Monitoring of Pesticide Residues in Commonly Used Fruits and Vegetables in Kuwait
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Collection
2.3. Sample Extraction and Clean-Up
2.4. Solutions and Standards
2.5. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.6. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Analysis
2.7. Quality Control
3. Results
3.1. Pesticide Residues in Analyzed Samples
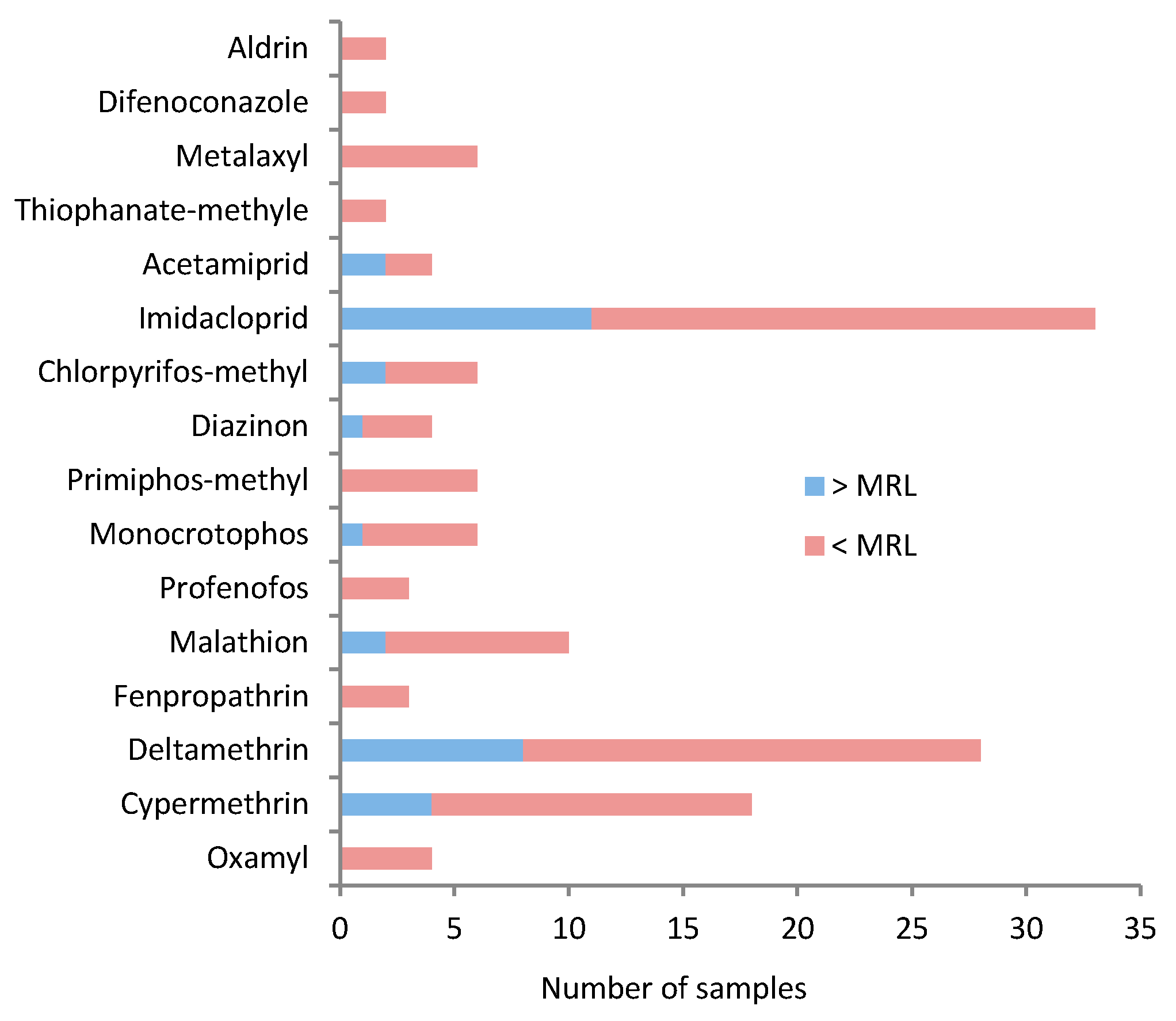
3.2. MRL Exceedances and Detection Frequencies of Pesticides in Analyzed Samples
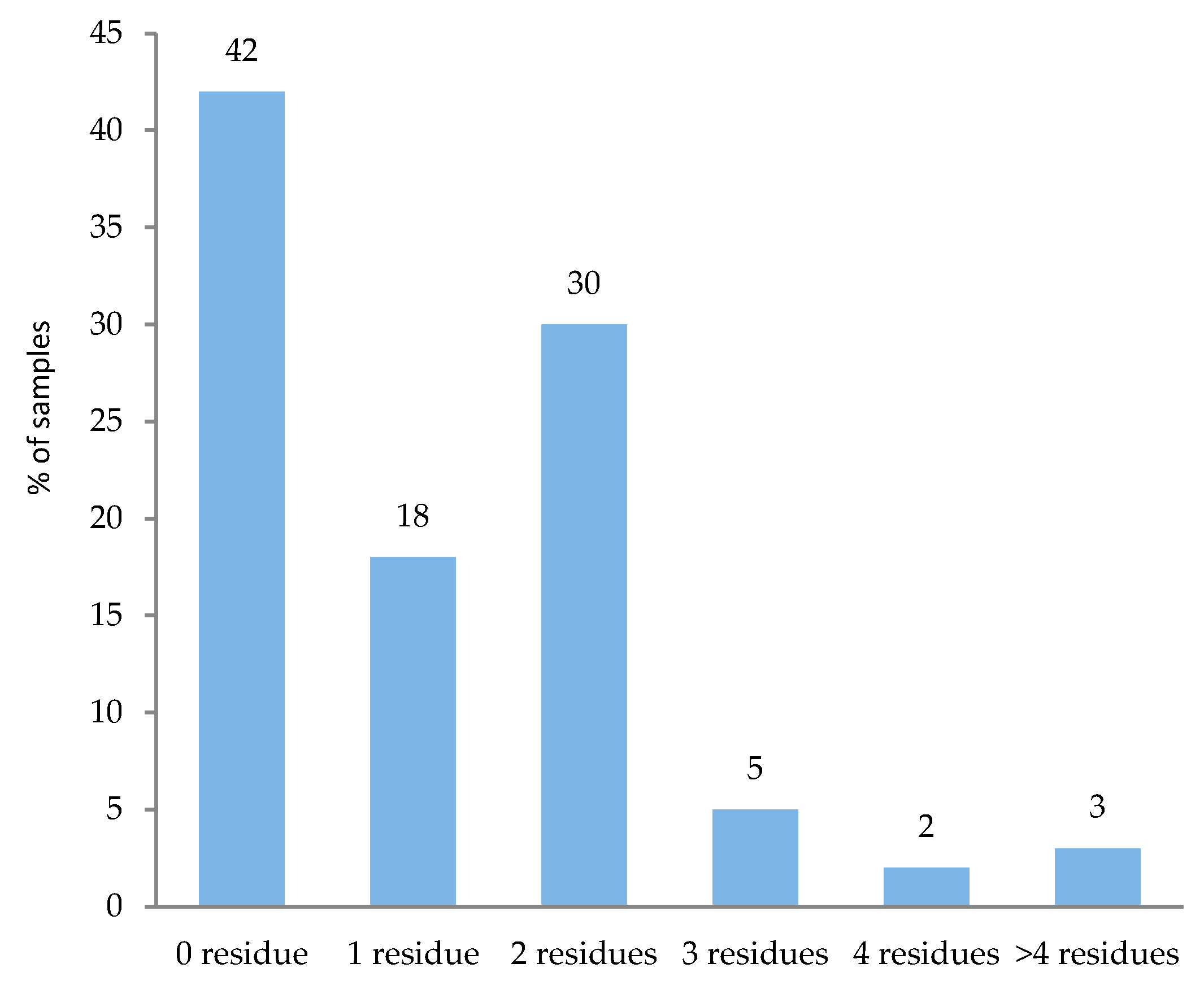
3.3. Multiple Residues in Analyzed Samples
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, D. Environmental and economic cost of the application of pesticides primarily in the United States. Environ. Dev. Sustain. 2005, 7, 229–252. [Google Scholar] [CrossRef]
- Jeyaratnam, J. Acute pesticide poisoning: A major global health problem. World Health Stat. Q 1990, 43, 139–144. [Google Scholar] [PubMed]
- Cecchi, A.; Rovedatti, G.M.; Sabino, G.; Magnarelli, G. Environmental exposure to organophosphate pesticides: Assessment of endocrine disruption and hepatotoxicity in pregnant women. Ecotoxicol. Environ. Saf. 2012, 80, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Alavanja, M.C.R.; Ross, M.K.; Bonner, M.R. Increased cancer burden among pesticide applicators and others due to pesticide exposure. Cancer J. Clin. 2013, 63, 120–142. [Google Scholar] [CrossRef] [PubMed]
- Lozowicka, B. Health risk for children and adults consuming apples with pesticide residue. Sci. Total Environ. 2015, 502, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Jallow, M.F.A.; Awadh, D.G.; Albaho, M.S.; Devi, V.Y.; Thomas, B.M. Pesticide risk behaviors and factors influencing pesticide use among farmers in Kuwait. Sci. Total Environ. 2017, 574, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Kuwait Agricultural Statistics. A Survey on Behalf of Kuwait Central Statistics Bureau, Safat, Kuwait, 2013–2014. Available online: http://www.csb.gov.kw/Socan_Statistic_ EN.aspx?ID=42 (accessed on 16 December 2016).
- Allen, G.; Halsall, C.J.; Ukpebor, J.; Paul, N.D.; Ridall, G.; Jason, J.; Wargent, J.J. Increased occurrence of pesticide residues on crops grown in protected environments compared to crops grown in open field conditions. Chemosphere 2015, 119, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Bashour, I. Pesticides, fertilizers and food safety. In Arab Environment Future Challenges; Tolba, M.K., Saab, N.W., Eds.; Arab Forum for Environment and Development: Beirut, Lebanon, 2008; pp. 137–145. [Google Scholar]
- Jallow, M.F.A.; Awadh, D.G.; Albaho, M.S.; Devi, V.Y.; Thomas, B.M. Pesticide Knowledge and safety practices among farm workers in Kuwait: Results of a survey. Int. J. Environ. Res. Public Health 2017, 14, 340. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, W.; Al-Awadhi, F.A.; Saeed, T.; Al-Omair, A.; Ahmad, N.; Husain, A.; Khalafawi, S.H.; Al-Omirah, B.; Dashti, H.; Al-Amiri, H.; et al. Kuwait total diet study: Dietary intake of organochhlorine, carbamate, benzimidazole and phenylurea pesticide residues. J. AOAC Int. 1999, 82, 1458–1465. [Google Scholar] [PubMed]
- Sawaya, W.; Al-Awadhi, F.A.; Saeed, T.; Al-Omair, A.; Ahmad, N.; Husain, A.; Khalafawi, S.; Al-Zenki, S.; Al-Amiri, H.; Al-Otaibi, J.; et al. Dietary intake of pesticides: Sate of Kuwait total diet study. Food Addit. Contam. 1999, 16, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, W.; Al-Awadhi, F.A.; Saeed, T.; Al-Omair, A.; Hussain, A.H.; Ahmad, N.; Al-Omair, H.; Al-Zenki, S.; Khalafawi, S.; Al-Otaibi, J.; et al. Dietary intake of organophosphate pesticides in Kuwait. Food Chem. 2000, 89, 331–338. [Google Scholar] [CrossRef]
- Saeed, T.; Sawaya, W.; Ahmad, N.; Rajagopal, S.; Al-Omair, A.; Al-Awadhi, S. Chlorinated pesticides residues in the total diet of Kuwait. Food Control 2001, 12, 91–98. [Google Scholar] [CrossRef]
- Saeed, T.; Sawaya, W.; Ahmad, N.; Rajagopal, S.; Al-Omair, A. Organophosphorus pesticide residue in the total diet of Kuwait. Arab J. Sci. Eng. 2005, 30, 17–27. [Google Scholar]
- Saeed, T.; Sawaya, W.; Ahmad, N.; Rajagopal, S.; Dashti, B.; Al-Awadhi, S. Assessment of the levels of chlorinated pesticides in breast milk in Kuwait. J. Food Addit. Contam. 2000, 12, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture. Maximum Residue Limit Database. Foreign Agricultural Service Department. Available online: http:www.fas.usda.gov/maximum-residue-limits-mrl-database (accessed on 18 December 2016).
- Codex Alimentarius Commission. Pesticide Residues in Food and Feed. Plant Production and Protection Division. Available online: http://www.fao.org/fao-who-codex alimentarius/standards/pestres/en (accessed on 4 January 2017).
- European Commission. Health and Food Safety, Regulation. Available online: http://ec.europa.eu/food/plant/pesticides/max_residue_levels/eu_rules/index_en.htm (accessed on 10 October 2016).
- Weaver, T.B.; Ghadiri, H.; Hulugalle, N.R.; Harden, S. Organochlorine pesticides in soil under irrigated cotton farming systems in Vertisols of the Namoi Valley, north-western New South Wales, Australia. Chemosphere 2012, 88, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Florence, C.; Philippe, L.; Magalie, L.J. Organochlorine (chlordecone) uptake by rootvegetables. Chemosphere 2015, 118, 96–102. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Establishing Community Methods of Sampling for the Official Control of Pesticide Residues in and on Products of Plant and Animal Origin and Repealing Directive. OJEU L 2002, 187, 30–43. [Google Scholar]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersivesolid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [PubMed]
- European Commission. Method Validation and Quality Control Procedures for Pesticide Residues Analysis in Food and Feed. Available online: http://ec.europa.eu/food/plant/protection/resources/qualcontrol_en.pdf (accessed on 10 October 2016).
- Berrada, H.; Fernández, M.; Ruiz, M.J.; Moltó, J.C.; Mañes, J.; Font, G. Surveillance of pesticide residues in fruits from Valencia during twenty months (2004/05). Food Control 2010, 21, 36–44. [Google Scholar] [CrossRef]
- Lehotay, S.J.; Son, K.A.; Kwon, H.; Koesukwiwat, U.; Fu, W.; Mastovska, K.; Hoh, E.; Leepipatpiboon, N. Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruits and vegetables. J. Chromatogr. A 2010, 1217, 2548–2560. [Google Scholar] [CrossRef] [PubMed]
- Osman, K.A.; Al-Humaid, A.M.; Al-Redhaiman, K.N. Monitoring of pesticide residues in vegetables marketed in Al-Qassim region, Saudi Arabia. Ecotoxicol. Environ. Saf. 2010, 73, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Bempah, C.K.; Donkor, A.; Yeboah, P.O.; Dubey, B.; Osei-Fosu, P. A preliminary assessment of consumer’s exposure to organochlorine pesticides in fruits and vegetables and the potential health risk in Accra Metropolis, Ghana. Food Chem. 2011, 128, 1058–1065. [Google Scholar] [CrossRef]
- World Health Organization. The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification. Available online: http://www.who.int/foodsafety/publications/classification-pesticides/en/ (accessed on 16 December 2016).
- Tomizawa, M.; Casida, J.E. Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu. Rev. Entomol. 2003, 48, 339–364. [Google Scholar] [CrossRef] [PubMed]
- Blocksom, K.A.; Walters, D.M.; Jicha, T.M.; Lazorchak, J.M.; Angradi, T.R.; Bolgrien, D.W. Persistent organic pollutants in fish tissue in the Mid-Continental Great Rivers of the United States. Sci. Total Environ. 2010, 408, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Shoiful, A.; Fujita, H.; Watanabe, I.; Honda, K. Concentrations of organochlorine pesticides (OCPs) residues in foodstuffs collected from traditional markets in Indonesia. Chemosphere 2013, 90, 1742–1750. [Google Scholar] [CrossRef] [PubMed]
- Ali, U.; Syed, J.; Malik, R.; Katsoyiannis, A.; Li, J.; Zhang, G.; Jones, K. Organochlorine pesticides (OCPs) in South Asian region: A review. Sci. Total Environ. 2014, 476–477, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Montory, M.; Ferrer, J.; Rivera, D.; Villouta, M.V.; Grimalt, J.O. First report onorganochlorine pesticides in water in a highly productive agro-industrial basin of the Central Valley, Chile. Chemosphere 2017, 173, 125–136. [Google Scholar]
- Osman, K.A.; Al-Humaid, A.I.; Al-Rehiayani, S.M.; Al-Redhaiman, K.N. Estimated daily intake of pesticide residues exposure by vegetables grown in greenhouses in Al-Qassim region, Saudi Arabia. Food Control 2011, 22, 947–953. [Google Scholar] [CrossRef]
- Qin, G.; Zou, K.; Li, Y.; Chen, Y.; He, F.; Ding, G. Pesticide residue determination in vegetables from western China applying gas chromatography with mass spectrometry. Biomed. Chromatogr. 2016, 30, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Li, S.L.; Ni, Z.L.; Qu, M.H.; Zhong, D.L.; Ye, C.F.; Tang, F.B. Pesticides in persimmons, jujubes and soil from China: Residue levels, risk assessment and relationship between fruits and soils. Sci. Total Environ. 2016, 542, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Savage, E.P.; Keefe, T.J.; Mounce, L.M.; Heaton, R.K.; Lewis, J.A.; Bucar., P.J. Chronic neurological sequelae of acute organophosphate pesticide poisoning. Arch. Environ. Health 1988, 43, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Shi, L.L.; Kong, D.Y.; Cai, D.J.; Cao, Y.Z.; Liu, Y.M.; Pang, G.; Yu, R. Accumulation levels and characteristics of some pesticides in human adipose tissue samples from Southeast China. Chemosphere 2011, 84, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Berlinger, M.J.; Jarvis, W.R.; Jewett, T.J.; Lebiush-Mordechi, S. Managing the greenhouse, crop and crop environment. In Integrated Pest and Disease Management in Greenhouse Crops; Albajes, R., Lodovica Gullino, M., van Lenteren, J.C., Eds.; Kluwer Academic Publishers: New York, NY, USA, 2002; pp. 97–123. [Google Scholar]
- Keikotlhaile, B.M.; Spanoghe, P.; Steurbaut, W. Effects of food processing on pesticide residues in fruits and vegetables: A meta-analysis approach. Food Chem. Toxicol. 2010, 48, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shabeer, A.T.P.; Kaushik, B.; Manjusha, J.; Rushali, G.; Sagar, U.; Sandip, H.; Dasharath, O. Residue dissipation and processing factor for dimethomorph, famoxadone andcymoxanil during raisin preparation. Food Chem. 2015, 170, 180–185. [Google Scholar] [CrossRef] [PubMed]
| Pesticide | % Recovery (RSD) a | LOD (mg·kg−1) b | LOQ (mg·kg−1) c | |
|---|---|---|---|---|
| Fortification Levels (mg·kg−1) | ||||
| 0.01 | 0.05 | |||
| Oxamyl | 94 (4.32) | 89 (8.45) | 0.0020–0.0031 | 0.0059–0.0212 |
| Cypermethrin | 86 (5.46) | 92 (6.42) | 0.0013–0.0030 | 0.0051–0.0098 |
| Deltamethrin | 89 (3.21) | 86 (4.98) | 0.0019–0.0028 | 0.0034–0.0061 |
| Fenpropathrin | 102 (6.80) | 94 (2.04) | 0.0700–0.1869 | 0.2140–0.4521 |
| Malathion | 98 (2.67) | 100 (3.98) | 0.0040–0.0092 | 0.0162–0.0312 |
| Profenofos | 100 (5.47) | 89 (4.21) | 0.0018–0.0021 | 0.0031–0.0074 |
| Monocrotophos | 96 (5.21) | 89 (6.21) | 0.0012–0.0021 | 0.0069–0.0123 |
| Primiphos-methyl | 98 (4.89) | 100 (10.18) | 0.0026–0.0089 | 0.0163–0.0281 |
| Diazinon | 85 (1.34) | 79 (4.51) | 0.0018–0.0025 | 0.0096–0.0184 |
| Chlorpyrifos-methyl | 106 (4.82) | 97 (8.23) | 0.0016–0.0027 | 0.0057–0.0098 |
| Imidacloprid | 95 (6.21) | 89 (6.18) | 0.0009–0.0014 | 0.0036–0.0075 |
| Acetamiprid | 99 (3.21) | 100 (2.98) | 0.0021–0.0031 | 0.0098–0.0123 |
| Thiophanate-methyl | 102 (6.18) | 87 (3.21) | 0.0035–0.0192 | 0.0632–0.0971 |
| Metalaxyl | 92 (3.41) | 85 (6.21) | 0.0007–0.0021 | 0.0029–0.0036 |
| Difenoconazole | 93 (4.29) | 90 (4.21) | 0.0019–0.0034 | 0.0067–0.0132 |
| Aldrin | 89 (3.19) | 85 (2.89) | 0.0008–0.0012 | 0.0049–0.0086 |
| Produce | Number of Samples | Without Residue | With Residue < MRL | With Residue > MRL |
|---|---|---|---|---|
| Tomato | 16 | 2 (12%) | 11 (69%) | 3 (19%) |
| Carrot | 10 | 9 (90%) | 1 (10%) | 0 (0%) |
| Bell pepper | 12 | 2 (17%) | 10 (83%) | 0 (0%) |
| Eggplant | 14 | 5 (36%) | 8 (57%) | 1 (7%) |
| Cucumber | 15 | 2 (13%) | 10 (67%) | 3 (20%) |
| Zucchini | 12 | 10 (83%) | 2 (17%) | 0 (0%) |
| Watermelon | 12 | 2 (17%) | 4 (33%) | 6 (50%) |
| Cabbage | 15 | 14 (93%) | 1 (7%) | 0 (0%) |
| Potato | 10 | 10 (100%) | 0 (0%) | 0 (0%) |
| Apple | 10 | 1 (10%) | 1 (10%) | 8 (80%) |
| Grapes | 14 | 2 (14%) | 5 (36%) | 7 (50%) |
| Strawberry | 10 | 3 (30%) | 3 (30%) | 4 (40%) |
| Total | 150 | 62 (42%) | 56 (37%) | 32 (21%) |
| Pesticide | Tom | Car | Bpep | EgP | Cuc | Zuc | WaM | Cab | Pot | App | Grap | Straw |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration Range (mg kg−1) | ||||||||||||
| Oxamyl | nd–0.09 | nd | nd | nd | nd–0.98 | nd | nd | nd | nd | nd | nd | nd |
| Cypermethrin | 0.02–0.24 * | nd | nd | nd–0.13 * | nd | nd | nd–0.09 | nd | nd | nd | nd–0.28 * | nd |
| Deltamethrin | nd | nd | nd–0.02 | nd | nd | nd | 0.06–0.29 * | nd | nd | 0.2–0.32 * | 0.032–0.38 * | nd–0.09 |
| Fenpropathrin | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd–1.2 |
| Malathion | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.09–0.58 * | nd | nd–0.98 |
| Profenofos | 0.02–0.39 | nd | nd–0.03 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Monocrotophos | nd–0.02 | nd | nd | nd | nd–0.04 | nd | nd–0.02 * | nd | nd | nd | nd | nd |
| Primiphos–methyl | nd–0.1 | nd | 0.091–0.2 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Diazinon | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd–0.08 | nd | nd–0.12 * |
| Chlorpyrifos–methyl | nd–0.08 | nd–0.03 | nd–0.02 | nd | nd | nd | nd | nd | nd | nd | nd–1.32 * | nd |
| Imidacloprid | nd–0.51 * | nd | nd–0.01 | nd–0.09 | 0.05–1.2 * | nd–0.08 | nd–0.23 * | nd | nd | 0.2–0.65 * | nd–0.98 | nd–0.2 |
| Acetamiprid | nd | nd | nd–0.05 | nd | nd | nd | nd | nd–0.1 | nd | nd | nd | nd–1.01 * |
| Thiophanate–methyl | nd–0.3 | nd | nd | nd | nb–0.19 | nd | nd | nd | nd | nd | nd | nd |
| Metalaxyl | nd–0.2 | nd | nd–0.01 | nd | 0.01–0.06 | nd | nd–0.08 | nd | nd | nd | nd | nd |
| Difenoconazole | nd | nd | nd | nd | nd | nd | 0.04–0.1 | nd | nd | nd | nd | nd |
| Aldrin | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd–0.02 | nd | nd |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jallow, M.F.A.; Awadh, D.G.; Albaho, M.S.; Devi, V.Y.; Ahmad, N. Monitoring of Pesticide Residues in Commonly Used Fruits and Vegetables in Kuwait. Int. J. Environ. Res. Public Health 2017, 14, 833. https://doi.org/10.3390/ijerph14080833
Jallow MFA, Awadh DG, Albaho MS, Devi VY, Ahmad N. Monitoring of Pesticide Residues in Commonly Used Fruits and Vegetables in Kuwait. International Journal of Environmental Research and Public Health. 2017; 14(8):833. https://doi.org/10.3390/ijerph14080833
Chicago/Turabian StyleJallow, Mustapha F. A., Dawood G. Awadh, Mohammed S. Albaho, Vimala Y. Devi, and Nisar Ahmad. 2017. "Monitoring of Pesticide Residues in Commonly Used Fruits and Vegetables in Kuwait" International Journal of Environmental Research and Public Health 14, no. 8: 833. https://doi.org/10.3390/ijerph14080833




