Benzene Exposure Alters Expression of Enzymes Involved in Fatty Acid β-Oxidation in Male C3H/He Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Antibodies
2.2. Animals and Treatment
2.3. Blood Routine
2.4. L-Carnitine Assay
2.5. Real-Time Polymerase Chain Reaction (PCR)
2.6. Western Blot
2.7. ATP Assay
2.8. Mitochondrial Membrane Potential Measurement
2.9. Measurement of Reactive Oxygen Species (ROS) Level
2.10. Measurement of H2O2 Level
2.11. Measurement of Malondialdehyde (MDA) Level
2.12. Statistical Analysis
3. Results
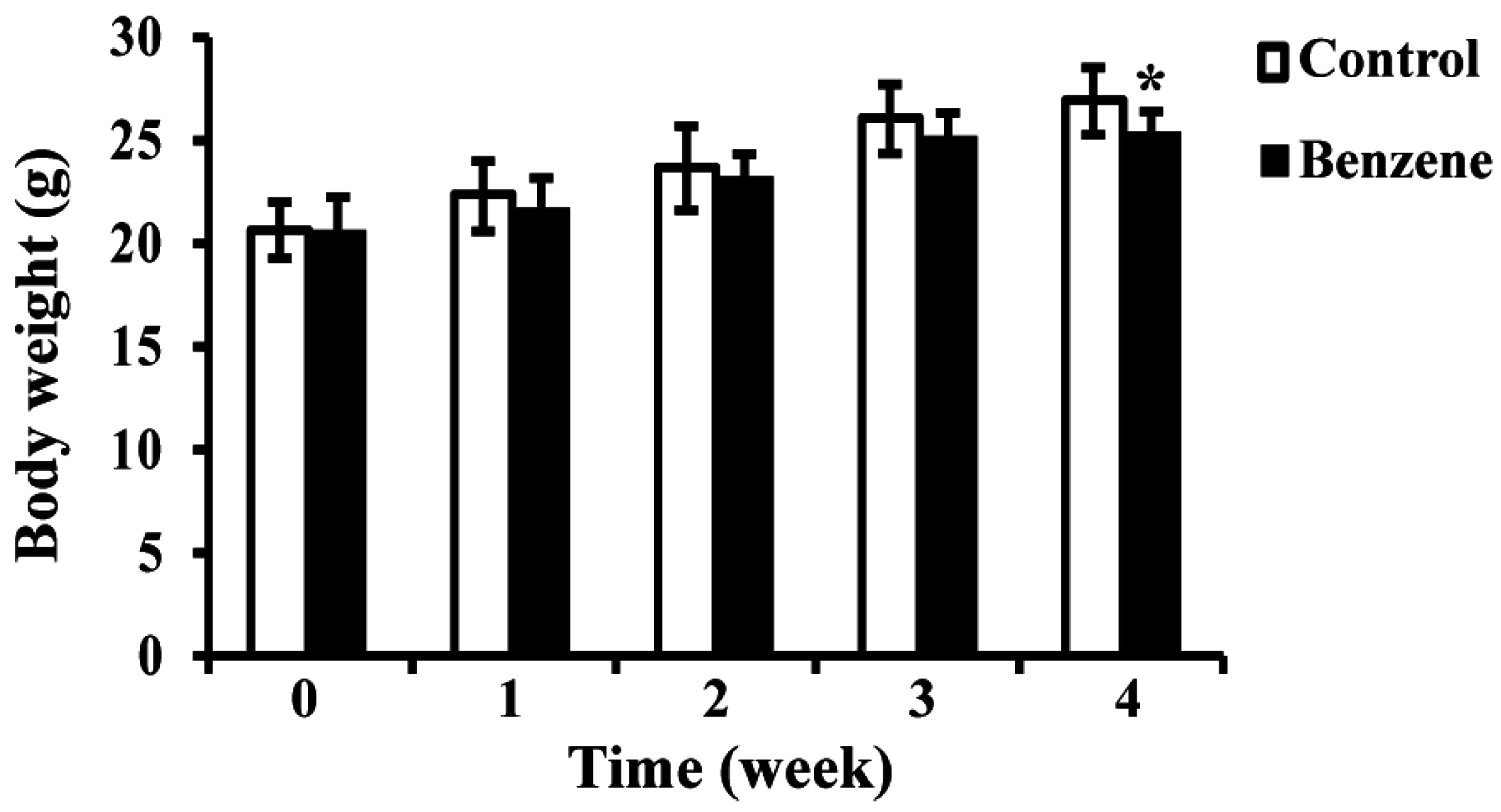
3.1. Effects of Benzene on Body Weight
3.2. Benzene Causes Declines in Mouse WBC, RBC, Pit Count, and Hgb Concentration
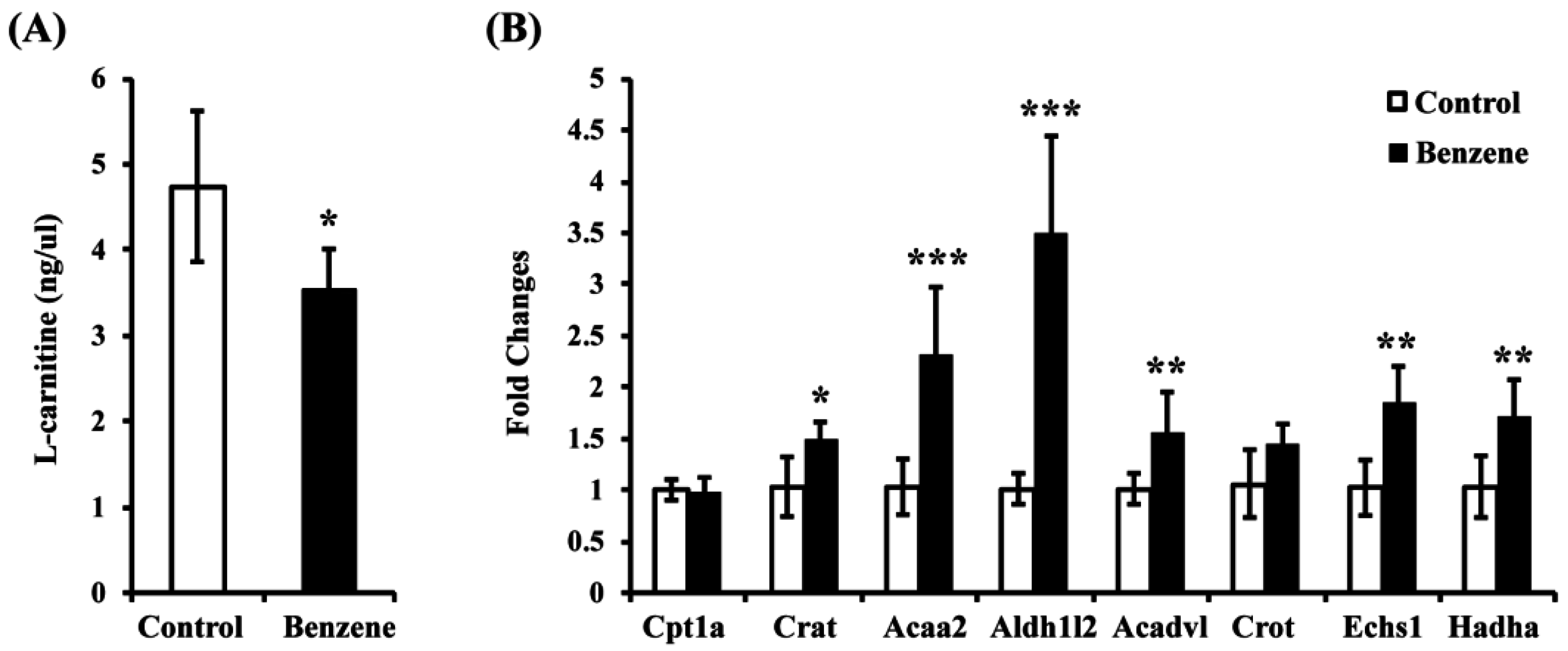
3.3. Benzene Induces Reduced L-Carnitine and Alters Expression of Key Genes in the FAO Pathway in Mouse BM Cells
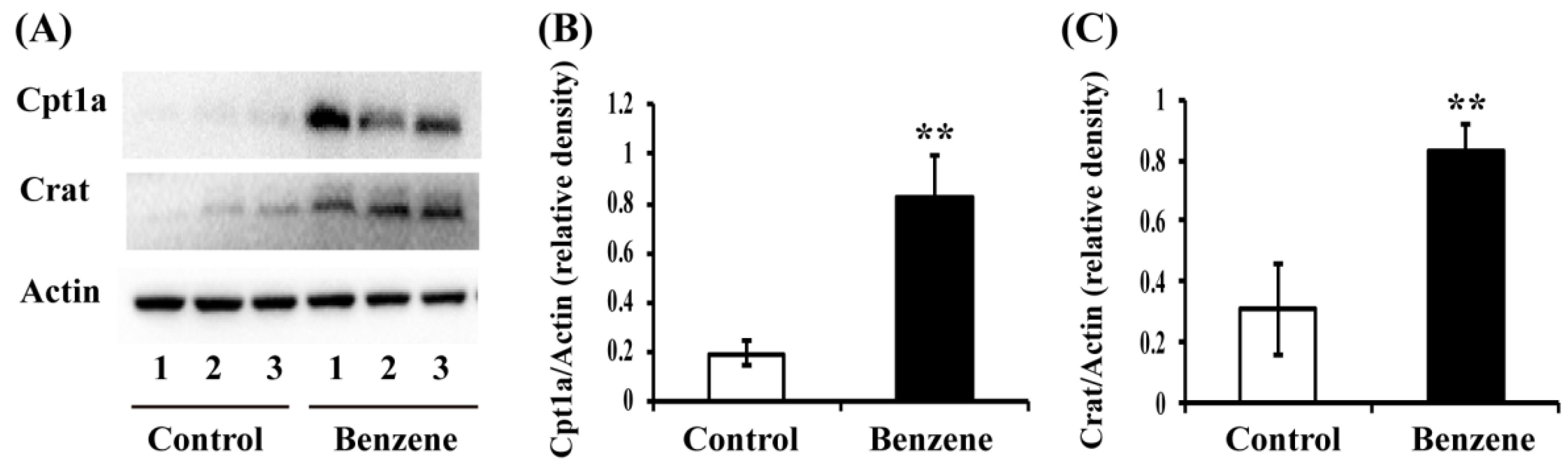
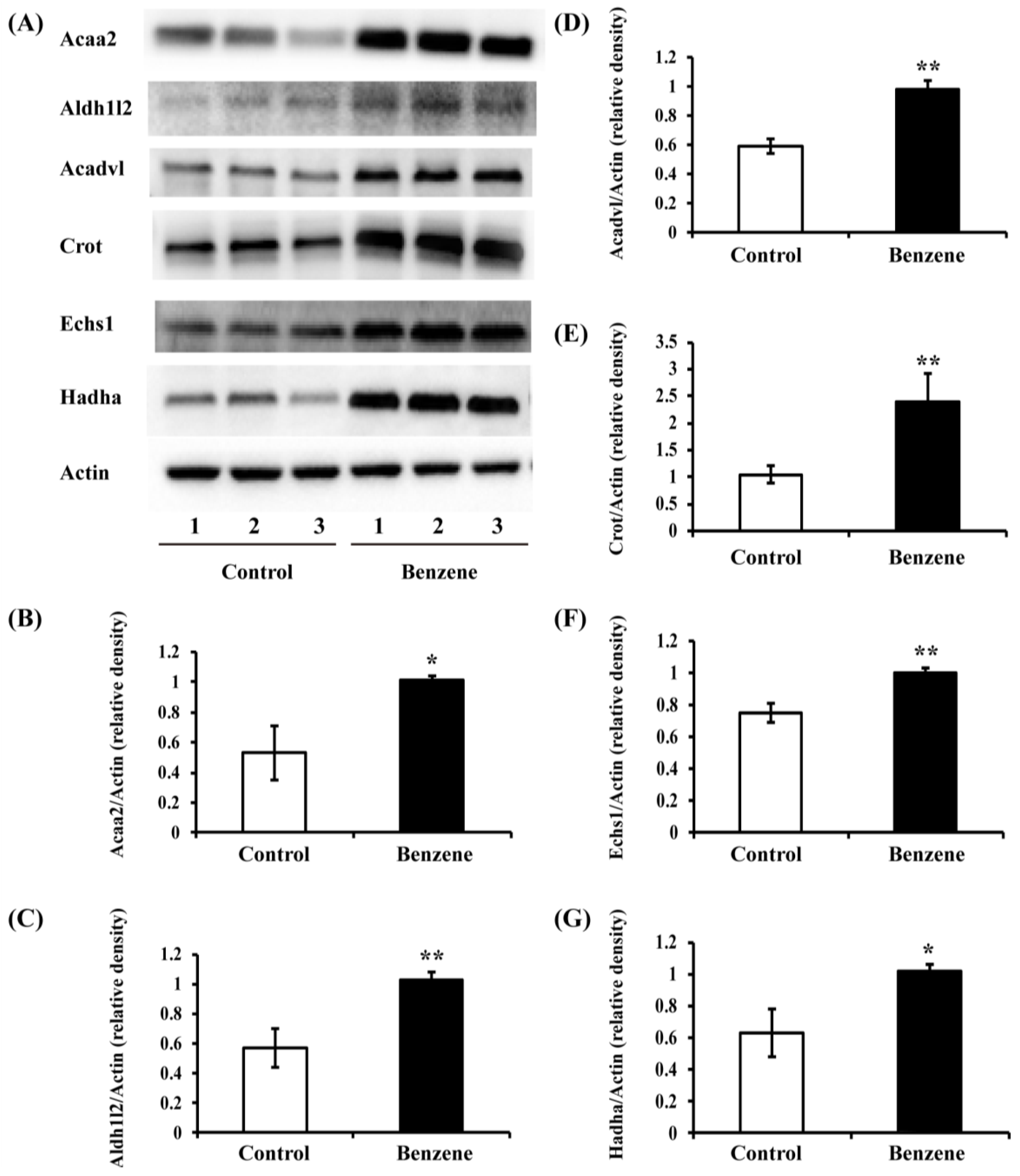
3.4. Benzene Induces Increased Expression of Key Fatty Acid (FA) Transport Proteins in Mouse BM Cells
3.5. Benzene Induces Increased Expression of Key FAO Proteins in Mouse BM Cells
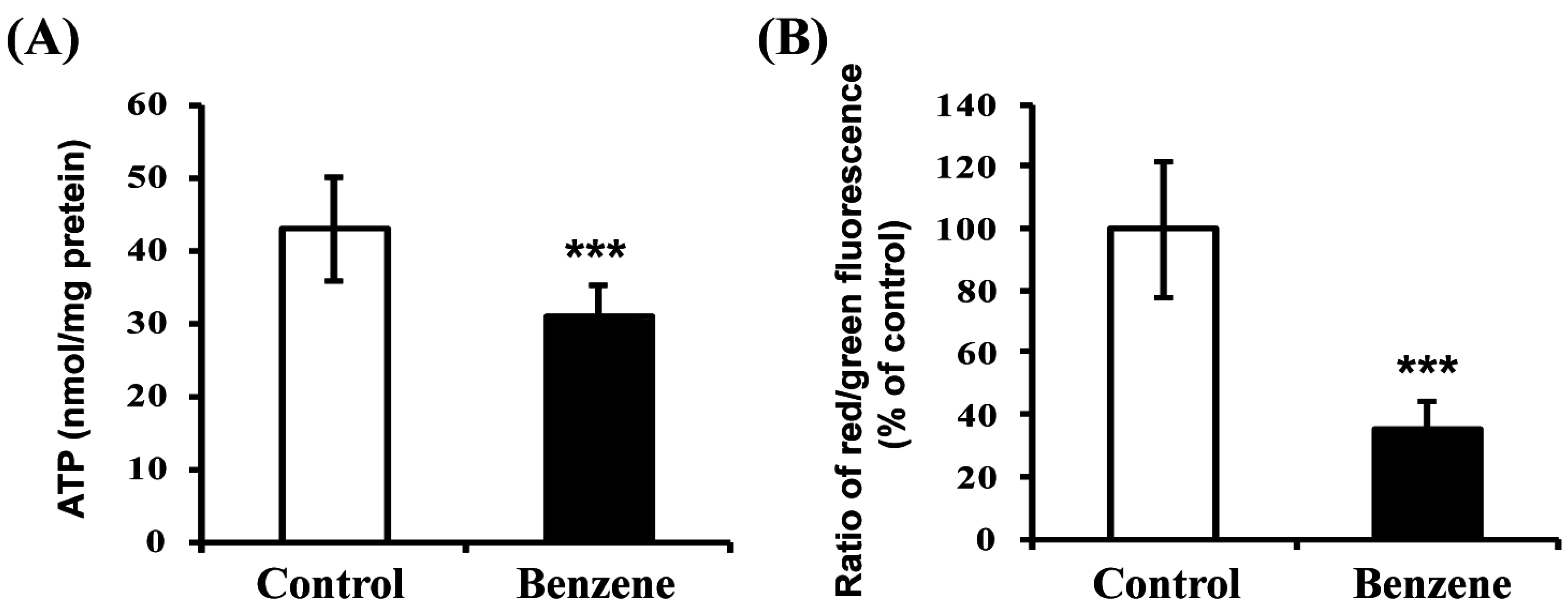
3.6. Benzene Induces Mitochondrial Dysfunction
3.7. Benzene Induces Oxidative Stress and Lipid Peroxidation
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Infante, P.F. The IARC October 2009 evaluation of benzene carcinogenicity was incomplete and needs to be reconsidered. Am. J. Ind. Med. 2011, 54, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Lan, Q.; Zhang, L.; Li, G.; Vermeulen, R.; Weinberg, R.S.; Dosemeci, M.; Rappaport, S.M.; Shen, M.; Alter, B.P.; Wu, Y.; et al. Hematotoxicity in workers exposed to low levels of benzene. Science 2004, 306, 1774–1776. [Google Scholar] [CrossRef] [PubMed]
- Carbonari, D.; Chiarella, P.; Mansi, A.; Pigini, D.; Iavicoli, S.; Tranfo, G. Biomarkers of susceptibility following benzene exposure: Influence of genetic polymorphisms on benzene metabolism and health effects. Biomark. Med. 2016, 10, 145–163. [Google Scholar] [CrossRef] [PubMed]
- Swaen, G.M.; van Amelsvoort, L.; Twisk, J.J.; Verstraeten, E.; Slootweg, R.; Collins, J.J.; Burns, C.J. Low level occupational benzene exposure and hematological parameters. Chem. Biol. Interact. 2010, 184, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Snyder, R. Leukemia and benzene. Int. J. Environ. Res. Public Health 2012, 9, 2875–2893. [Google Scholar] [CrossRef] [PubMed]
- Schnatter, A.R.; Glass, D.C.; Tang, G.; Irons, R.D.; Rushton, L. Myelodysplastic syndrome and benzene exposure among petroleum workers: An international pooled analysis. J. Natl. Cancer Inst. 2012, 104, 1724–1737. [Google Scholar] [CrossRef] [PubMed]
- Ross, D. The role of metabolism and specific metabolites in benzene-induced toxicity: Evidence and issues. J. Toxicol. Environ. Health A 2000, 61, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Yang, J.; Yang, G.; Niu, P.; Tian, L. Differential gene expression profiling analysis in workers occupationally exposed to benzene. Sci. Total Environ. 2014, 472, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Rothman, N.; Li, G.; Guo, W.; Yang, W.; Hubbard, A.E.; Hayes, R.B.; Yin, S.; Lu, W.; Smith, M.T. Aberrations in chromosomes associated with lymphoma and therapy-related leukemia in benzene-exposed workers. Environ. Mol. Mutagen. 2007, 48, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Wang, Q.F.; Li, B.; Tian, H.; Ni, Y.; Yin, S.; Li, G. Methylation and expression analysis of tumor suppressor genes p15 and p16 in benzene poisoning. Chem. Biol. Interact. 2010, 184, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Gowans, I.D.; Lorimore, S.A.; McIlrath, J.M.; Wright, E.G. Genotype-dependent induction of transmissible chromosomal instability by gamma-radiation and the benzene metabolite hydroquinone. Cancer Res. 2005, 65, 3527–3530. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Dehn, D.; Kepa, J.K.; Siegel, D.; Scott, D.E.; Tan, W.; Ross, D. NAD(P)H:quinone oxidoreductase 1-compromised human bone marrow endothelial cells exhibit decreased adhesion molecule expression and CD34+ hematopoietic cell adhesion. J. Pharmacol. Exp. Ther. 2010, 334, 260–268. [Google Scholar] [CrossRef] [PubMed]
- McHale, C.M.; Zhang, L.; Smith, M.T. Current understanding of the mechanism of benzene-induced leukemia in humans: Implications for risk assessment. Carcinogenesis 2012, 33, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Suda, T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat. Rev. Mol. Cell Biol. 2014, 15, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Carracedo, A.; Weiss, D.; Arai, F.; Ala, U.; Avigan, D.E.; Schafer, Z.T.; Evans, R.M.; Suda, T.; Lee, C.H.; et al. A PML-PPAR-delta pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat. Med. 2012, 18, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, M.R.; Mirabilii, S. Targeting the leukemia cell metabolism by the CPT1a inhibition: Functional preclinical effects in leukemias. Boold 2015, 126, 1925–1929. [Google Scholar] [CrossRef] [PubMed]
- Samudio, I.; Harmancey, R.; Fiegl, M.; Kantarjian, H.; Konopleva, M.; Korchin, B.; Kaluarachchi, K.; Bornmann, W.; Duvvuri, S.; Taegtmeyer, H.; et al. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. J. Clin. Investig. 2010, 120, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Zhang, J.; Yin, L.; Pu, Y. Investigation into variation of endogenous metabolites in bone marrow cells and plasma in C3H/He mice exposed to benzene. Int. J. Mol. Sci. 2014, 15, 4994–5010. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Hirabayashi, Y. Hematopoietic neoplastic diseases develop in C3H/He and C57BL/6 mice after benzene exposure: Strain differences in bone marrow tissue responses observed using microarrays. Chem. Biol. Interact. 2010, 184, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Hirabayashi, Y.; Kaneko, T.; Kanno, J.; Kodama, Y.; Matsushima, Y.; Ogawa, Y.; Saitoh, M.; Sekita, K.; Uchida, O.; et al. Benzene-induced hematopoietic neoplasms including myeloid leukemia in Trp53-deficient C57BL/6 and C3H/He mice. Toxicol. Sci. 2009, 110, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Cogliano, V.J.; Baan, R.; Straif, K. Updating IARC’s carcinogenicity assessment of benzene. Am. J. Ind. Med. 2011, 54, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Zhang, J.; Xiong, M.; Chen, Y.; Yin, L.; Pu, Y. Metabonomics biomarkers for subacute toxicity screening for benzene exposure in mice. J. Toxicol. Environ. Health Part A 2012, 75, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Zhang, J.; Xiong, M.; Wei, H.; Tan, K.; Yin, L.; Pu, Y. Altered expression of genes in signaling pathways regulating proliferation of hematopoietic stem and progenitor cells in mice with subchronic benzene exposure. Int. J. Environ. Res. Public Health 2015, 12, 9298–9313. [Google Scholar] [CrossRef] [PubMed]
- Houten, S.M.; Wanders, R.J. A general introduction to the biochemistry of mitochondrial fatty acid beta-oxidation. J. Inherit. Metab. Dis. 2010, 33, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Mihalik, S.J.; Goodpaster, B.H.; Kelley, D.E.; Chace, D.H.; Vockley, J.; Toledo, F.G.; DeLany, J.P. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity 2010, 18, 1695–1700. [Google Scholar] [CrossRef] [PubMed]
- Staubert, C.; Bhuiyan, H.; Lindahl, A.; Broom, O.J.; Zhu, Y.; Islam, S.; Linnarsson, S.; Lehtio, J.; Nordstrom, A. Rewired metabolism in drug-resistant leukemia cells: A metabolic switch hallmarked by reduced dependence on exogenous glutamine. J. Biol. Chem. 2015, 290, 8348–8359. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, A.; Cantley, L.C.; Pandolfi, P.P. Cancer metabolism: Fatty acid oxidation in the limelight. Nat. Rev. Cancer 2013, 13, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Lehne, G.; Grasmo-Wendler, U.H.; Berner, J.M.; Meza-Zepeda, L.A.; Adamsen, B.L.; Flack, A.; Reiner, A.; Clausen, O.P.; Hovig, E.; Myklebost, O. Upregulation of stem cell genes in multidrug resistant K562 leukemia cells. Leuk. Res. 2009, 33, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Katunga, L.A.; Willis, M.S. Mitochondria as a source and target of lipid peroxidation products in healthy and diseased heart. Clin. Exp. Pharmacol. Physiol. 2012, 39, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Schafer, Z.T.; Grassian, A.R.; Song, L.; Jiang, Z.; Gerhart-Hines, Z.; Irie, H.Y.; Gao, S.; Puigserver, P.; Brugge, J.S. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature 2009, 461, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M.; Chandel, N.S.; Hay, N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 2012, 485, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, K.; Arai, F.; Yoshihara, H.; Nakamura, Y.; Gomei, Y.; Iwasaki, H.; Miyamoto, K.; Shima, H.; Ito, K.; Suda, T. Function of oxidative stress in the regulation of hematopoietic stem cell-niche interaction. Biochem. Biophys. Res. Commun. 2007, 363, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, P.; Sabitha, K.E.; Shyamaladevi, C.S. Attenuation of 4-nitroquinoline 1-oxide induced in vitro lipid peroxidation by green tea polyphenols. Life Sci. 2007, 80, 1080–1086. [Google Scholar] [CrossRef] [PubMed]

| Primer Name | Primer Sequence (5′-3′) |
|---|---|
| Actin | Forward: CGAGCGGTTCCGATGCCCTG; Reverse: ACGCAGCTCAGTAACAGTCCGC |
| Cpt1a | Forward: CGATCATCATGACTATGCGCTACT; Reverse: GCCGTGCTCTGCAAACATC |
| Crat | Forward: GCTTGTGCGGTCTCCTATGGT; Reverse: TTGGCTTTCTCTATGTCGTTCTTG |
| Hadha | Forward: AAGGGGATGTGGCAGTTATT; Reverse: ACTCCTGATTTGGTCGTTGG |
| Acaa2 | Forward: CAATGAGGAGATGGCACCC; Reverse: CTTTCTTGAACACGGACGG |
| Acadvl | Forward: GTGGCTCTGCAAGGCTGTA; Reverse: CGATTCCTGTCCTCCGTCTC |
| Echs1 | Forward: AAGCAGGCAGGTCTTGTAAGC; Reverse: CGGTGGCAAAGGTGGAATA |
| Aldh1l2 | Forward: ACCAGCCGGGTTTATTTCAAA; Reverse: ACTCCCACTACTCGGTGGC |
| Crot | Forward: GGTGGCTCAATGTTGCCTAC; Reverse: TCCTCTTTCTAACTGAGTGCCT |
| Group | WBC (109/L) | RBC (1012/L) | Hgb (g/L) | Pit (109/L) |
|---|---|---|---|---|
| Control | 7.36 ± 1.45 | 8.04 ± 0.18 | 127.75 ± 11.32 | 593.38 ± 87.43 |
| Benzene | 3.08 ± 0.80 *** | 6.23 ± 0.33 * | 109.50 ± 5.50 * | 368.38 ± 82.63 * |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, R.; Cao, M.; Zhang, J.; Yang, W.; Wei, H.; Meng, X.; Yin, L.; Pu, Y. Benzene Exposure Alters Expression of Enzymes Involved in Fatty Acid β-Oxidation in Male C3H/He Mice. Int. J. Environ. Res. Public Health 2016, 13, 1068. https://doi.org/10.3390/ijerph13111068
Sun R, Cao M, Zhang J, Yang W, Wei H, Meng X, Yin L, Pu Y. Benzene Exposure Alters Expression of Enzymes Involved in Fatty Acid β-Oxidation in Male C3H/He Mice. International Journal of Environmental Research and Public Health. 2016; 13(11):1068. https://doi.org/10.3390/ijerph13111068
Chicago/Turabian StyleSun, Rongli, Meng Cao, Juan Zhang, Wenwen Yang, Haiyan Wei, Xing Meng, Lihong Yin, and Yuepu Pu. 2016. "Benzene Exposure Alters Expression of Enzymes Involved in Fatty Acid β-Oxidation in Male C3H/He Mice" International Journal of Environmental Research and Public Health 13, no. 11: 1068. https://doi.org/10.3390/ijerph13111068






