Plasma miRNAs as Biomarkers to Identify Patients with Castration-Resistant Metastatic Prostate Cancer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Detection of Differentially Expressed miRNAs in PCa Patients’ Plasma Samples
2.2. Deregulated Expression of Plasma miRNAs in Prostate Tumor Tissue
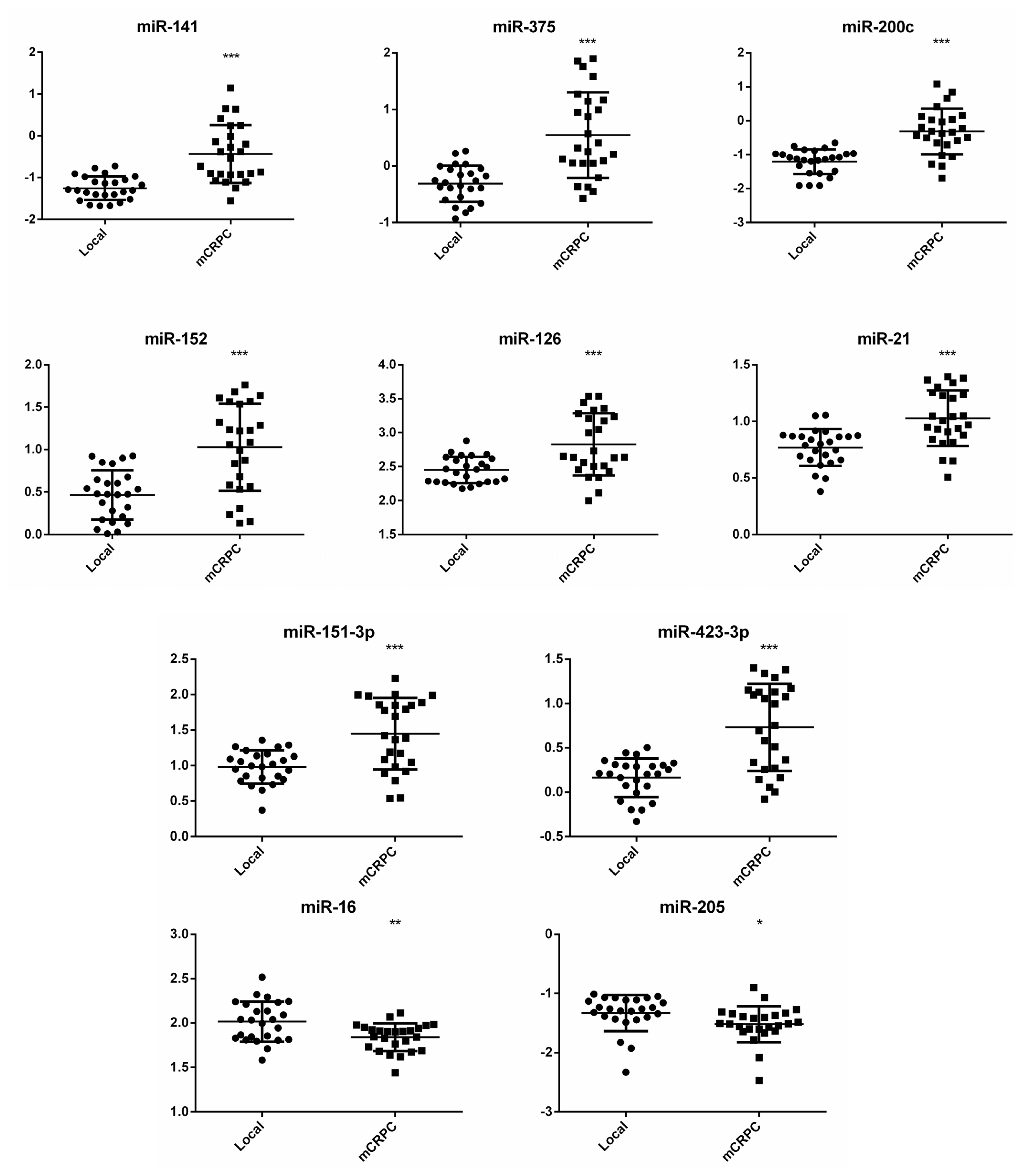
2.3. Expression Analysis of Selected miRNAs in Individual Plasma Samples
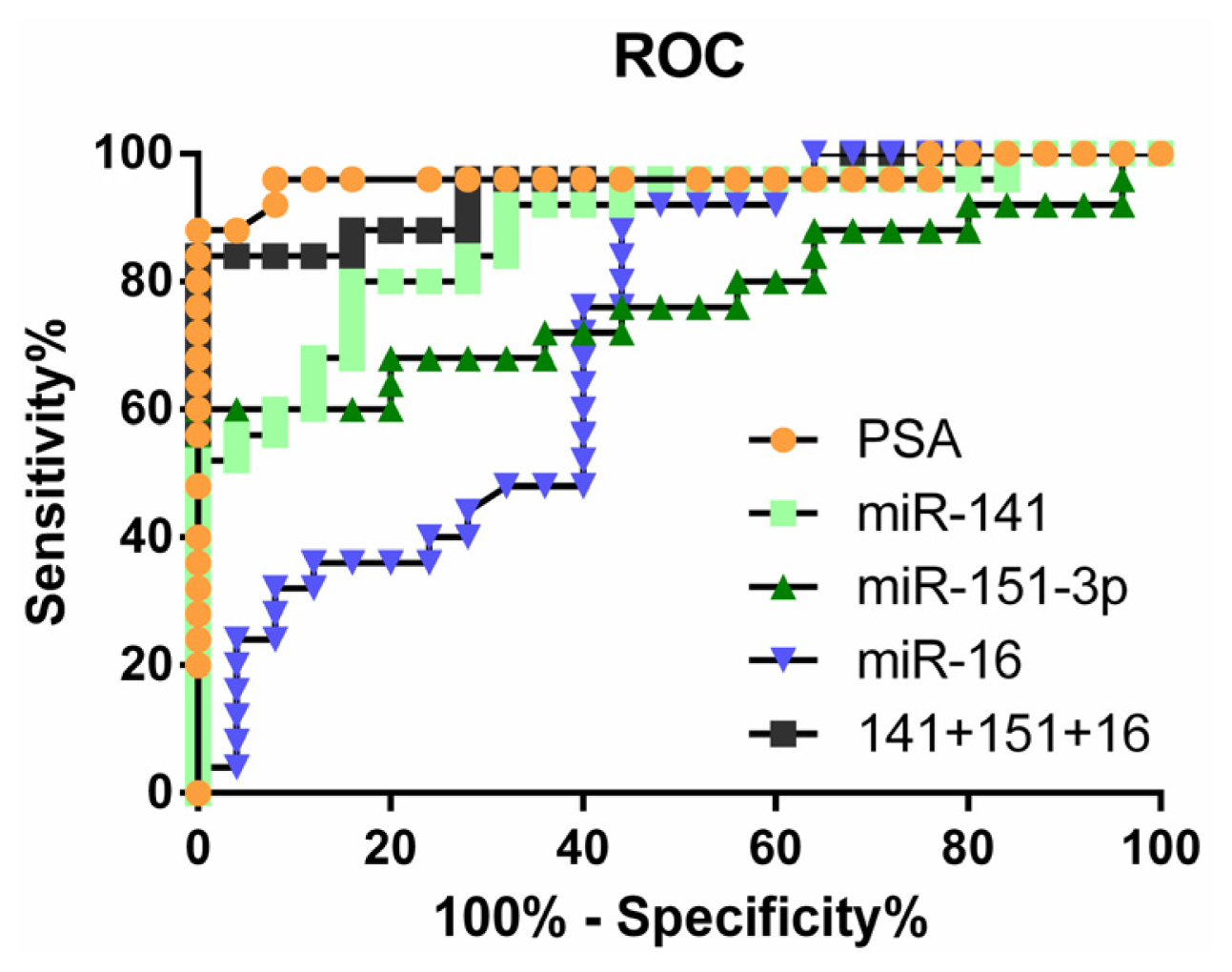
2.4. Clinical Significance of Differentially Expressed miRNAs
2.5. Discussion
3. Experimental Section
3.1. Human Plasma Samples
3.2. RNA Extraction, cDNA Preparation, qRT-PCR
3.3. Analysis of Differentially Expressed miRNAs in Human Tumor Tissues
3.4. Statistical Analysis
4. Conclusions
Supplementary Information
ijms-14-07757-s001.pdfAcknowledgments
Conflict of Interest
References
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Murray, T.; Thun, M.J. Cancer statistics, 2008. CA Cancer J. Clin 2008, 58, 71–96. [Google Scholar]
- Kohli, M.; Tindall, D.J. New developments in the medical management of prostate cancer. Mayo Clin. Proc 2010, 85, 77–86. [Google Scholar]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar]
- Bushati, N.; Cohen, S.M. microRNA functions. Annu. Rev. Cell Dev. Biol 2007, 23, 175–205. [Google Scholar]
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 2009, 19, 92–105. [Google Scholar]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res 2011, 39, D152–D157. [Google Scholar]
- Miska, E.A. How microRNAs control cell division, differentiation and death. Curr. Opin. Genet. Dev 2005, 15, 563–568. [Google Scholar]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838. [Google Scholar]
- Tam, W. The emergent role of microRNAs in molecular diagnostics of cancer. J. Mol. Diagn 2008, 10, 411–414. [Google Scholar]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar]
- Lodes, M.J.; Caraballo, M.; Suciu, D.; Munro, S.; Kumar, A.; Anderson, B. Detection of cancer with serum miRNAs on an oligonucleotide microarray. PLoS One 2009, 4, e6229. [Google Scholar]
- Brase, J.C.; Johannes, M.; Schlomm, T.; Falth, M.; Haese, A.; Steuber, T.; Beissbarth, T.; Kuner, R.; Sultmann, H. Circulating miRNAs are correlated with tumor progression in prostate cancer. Int. J. Cancer 2011, 128, 608–616. [Google Scholar]
- Zhang, H.L.; Yang, L.F.; Zhu, Y.; Yao, X.D.; Zhang, S.L.; Dai, B.; Zhu, Y.P.; Shen, Y.J.; Shi, G.H.; Ye, D.W. Serum miRNA-21: Elevated levels in patients with metastatic hormone-refractory prostate cancer and potential predictive factor for the efficacy of docetaxel-based chemotherapy. Prostate 2011, 71, 326–331. [Google Scholar]
- Selth, L.A.; Townley, S.; Gillis, J.L.; Ochnik, A.M.; Murti, K.; Macfarlane, R.J.; Chi, K.N.; Marshall, V.R.; Tilley, W.D.; Butler, L.M. Discovery of circulating microRNAs associated with human prostate cancer using a mouse model of disease. Int. J. Cancer 2012, 131, 652–661. [Google Scholar]
- Moltzahn, F.; Olshen, A.B.; Baehner, L.; Peek, A.; Fong, L.; Stoppler, H.; Simko, J.; Hilton, J.F.; Carroll, P.; Blelloch, R. Microfluidic-based multiplex qRT-PCR identifies diagnostic and prognostic microRNA signatures in the sera of prostate cancer patients. Cancer Res 2011, 71, 550–560. [Google Scholar]
- Watahiki, A.; Wang, Y.; Morris, J.; Dennis, K.; O’Dwyer, H.M.; Gleave, M.; Gout, P.W. MicroRNAs associated with metastatic prostate cancer. PLoS One 2011, 6, e24950. [Google Scholar]
- Zhu, C.; Li, J.; Ding, Q.; Cheng, G.; Zhou, H.; Tao, L.; Cai, H.; Li, P.; Cao, Q.; Ju, X.; et al. miR-152 controls migration and invasive potential by targeting TGFalpha in prostate cancer cell lines. Prostate 2013. [Google Scholar] [CrossRef]
- Taylor, B.S.; Schultz, N.; Hieronymus, H.; Gopalan, A.; Xiao, Y.; Carver, B.S.; Arora, V.K.; Kaushik, P.; Cerami, E.; Reva, B.; et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010, 18, 11–22. [Google Scholar]
- Brase, J.C.; Wuttig, D.; Kuner, R.; Sultmann, H. Serum microRNAs as non-invasive biomarkers for cancer. Mol. Cancer 2010, 9, 306. [Google Scholar]
- Ribas, J.; Ni, X.; Haffner, M.; Wentzel, E.A.; Salmasi, A.H.; Chowdhury, W.H.; Kudrolli, T.A.; Yegnasubramanian, S.; Luo, J.; Rodriguez, R.; et al. miR-21: An androgen receptor-regulated microRNA that promotes hormone-dependent and hormone-independent prostate cancer growth. Cancer Res 2009, 69, 7165–7169. [Google Scholar]
- Hao, Y.; Zhao, Y.; Zhao, X.; He, C.; Pang, X.; Wu, T.C.; Califano, J.A.; Gu, X. Improvement of prostate cancer detection by integrating the PSA test with miRNA expression profiling. Cancer Invest 2011, 29, 318–324. [Google Scholar]
- Saito, Y.; Friedman, J.M.; Chihara, Y.; Egger, G.; Chuang, J.C.; Liang, G. Epigenetic therapy upregulates the tumor suppressor microRNA-126 and its host gene EGFL7 in human cancer cells. Biochem. Biophys. Res. Commun 2009, 379, 726–731. [Google Scholar]
- Szczyrba, J.; Loprich, E.; Wach, S.; Jung, V.; Unteregger, G.; Barth, S.; Grobholz, R.; Wieland, W.; Stohr, R.; Hartmann, A.; et al. The microRNA profile of prostate carcinoma obtained by deep sequencing. Mol. Cancer Res 2010, 8, 529–538. [Google Scholar]
- U. S. National Institutes of Health. Available online: http://edrn.nci.nih.gov/resources/standardoperating-procedures/biological-specimens (accessed on 2 January 2013).
- D’Amico, A.V.; Whittington, R.; Malkowicz, S.B.; Schultz, D.; Blank, K.; Broderick, G.A.; Tomaszewski, J.E.; Renshaw, A.A.; Kaplan, I.; Beard, C.J.; et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998, 280, 969–974. [Google Scholar]
- Graph Pad Prism Software, 6 ed; GraphPad Software Inc: La Jolla, CA, USA, 2012.
- Institute for Statistics and Mathematics of WU. The R Project for Statistical Computing. Available online: http://www.r-project.org (accessed on 2 January 2013).
- De Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B., Jr; Saad, F.; et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med 2011, 364, 1995–2005. [Google Scholar]
- De Bono, J.S.; Fizazi, K.; Saad, F.; Taplin, M.-E.; Sternberg, C.N.; Miller, K.; Mulders, P.; Chi, K.N.; Armstrong, A.J.; Hirmand, M.; et al. Primary, secondary, and quality-of-life endpoint results from the phase III AFFIRM study of MDV3100, an androgen receptor signaling inhibitor. J. Clin. Oncol. 2012, 30. Abstract 4519. [Google Scholar]


| Increased miRNA | Localized | mCRPC | Notes 1 |
|---|---|---|---|
| miRNA-125b-2 * | ND | 33.88 | >2 |
| miRNA-136 | 34.58 | 31.35 | >2 |
| miRNA-151-3p | 31.61 | 29.26 | >2 |
| miRNA-200a | 34.27 | 31.52 | >2 |
| miRNA-744a * | 38.09 | 34.51 | >2 |
| miRNA-9 | 39.09 | 34.79 | >2 |
| miRNA-9 * | ND | 34.12 | >2 |
| miRNA-99a | 34.28 | 32.02 | >2 |
| Let-7d | 33.21 | 31.37 | >1 |
| miRNA-126 | 26.49 | 24.93 | >1 |
| miRNA-142-5p | 30.35 | 29.16 | >1 |
| miRNA-18b | 30.14 | 28.98 | >1 |
| miRNA-27a | 29.07 | 27.31 | >1 |
| miRNA-27b | 29.15 | 27.25 | >1 |
| miRNA-30a * | 35.48 | 34.33 | >1 |
| Decreased miRNA | Local | mCRPC | Notes |
| miRNA-205 | 32.43 | 34.90 | >2 |
| miRNA-106b | 30.38 | 31.50 | >1 |
| miRNA-16 | 22.64 | 23.97 | >1 |
| miRNA-363 | 29.93 | 31.08 | >1 |
| AUC | 95% Confidence interval | Sensitivity | Specificity | |
|---|---|---|---|---|
| miR-141 | 0.8784 | 0.7832 to 0.9736 | 56% | 96% |
| miR-375 | 0.8576 | 0.7499 to 0.9653 | 56% | 96% |
| miR-151-3p | 0.7696 | 0.6310 to 0.9082 | 60% | 96% |
| miR-126 | 0.7488 | 0.6100 to 0.8876 | 48% | 96% |
| miR-16 | 0.7160 | 0.5708 to 0.8612 | 24% | 96% |
| miR-205 | 0.7536 | 0.6094 to 0.8978 | 60% | 88% |
| miR141 + 151-3p + 16 | 0.9440 | 0.8795 to 1.008 | 84% | 96% |
| PSA | 0.9640 | 0.9040 to 1.024 | 88% | 96% |
| PSA + miR141 + 151-3p + 16 | 0.9680 | 0.9087 to 1.027 | 96% | 96% |
| Upregulated in CRPC | Downregulated in CRPC | |||||
|---|---|---|---|---|---|---|
| miR-141 | miR-151-3p | miR-152 | miR-423-3p | miR-205 | ||
| Pathological Gleason score | p-value | NS | NS | NS | 0.002 | 0.004 |
| 7 or less (87) | 13.186 | 7.734 | 7.165 | 3.709 | 10.68 | |
| 8 or more (19) | 13.389 | 8.152 | 6.919 | 4.26 | 7.093 | |
| Lymph node involvement | p-value | NS | NS | NS | 0.003 | NS |
| Abnormal N1 (13) | 13.692 | 8.193 | 6.707 | 4.423 | 5.744 | |
| Normal N0 (79) | 13.139 | 7.762 | 7.164 | 3.757 | 10.339 | |
| Time to tumor recurrence | p-value | 0.003 | NS | 0.01 | NS | NS |
| <1 year (19) | 13.641 | 8.101 | 6.736 | 4.192 | 7.218 | |
| >1 year (81) | 13.144 | 7.754 | 7.181 | 3.727 | 10.568 | |
| PSA at diagnosis | p-value | NS | NS | NS | 0.003 | 0.005 |
| <20 (100) | 13.198 | 7.787 | 7.125 | 3.79 | 10.17 | |
| >20 (11) | 13.603 | 8.151 | 6.568 | 4.337 | 5.748 | |
| Probability of freedom of biochemical recurrence (5 years) | p-value | <0.001 | NS | 0.002 | NS | NS |
| <90% (21) | 13.715 | 8.118 | 6.647 | 4.133 | 6.77 | |
| >90% (74) | 13.155 | 7.713 | 7.203 | 3.678 | 10.74 | |
| Metastatic events | p-value | NS | NS | NS | <0.001 | <0.001 |
| Clinical (19) | 13.149 | 8.145 | 6.466 | 4.529 | 5.045 | |
| none (94) | 13.231 | 7.764 | 7.169 | 3.723 | 10.657 | |
| Biochemical recurrence event | p-value | NS | <0.001 | NS | <0.001 | 0.006 |
| yes (27) | 13.36 | 8.125 | 6.923 | 4.177 | 8.03 | |
| no (80) | 13.189 | 7.711 | 7.162 | 3.685 | 10.629 | |
| Clinically Localized Prostate Cancer Patients (N= 25) | |
| Median Age = 67 years; Range = 51–82 years | |
| Patient Characteristics | Number of Patients |
| T Stage | |
| T1C | 11 |
| T2a | 7 |
| T2b | 6 |
| T3 | 1 |
| PSA at Diagnosis | |
| ≤10 | 15 |
| >10 to ≤20 | 9 |
| >20 | 1 |
| Gleason Score | |
| ≤6 | 7 |
| 7 | 15 |
| ≥8 | 3 |
| Risk Group [25] | |
| Low | 4 |
| Intermediate | 17 |
| High | 4 |
| mCRPC Patients (N= 25) | |
| Median Age = 73 years; Range = 45–86 years | |
| Patient Characteristics | Number of Patients |
| PSA | |
| <50 | 6 |
| >50 to ≤300 | 14 |
| ≥300 | 5 |
| Metastatic Site | |
| Bone | 17 |
| Lymph Node ± Other | 5 |
| Other | 3 |
| Therapy at Time of Sampling | |
| Observation * | 15 (1 was not on LHRH-A) |
| Complete Androgen Blockade | 4 |
| Prednisone ± LHRH-A | 4 |
| Abiraterone | 2 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Watahiki, A.; Macfarlane, R.J.; Gleave, M.E.; Crea, F.; Wang, Y.; Helgason, C.D.; Chi, K.N. Plasma miRNAs as Biomarkers to Identify Patients with Castration-Resistant Metastatic Prostate Cancer. Int. J. Mol. Sci. 2013, 14, 7757-7770. https://doi.org/10.3390/ijms14047757
Watahiki A, Macfarlane RJ, Gleave ME, Crea F, Wang Y, Helgason CD, Chi KN. Plasma miRNAs as Biomarkers to Identify Patients with Castration-Resistant Metastatic Prostate Cancer. International Journal of Molecular Sciences. 2013; 14(4):7757-7770. https://doi.org/10.3390/ijms14047757
Chicago/Turabian StyleWatahiki, Akira, Robyn J. Macfarlane, Martin E. Gleave, Francesco Crea, Yuzhuo Wang, Cheryl D. Helgason, and Kim N. Chi. 2013. "Plasma miRNAs as Biomarkers to Identify Patients with Castration-Resistant Metastatic Prostate Cancer" International Journal of Molecular Sciences 14, no. 4: 7757-7770. https://doi.org/10.3390/ijms14047757





