A Stratified Meta-Analysis of the Association between Exposure to Environmental Tobacco Smoke during Childhood and Adulthood and Urothelial Bladder Cancer Risk
Abstract
:1. Introduction
2. Methods
2.1. Literature Search
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Study Characteristics
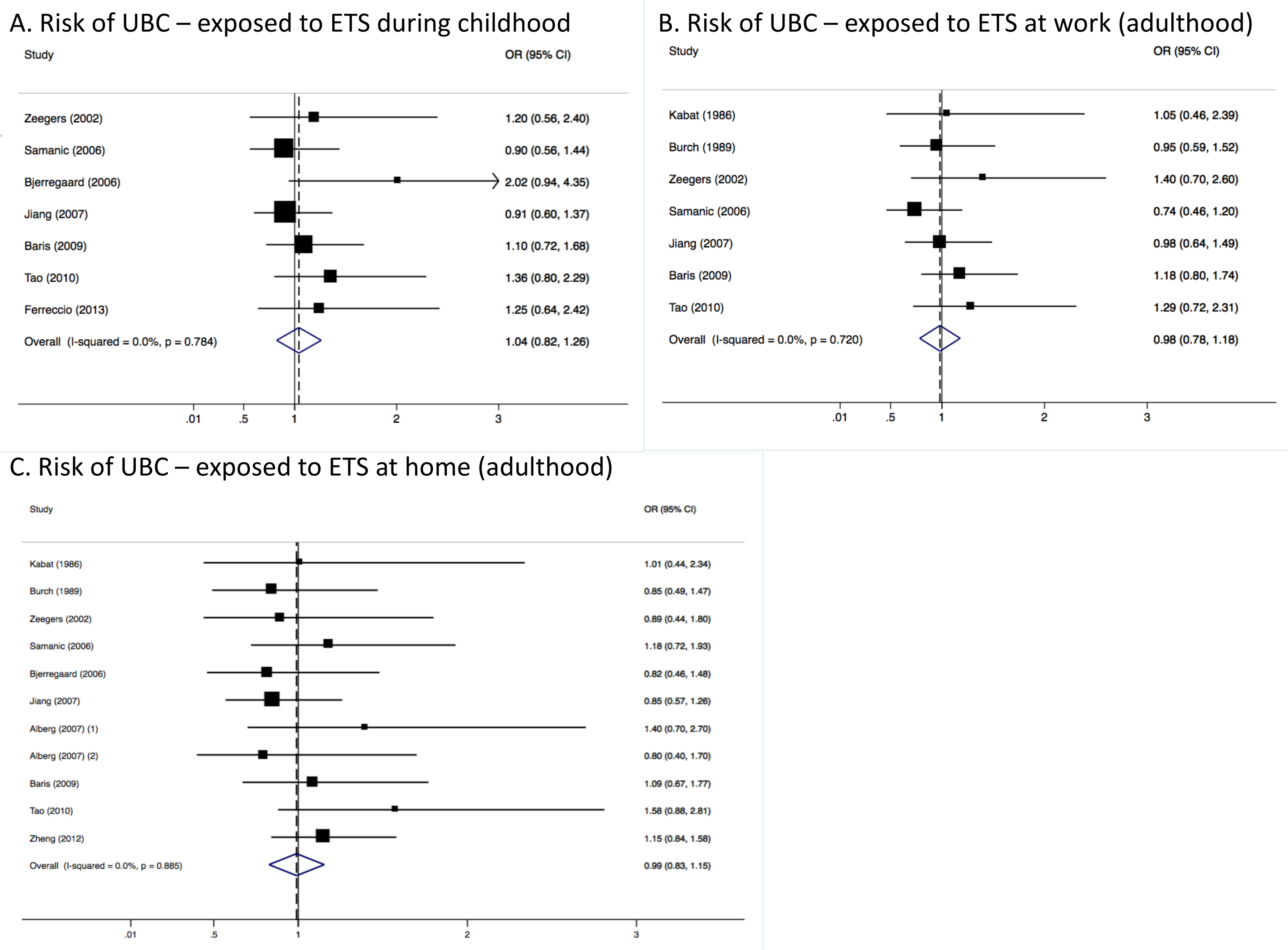
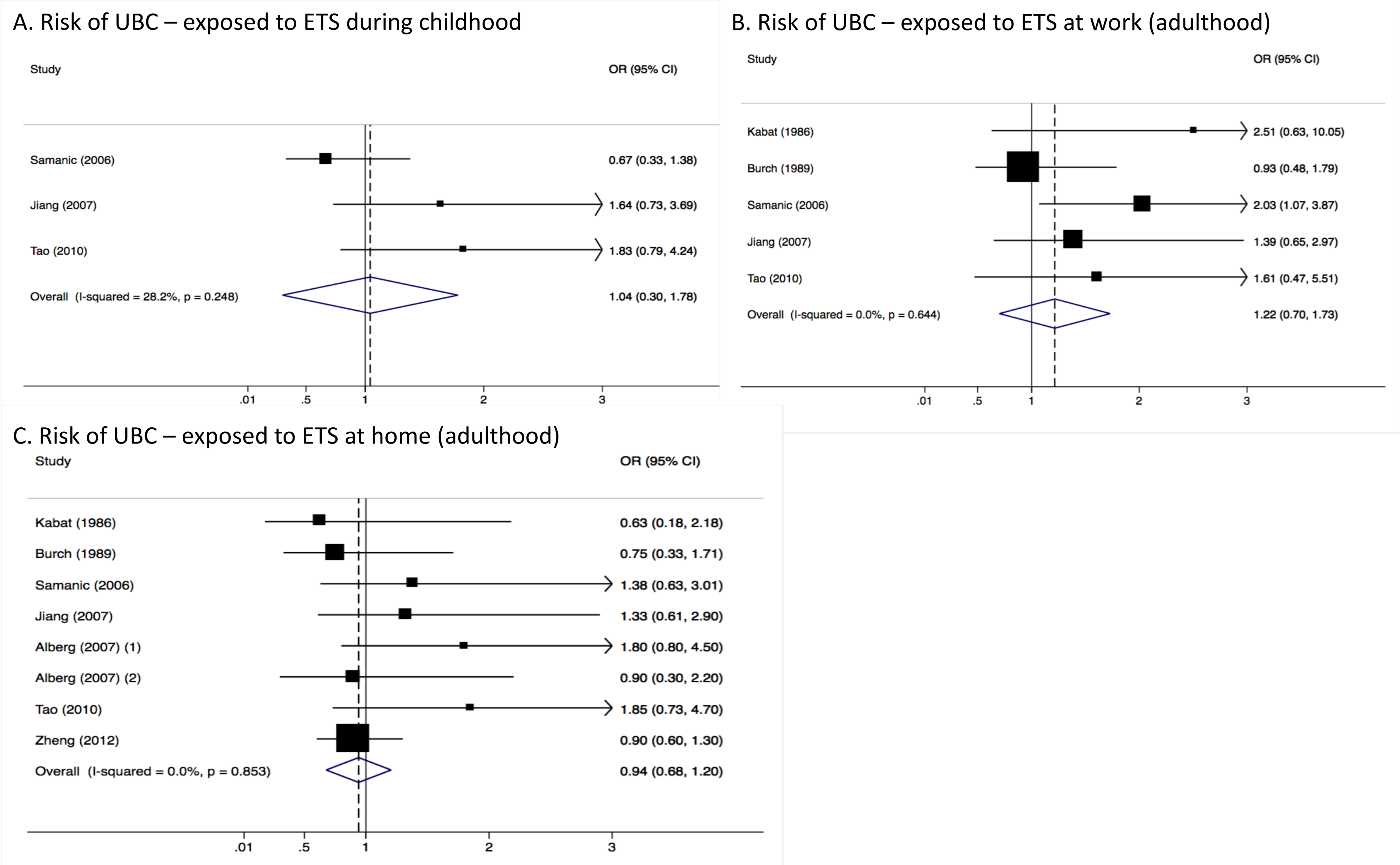
3.2. Pooled Results from Stratified Analysis
3.3. Heterogeneity and Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Van Osch, F.H.; Jochems, S.H.; van Schooten, F.-J.; Bryan, R.T.; Zeegers, M.P. Quantified relations between exposure to tobacco smoking and bladder cancer risk: A meta-analysis of 89 observational studies. Int. J. Epidemiol. 2016, 45, 857–870. [Google Scholar] [CrossRef] [PubMed]
- The Organisation for Economic Co-operation (OECD). Health at a Glance 2017; OECD Publishing: Paris, France, 2017; ISBN 9789264280397. [Google Scholar]
- U.S. Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2006.
- Oberg, M.; Jaakkola, M.S.; Woodward, A.; Peruga, A.; Prüss-Ustün, A. Worldwide burden of disease from exposure to second-hand smoke: A retrospective analysis of data from 192 countries. Lancet 2011, 377, 139–146. [Google Scholar] [CrossRef]
- Lee, P.N.; Thornton, A.J.; Hamling, J.S.; Lee, P.N. Epidemiological evidence on environmental tobacco smoke and cancers other than lung or breast. Regul. Toxicol. Pharmacol. 2016, 80, 134–163. [Google Scholar] [CrossRef] [PubMed]
- Agaku, I.T.; Singh, T.; Rolle, I.; Olalekan, A.-Y.; King, B.A. Prevalence and Determinants of Secondhand Smoke Exposure among Middle and High School Students. Pediatrics 2016, 137, e20151985. [Google Scholar] [CrossRef] [PubMed]
- Rachiotis, G.; Barbouni, A.; Katsioulis, A.; Antoniadou, E.; Kostikas, K.; Merakou, K.; Kourea, K.; Khoury, R.N.; Tsouros, A.; Kremastinou, J.; et al. Prevalence and determinants of current and secondhand smoking in Greece: Results from the Global Adult Tobacco Survey (GATS) study. BMJ Open 2017, 7, e013150. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O ’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2016. [Google Scholar]
- MedCalc MedCalc’s Odds Ratio Calculator. Available online: https://www.medcalc.org/calc/odds_ratio.php (accessed on 20 March 2018).
- Hamling, J.; Lee, P.; Weitkunat, R.; Ambühl, M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat. Med. 2008, 27, 954–970. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Su, H.-J.J.; Guo, Y.-L.L.; Houseman, E.A.; Christiani, D.C. Interaction between environmental tobacco smoke and arsenic methylation ability on the risk of bladder cancer. Cancer Causes Control 2005, 16, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-C.; Chen, M.-C.; Huang, Y.-K.; Huang, C.-Y.; Lai, L.-A.; Chung, C.-J.; Shiue, H.-S.; Pu, Y.-S.; Lin, Y.-C.; Han, B.-C.; et al. Environmental tobacco smoke and arsenic methylation capacity are associated with urothelial carcinoma. J. Formos. Med. Assoc. 2013, 112, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Sandler, D.P.; Everson, R.B.; Wilcox, A.J. Passive smoking in adulthood and cancer risk. Am. J. Epidemiol. 1985, 121, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Xiang, Y.-B.; Wang, R.; Nelson, H.H.; Gao, Y.-T.; Chan, K.K.; Mimi, C.Y.; Yuan, J.-M. Environmental tobacco smoke in relation to bladder cancer risk—The Shanghai bladder cancer study. Cancer Epidemiol. Biomark. Prev. 2010, 19, 3087–3095. [Google Scholar] [CrossRef] [PubMed]
- Zeegers, M.P.A.; Goldbohm, R.A.; van den Brandt, P.A. A prospective study on active and environmental tobacco smoking and bladder cancer risk (The Netherlands). Cancer Causes Control 2002, 13, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Bjerregaard, B.K.; Raaschou-Nielsen, O.; Sørensen, M.; Frederiksen, K.; Christensen, J.; Tjønneland, A.; Overvad, K.; Chapelon, F.C.; Nagel, G.; Chang-Claude, J.; et al. Tobacco smoke and bladder cancer-in the European prospective investigation into cancer and nutrition. Int. J. Cancer 2006, 119, 2412–2416. [Google Scholar] [CrossRef] [PubMed]
- Alberg, A.J.; Kouzis, A.; Genkinger, J.M.; Gallicchio, L.; Burke, A.E.; Hoffman, S.C.; Diener-West, M.; Helzlsouer, K.J.; Comstock, G.W. A prospective cohort study of bladder cancer risk in relation to active cigarette smoking and household exposure to secondhand cigarette smoke. Am. J. Epidemiol. 2007, 165, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Kabat, G.C.; Dieck, G.S.; Wynder, E.L. Bladder cancer in nonsmokers. Cancer 1986, 57, 362–367. [Google Scholar] [CrossRef]
- Burch, J.D.; Rohan, T.E.; Howe, G.R.; Risch, H.A.; Hill, G.B.; Steele, R.; Miller, A.B. Risk of bladder cancer by source and type of tobacco exposure: A case-control study. Int. J. Cancer 1989, 44, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Samanic, C.; Kogevinas, M.; Dosemeci, M.; Malats, N.; Real, F.X.; Garcia-Closas, M.; Serra, C.; Carrato, A.; García-Closas, R.; Sala, M. Smoking and bladder cancer in Spain: Effects of tobacco type, timing, environmental tobacco smoke, and gender. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yuan, J.-M.; Skipper, P.L.; Tannenbaum, S.R.; Mimi, C.Y. Environmental tobacco smoke and bladder cancer risk in never smokers of Los Angeles County. Cancer Res. 2007, 67, 7540–7545. [Google Scholar] [CrossRef] [PubMed]
- Baris, D.; Karagas, M.R.; Verrill, C.; Johnson, A.; Andrew, A.S.; Marsit, C.J.; Schwenn, M.; Colt, J.S.; Cherala, S.; Samanic, C.; et al. A case-control study of smoking and bladder cancer risk: Emergent patterns over time. J. Natl. Cancer Inst. 2009, 101, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-L.; Amr, S.; Saleh, D.A.; Dash, C.; Ezzat, S.; Mikhail, N.N.; Gouda, I.; Loay, I.; Hifnawy, T.; Abdel-Hamid, M.; et al. Urinary bladder cancer risk factors in Egypt: A multicenter case-control study. Cancer Epidemiol. Biomark. Prev. 2012, 21, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Ferreccio, C.; Yuan, Y.; Calle, J.; Benítez, H.; Parra, R.L.; Acevedo, J.; Smith, A.H.; Liaw, J.; Steinmaus, C. Arsenic, tobacco smoke, and occupation: Associations of multiple agents with lung and bladder cancer. Epidemiology 2013, 24, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.G.; Hawkins, J.D.; Catalano, R.F.; Abbott, R.D.; Guo, J. Family influences on the risk of daily smoking initiation. J. Adolesc. Health 2005, 37, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Brody, A.L.; Mandelkern, M.A.; London, E.D.; Khan, A.; Kozman, D.; Costello, M.R.; Vellios, E.E.; Archie, M.M.; Bascom, R.; Mukhin, A.G. Effect of Secondhand Smoke on Occupancy of Nicotinic Acetylcholine Receptors in Brain. Arch. Gen. Psychiatr. 2011, 68, 953. [Google Scholar] [CrossRef] [PubMed]

| Reference | First Author | Year | Country | Never Smokers * | Study Design | Cigarette Smoking Assessment | Exposure to ETS | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort Study | Case-Control Study | Childhood Exposure | Adulthood Exposure | ||||||||
| Case Source | Control Source | At Home | At Work | ||||||||
| [18] | Kabat | 1986 | USA | 644 | - | Hospital | Hospital | Structured interview | - | Yes | Yes |
| [19] | Burch | 1989 | Canada | 359 | - | Hospital | Population | Structured interview | - | Yes | Yes |
| [15] | Zeegers | 2002 | The Netherlands | 1233 | Yes | - | - | Postal questionnaire | Yes | Yes | Yes |
| [20] | Samanic | 2006 | Spain | 528 | - | Hospital | Hospital | Postal questionnaire | Yes | Yes | Yes |
| [16] | Bjerregaard | 2006 | Europe | 220,790 | Yes | - | - | Postal questionnaire | Yes | Yes | - |
| [21] | Jiang | 2007 | USA | 440 | - | Population | Population | Structured interview | Yes | Yes | Yes |
| [17] | Alberg * | 2007 | USA | 18,839/20,181 | Yes | - | - | Postal questionnaire | - | Yes | - |
| [22] | Baris | 2009 | USA | 547 | - | Population | Population | Structured interview | Yes | Yes | - |
| [14] | Tao | 2010 | China | 456 | - | Population | Population | Structured interview | Yes | Yes | Yes |
| [23] | Zheng | 2012 | Egypt | 678 | - | Hospital | Population | Structured interview | - | Yes | Yes |
| [24] | Ferreccio | 2013 | Chile | 307 | - | Population | Population | Structured interview | Yes | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Osch, F.H.M.; Jochems, S.H.J.; Wesselius, A.; Van Schooten, F.J.; Bryan, R.T.; Zeegers, M.P. A Stratified Meta-Analysis of the Association between Exposure to Environmental Tobacco Smoke during Childhood and Adulthood and Urothelial Bladder Cancer Risk. Int. J. Environ. Res. Public Health 2018, 15, 569. https://doi.org/10.3390/ijerph15040569
Van Osch FHM, Jochems SHJ, Wesselius A, Van Schooten FJ, Bryan RT, Zeegers MP. A Stratified Meta-Analysis of the Association between Exposure to Environmental Tobacco Smoke during Childhood and Adulthood and Urothelial Bladder Cancer Risk. International Journal of Environmental Research and Public Health. 2018; 15(4):569. https://doi.org/10.3390/ijerph15040569
Chicago/Turabian StyleVan Osch, Frits H. M., Sylvia H. J. Jochems, Anke Wesselius, Frederik J. Van Schooten, Richard T. Bryan, and Maurice P. Zeegers. 2018. "A Stratified Meta-Analysis of the Association between Exposure to Environmental Tobacco Smoke during Childhood and Adulthood and Urothelial Bladder Cancer Risk" International Journal of Environmental Research and Public Health 15, no. 4: 569. https://doi.org/10.3390/ijerph15040569






