef] [PubMed]
- Scribano, M.L.; Prantera, C. Use of antibiotics in the treatment of Crohn’s disease. World J. Gastroenterol. 2013, 19, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Thorkildsen, L.T.; Nwosu, F.C.; Avershina, E.; Ricanek, P.; Perminow, G.; Brackmann, S.; Vatn, M.H.; Rudi, K. Dominant fecal microbiota in newly diagnosed untreated inflammatory bowel disease patients. Gastroenterol. Res. Pract. 2013, 2013, 636785. [Google Scholar] [CrossRef] [PubMed]
- Kerman, D.H.; Deshpande, A.R. Gut microbiota and inflammatory bowel disease: The role of antibiotics in disease management. Postgrad. Med. 2014, 126, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, L.; Huse, S.; Sogin, M.L.; Relman, D.A. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008, 6, e280. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.; Clemente, J.C.; Colombel, J.F. Can inflammatory bowel disease be permanently treated with short-term interventions on the microbiome? Expert Rev. Gastroenterol. Hepatol. 2015, 9, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Loening-Baucke, V.; Bengmark, S.; Scholze, J.; Doerffel, Y. Bacterial biofilm suppression with antibiotics for ulcerative and indeterminate colitis: Consequences of aggressive treatment. Arch. Med. Res. 2008, 39, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Maccaferri, S.; Vitali, B.; Klinder, A.; Kolida, S.; Ndagijimana, M.; Laghi, L.; Calanni, F.; Brigidi, P.; Gibson, G.R.; Costabile, A. Rifaximin modulates the colonic microbiota of patients with Crohn’s disease: An in vitro approach using a continuous culture colonic model system. J. Antimicrob. Chemother. 2010, 65, 2556–2565. [Google Scholar] [CrossRef] [PubMed]
- Blichfeldt, P.; Blomhoff, J.P.; Myhre, E.; Gjone, E. Metronidazole in Crohn’s disease. A double blind cross-over clinical trial. Scand. J. Gastroenterol. 1978, 13, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Ursing, B.; Alm, T.; Barany, F.; Bergelin, I.; Ganrot-Norlin, K.; Hoevels, J.; Huitfeldt, B.; Jarnerot, G.; Krause, U.; Krook, A.; et al. A comparative study of metronidazole and sulfasalazine for active Crohn’s disease: The cooperative Crohn’s disease study in Sweden. II. Result. Gastroenterology 1982, 83, 550–562. [Google Scholar] [PubMed]
- Sutherland, L.; Singleton, J.; Sessions, J.; Hanauer, S.; Krawitt, E.; Rankin, G.; Summers, R.; Mekhjian, H.; Greenberger, N.; Kelly, M.; et al. Double blind, placebo controlled trial of metronidazole in Crohn’s disease. Gut 1991, 32, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Thia, K.T.; Mahadevan, U.; Feagan, B.G.; Wong, C.; Cockeram, A.; Bitton, A.; Bernstein, C.N.; Sandborn, W.J. Ciprofloxacin or metronidazole for the treatment of perianal fistulas in patients with Crohn’s disease: A randomized, double-blind, placebo-controlled pilot study. Inflamm. Bowel Dis. 2009, 15, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.W.; Ji, H.Z.; Wang, F.Y. Meta-analysis of ciprofloxacin in treatment of Crohn’s disease. Biomed. Rep. 2015, 3, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Prantera, C.; Lochs, H.; Campieri, M.; Scribano, M.L.; Sturniolo, G.C.; Castiglione, F.; Cottone, M. Antibiotic treatment of Crohn’s disease: Results of a multicentre, double blind, randomized, placebo-controlled trial with rifaximin. Aliment. Pharmacol. Ther. 2006, 23, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, F.; Rispo, A.; Di, G.E.; Cozzolino, A.; Manguso, F.; Grassia, R.; Mazzacca, G. Antibiotic treatment of small bowel bacterial overgrowth in patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2003, 18, 1107–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickinson, R.J.; O’Connor, H.J.; Pinder, I.; Hamilton, I.; Johnston, D.; Axon, A.T. Double blind controlled trial of oral vancomycin as adjunctive treatment in acute exacerbations of idiopathic colitis. Gut 1985, 26, 1380–1384. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.W.; Selby, W.S.; Jewell, D.P. Controlled trial of intravenous metronidazole as an adjunct to corticosteroids in severe ulcerative colitis. Gut 1986, 27, 1210–1212. [Google Scholar] [CrossRef] [PubMed]
- Gionchetti, P.; Rizzello, F.; Ferrieri, A.; Venturi, A.; Brignola, C.; Ferretti, M.; Peruzzo, S.; Miglioli, M.; Campieri, M. Rifaximin in patients with moderate or severe ulcerative colitis refractory to steroid-treatment: A double-blind, placebo-controlled trial. Dig. Dis. Sci. 1999, 44, 1220–1221. [Google Scholar] [PubMed]
- Turunen, U.M.; Farkkila, M.A.; Hakala, K.; Seppala, K.; Sivonen, A.; Ogren, M.; Vuoristo, M.; Valtonen, V.V.; Miettinen, T.A. Long-term treatment of ulcerative colitis with ciprofloxacin: A prospective, double-blind, placebo-controlled study. Gastroenterology 1998, 115, 1072–1078. [Google Scholar] [CrossRef]
- Turner, D.; Levine, A.; Kolho, K.L.; Shaoul, R.; Ledder, O. Combination of oral antibiotics may be effective in severe pediatric ulcerative colitis: A preliminary report. J. Crohns Colitis 2014, 8, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Steinhart, A.H. Antibiotic therapy for Crohn’s disease: A review. Can. J. Gastroenterol. 2006, 20, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Neurath, M.F.; Wirtz, S. The intestinal microbiota in inflammatory bowel disease. ILAR J. 2015, 56, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Hormannsperger, G.; Schaubeck, M.; Haller, D. Intestinal Microbiota in Animal Models of Inflammatory Diseases. ILAR J. 2015, 56, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Grehan, M.J.; Borody, T.J.; Leis, S.M.; Campbell, J.; Mitchell, H.; Wettstein, A. Durable alteration of the colonic microbiota by the administration of donor fecal flora. J. Clin. Gastroenterol. 2010, 44, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Rohlke, F.; Stollman, N. Fecal microbiota transplantation in relapsing Clostridium difficile infection. Ther. Adv. Gastroenterol. 2012, 5, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.M.; Wang, H.G.; Wang, M.; Cui, B.T.; Fan, Z.N.; Ji, G.Z. Fecal microbiota transplantation for severe enterocolonic fistulizing Crohn’s disease. World J. Gastroenterol. 2013, 19, 7213–7216. [Google Scholar] [CrossRef] [PubMed]
- Lewin, R.A. More on Merde. Perspect. Biol. Med. 2001, 44, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Eiseman, B.; Silen, W.; Bascom, G.S.; Kauvar, A.J. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 1958, 44, 854–859. [Google Scholar] [PubMed]
- Bowden, T.A., Jr.; Mansberger, A.R., Jr.; Lykins, L.E. Pseudomembraneous enterocolitis: Mechanism for restoring floral homeostasis. Am. Surg. 1981, 47, 178–183. [Google Scholar] [PubMed]
- McFarland, L.V. Alternative treatments for Clostridium difficile disease: What really works? J. Med. Microbiol. 2005, 54, 101–111. [Google Scholar] [PubMed]
- Schwan, A.; Sjolin, S.; Trottestam, U.; Aronsson, B. Relapsing Clostridium difficile enterocolitis cured by rectal infusion of homologous faeces. Lancet 1983, 2, 845. [Google Scholar] [CrossRef]
- Khoruts, A.; Dicksved, J.; Jansson, J.K.; Sadowsky, M.J. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J. Clin. Gastroenterol. 2010, 44, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Gough, E.; Shaikh, H.; Manges, A.R. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin. Infect. Dis. 2011, 53, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; de Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.; Tijssen, J.G.; et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Surawicz, C.M.; Brandt, L.J.; Binion, D.G.; Ananthakrishnan, A.N.; Curry, S.R.; Gilligan, P.H.; McFarland, L.V.; Mellow, M.; Zuckerbraun, B.S. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am. J. Gastroenterol. 2013, 108, 478–498. [Google Scholar] [CrossRef] [PubMed]
- Debast, S.B.; Bauer, M.P.; Kuijper, E.J. European Society of Clinical Microbiology and Infectious Diseases: Update of the treatment guidance document for Clostridium difficile infection. Clin. Microbiol. Infect. 2014, 20, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Bennet, J.D.; Brinkman, M. Treatment of ulcerative colitis by implantation of normal colonic flora. Lancet 1989, 1, 164. [Google Scholar] [CrossRef]
- Borody, T.J.; George, L.; Andrews, P.; Brandl, S.; Noonan, S.; Cole, P.; Hyland, L.; Morgan, A.; Maysey, J.; Moore-Jones, D. Bowel-flora alteration: A potential cure for inflammatory bowel disease and irritable bowel syndrome? Med. J. Aust. 1989, 150, 604. [Google Scholar] [PubMed]
- Borody, T.J.; Warren, E.F.; Leis, S.; Surace, R.; Ashman, O. Treatment of ulcerative colitis using fecal bacteriotherapy. J. Clin. Gastroenterol. 2003, 37, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Colman, R.J.; Rubin, D.T. Fecal microbiota transplantation as therapy for inflammatory bowel disease: A systematic review and meta-analysis. J. Crohns Colitis 2014, 8, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- De Leon, L.M.; Watson, J.B.; Kelly, C.R. Transient flare of ulcerative colitis after fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin. Gastroenterol. Hepatol. 2013, 11, 1036–1038. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.R.; Ihunnah, C.; Fischer, M.; Khoruts, A.; Surawicz, C.; Afzali, A.; Aroniadis, O.; Barto, A.; Borody, T.; Giovanelli, A.; et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am. J. Gastroenterol. 2014, 109, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015, 149, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Rossen, N.G.; Fuentes, S.; van der Spek, M.J.; Tijssen, J.G.; Hartman, J.H.; Duflou, A.; Lowenberg, M.; van den Brink, G.R.; Mathus-Vliegen, E.M.; de Vos, W.M.; et al. Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients With Ulcerative Colitis. Gastroenterology 2015, 149, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Lawley, T.D.; Clare, S.; Walker, A.W.; Stares, M.D.; Connor, T.R.; Raisen, C.; Goulding, D.; Rad, R.; Schreiber, F.; Brandt, C.; et al. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS Pathog. 2012, 8, e1002995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrof, E.O.; Gloor, G.B.; Vanner, S.J.; Weese, S.J.; Carter, D.; Daigneault, M.C.; Brown, E.M.; Schroeter, K.; Allen-Vercoe, E. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: “RePOOPulating” the gut. Microbiome 2013, 1. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Ding, C.; Gong, J.; Wei, Y.; McFarland, L.V.; Li, N. Freeze-dried, capsulized fecal microbiota transplantation for relapsing Clostridium difficile infection. J. Clin. Gastroenterol. 2015, 49, 537–538. [Google Scholar] [CrossRef] [PubMed]
- Youngster, I.; Russell, G.H.; Pindar, C.; Ziv-Baran, T.; Sauk, J.; Hohmann, E.L. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 2014, 312, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Szkudlapski, D.; Labuzek, K.; Pokora, Z.; Smyla, N.; Gonciarz, M.; Mularczyk, A.; Maluch, P.; Okopien, B. The emering role of helminths in treatment of the inflammatory bowel disorders. J. Physiol. Pharmacol. 2014, 65, 741–751. [Google Scholar] [PubMed]
- Versini, M.; Jeandel, P.Y.; Bashi, T.; Bizzaro, G.; Blank, M.; Shoenfeld, Y. Unraveling the Hygiene Hypothesis of helminthes and autoimmunity: Origins, pathophysiology, and clinical applications. BMC Med. 2015, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Zaccone, P.; Fehervari, Z.; Phillips, J.M.; Dunne, D.W.; Cooke, A. Parasitic worms and inflammatory diseases. Parasite Immunol. 2006, 28, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, J.V. Do we need worms to promote immune health? Clin. Rev. Allergy Immunol. 2015, 49, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Bashi, T.; Blank, M.; Shoenfeld, Y. Treating inflammatory bowel disease: From helminths to ova. Isr. Med. Assoc. J. 2014, 16, 627–628. [Google Scholar] [PubMed]
- Weinstock, J.V.; Elliott, D.E. Translatability of helminth therapy in inflammatory bowel diseases. Int. J. Parasitol. 2013, 43, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Giacomin, P.; Croese, J.; Krause, L.; Loukas, A.; Cantacessi, C. Suppression of inflammation by helminths: A role for the gut microbiota? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.; Ferreira, I.; Loukas, A. The hookworm pharmacopoeia for inflammatory diseases. Int. J. Parasitol. 2013, 43, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Wammes, L.J.; Mpairwe, H.; Elliott, A.M.; Yazdanbakhsh, M. Helminth therapy or elimination: Epidemiological, immunological, and clinical considerations. Lancet Infect. Dis. 2014, 14, 1150–1162. [Google Scholar] [CrossRef]
- Glendinning, L.; Nausch, N.; Free, A.; Taylor, D.W.; Mutapi, F. The microbiota and helminths: Sharing the same niche in the human host. Parasitology 2014, 141, 1255–1271. [Google Scholar] [CrossRef] [PubMed]
- Foth, B.J.; Tsai, I.J.; Reid, A.J.; Bancroft, A.J.; Nichol, S.; Tracey, A.; Holroyd, N.; Cotton, J.A.; Stanley, E.J.; Zarowiecki, M.; et al. Whipworm genome and dual-species transcriptome analyses provide molecular insights into an intimate host-parasite interaction. Nat. Genet. 2014, 46, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Beer, R.J. The relationship between Trichuris trichiura (Linnaeus 1758) of man and Trichuris suis (Schrank 1788) of the pig. Res. Vet. Sci. 1976, 20, 47–54. [Google Scholar] [PubMed]
- Helmby, H. Human helminth therapy to treat inflammatory disorders—Where do we stand? BMC Immunol. 2015, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Summers, R.W.; Elliott, D.E.; Qadir, K.; Urban, J.F., Jr.; Thompson, R.; Weinstock, J.V. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am. J. Gastroenterol. 2003, 98, 2034–2041. [Google Scholar] [CrossRef] [PubMed]
- Summers, R.W.; Elliott, D.E.; Urban, J.F., Jr.; Thompson, R.; Weinstock, J.V. Trichuris suis therapy in Crohn’s disease. Gut 2005, 54, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Summers, R.W.; Elliott, D.E.; Urban, J.F., Jr.; Thompson, R.A.; Weinstock, J.V. Trichuris suis therapy for active ulcerative colitis: A randomized controlled trial. Gastroenterology 2005, 128, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Elliott, D.E.; Weinstock, J.; Summers, R.W.; Landry-Wheeler, A.; Silver, N.; Harnett, M.D.; Hanauer, S.B. Randomised clinical trial: The safety and tolerability of Trichuris suis ova in patients with Crohn's disease. Aliment. Pharmacol. Ther. 2013, 38, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.T.; Gao, X.; Rosa, B.A.; Abubucker, S.; Hallsworth-Pepin, K.; Martin, J.; Tyagi, R.; Heizer, E.; Zhang, X.; Bhonagiri-Palsikar, V.; et al. Genome of the human hookworm Necator americanus. Nat. Genet. 2014, 46, 261–269. [Google Scholar] [PubMed]
- Hotez, P.J.; Brooker, S.; Bethony, J.M.; Bottazzi, M.E.; Loukas, A.; Xiao, S. Hookworm infection. N. Engl. J. Med. 2004, 351, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Croese, J.; O’neil, J.; Masson, J.; Cooke, S.; Melrose, W.; Pritchard, D.; Speare, R. A proof of concept study establishing Necator americanus in Crohn’s patients and reservoir donors. Gut 2006, 55, 136–137. [Google Scholar] [CrossRef] [PubMed]
- Croese, J.; Giacomin, P.; Navarro, S.; Clouston, A.; McCann, L.; Dougall, A.; Ferreira, I.; Susianto, A.; O'Rourke, P.; Howlett, M.; et al. Experimental hookworm infection and gluten microchallenge promote tolerance in celiac disease. J. Allergy Clin. Immunol. 2015, 135, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Gaze, S.; McSorley, H.J.; Daveson, J.; Jones, D.; Bethony, J.M.; Oliveira, L.M.; Speare, R.; McCarthy, J.S.; Engwerda, C.R.; Croese, J.; et al. Characterising the mucosal and systemic immune responses to experimental human hookworm infection. PLoS Pathog. 2012, 8, e1002520. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Tang, M.S.; Lim, Y.A.; Choy, S.H.; Kurtz, Z.D.; Cox, L.M.; Gundra, U.M.; Cho, I.; Bonneau, R.; Blaser, M.J.; et al. Helminth colonization is associated with increased diversity of the gut microbiota. PLoS Negl. Trop. Dis. 2014, 8, e2880. [Google Scholar] [CrossRef] [PubMed]
- Sepehri, S.; Kotlowski, R.; Bernstein, C.N.; Krause, D.O. Microbial diversity of inflamed and noninflamed gut biopsy tissues in inflammatory bowel disease. Inflamm. Bowel Dis. 2007, 13, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Cantacessi, C.; Giacomin, P.; Croese, J.; Zakrzewski, M.; Sotillo, J.; McCann, L.; Nolan, M.J.; Mitreva, M.; Krause, L.; Loukas, A. Impact of experimental hookworm infection on the human gut microbiota. J. Infect. Dis. 2014, 210, 1431–1434. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matijašić, M.; Meštrović, T.; Perić, M.; Čipčić Paljetak, H.; Panek, M.; Vranešić Bender, D.; Ljubas Kelečić, D.; Krznarić, Ž.; Verbanac, D. Modulating Composition and Metabolic Activity of the Gut Microbiota in IBD Patients. Int. J. Mol. Sci. 2016, 17, 578. https://doi.org/10.3390/ijms17040578
Matijašić M, Meštrović T, Perić M, Čipčić Paljetak H, Panek M, Vranešić Bender D, Ljubas Kelečić D, Krznarić Ž, Verbanac D. Modulating Composition and Metabolic Activity of the Gut Microbiota in IBD Patients. International Journal of Molecular Sciences. 2016; 17(4):578. https://doi.org/10.3390/ijms17040578
Chicago/Turabian StyleMatijašić, Mario, Tomislav Meštrović, Mihaela Perić, Hana Čipčić Paljetak, Marina Panek, Darija Vranešić Bender, Dina Ljubas Kelečić, Željko Krznarić, and Donatella Verbanac. 2016. "Modulating Composition and Metabolic Activity of the Gut Microbiota in IBD Patients" International Journal of Molecular Sciences 17, no. 4: 578. https://doi.org/10.3390/ijms17040578






