Survival and Risk Comparison of Campylobacter jejuni on Various Processed Meat Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Preparation of Sample and Inoculation
2.3. Enumeration of C. jejuni
2.4. Primary Survival Modeling
2.5. Secondary Survival Modeling
2.6. Water Activity (Aw), Salinity, and pH Analysis
2.7. Risk Comparison for Various Processed Meat Products Using FDA-iRISK
2.8. Statistical Analysis
3. Results and Discussion
3.1. Measurement of Water Activity (Aw), Salinity, and pH on Processed Meat Products
3.2. Primary Survival Model of C. jejuni on Processed Meat Products
3.3. Effect of Temperature and Seasoning on the Survival Ability of C. jejuni in Processed Meat Products
3.4. Risk Comparison of Various Processed Meat Products Using FDA-iRisk
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- EFSA. Trends and Sources of Zoonoses, Zoonotic Agents and Food-Borne Outbreaks in 2012. Available online: http://www.efsa.europa.eu/en/efsajournal/pub/3547.htm (accessed on 16 July 2014).
- CDC. Campylobacter. Available online: http://www.cdc.gov/nczved/divisions/dfbmd/diseases/campylobacter/ (accessed on 23 October 2014).
- Kirkpatrick, B.D.; Tribble, D.R. Update on human Campylobacter jejuni infections. Curr. Opin. Gastroenterol. 2011, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dasti, J.I. Campylobacter jejuni: A brief overview on pathogenicity-associated factors and disease-mediating mechanisms. Int. J. Med. Microbiol. 2010, 300, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Chamovitz, B.N.; Hartstein, A.I.; Alexander, S.R.; Terry, A.B.; Short, P.; Katon, R. Campylobacter jejuni-associated hemolytic-uremic syndrome in a mother and daughter. Pediatrics 1983, 71, 253–256. [Google Scholar] [PubMed]
- Nachamkin, I.; Allos, B.M.; Ho, T. Campylobacter species and Guillain-Barre syndrome. Clin. Microbiol. Rev. 1998, 11, 555–567. [Google Scholar] [PubMed]
- Altekruse, S.F.; Stern, N.J.; Fields, P.I.; Swerdlow, D.L. Campylobacter jejuni—An emerging foodborne pathogen. Emerg. Infect. Dis. 1999, 5, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Mead, G. Campylobacter update-the challenge. Int. Poult. Prod. 2004, 12, 26–29. [Google Scholar]
- Birk, T.; Ingmer, H.; Andersen, M.T.; Jørgensen, K.; Brøndsted, L. Chicken juice, a food-based model system suitable to study survival of Campylobacter jejuni. Lett. Appl. Microbiol. 2004, 38, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Schiellerup, P.; Krogfelt, K.A.; Locht, H. A comparison of self-reported joint symptoms following infection with different enteric pathogens: Effect of HLA-B27. J. Rheumatol. 2008, 35, 480–487. [Google Scholar] [PubMed]
- EFSA. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2013. EFSA J. 2015, 13, 3991–4153. [Google Scholar]
- Thomas, M.K.; Murray, R.; Flockhart, L.; Pintar, K.; Pollari, F.; Fazil, A.; Nesbitt, A.; Marshall, B. Estimates of the burden of foodborne illness in Canada for 30 specified pathogens and unspecified agents, circa 2006. Foodborne Pathog. Dis. 2013, 10, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Crim, S.M.; Griffin, P.M.; Tauxe, R.; Marder, E.P.; Gilliss, D.; Cronquist, A.B.; Cartter, M.; Tobin-D’Angelo, M.; Blythe, D.; Smith, K.; et al. Preliminary incidence and trends of infection with pathogens transmitted commonly through food—Foodborne Diseases Active Surveillance Network, 10 U.S. sites, 2006–2014. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 495–499. [Google Scholar] [PubMed]
- MFDS. Available online: http://www.mfds.go.kr/e-stat/index.do?nMenuCode=16 (accessed on 3 March 2016).
- KMIA. Available online: http://www.kmia.or.kr/infocenter/infocenter2.html (accessed on 11 September 2014).
- Vandendriessche, F. Meat products in the past, today and in the future. Meat Sci. 2008, 78, 104–113. [Google Scholar] [CrossRef] [PubMed]
- aTFIS. Available online: http://www.atfis.or.kr/article/M001020000/view.do?articleId=615&page=6&searchKey=&searchString=&searchCategory= (accessed on 3 October 2015).
- Wen, X.; Dickson, J.S. Survival of Campylobacter jejuni and Salmonella enterica Typhimurium in vacuum-packed, moisture-enhanced pork. J. Food Prot. 2012, 75, 576–579. [Google Scholar] [CrossRef] [PubMed]
- ESR. Risk Profile: Campylobacter jejuni/coli in Red Meat. Available online: http://www.foodsafety.govt.nz/elibrary/industry/Risk_Profile_Campylobacter_Jejuni_Coli-Science_Research.pdf (accessed on 25 June 2014).
- Nachamkin, I.; Blaser, M.J. Campylobacter; ASM Press: Washington, MD, USA, 2000. [Google Scholar]
- Lee, A.; Smith, S.C.; Coloe, P.J. Survival and growth of Campylobacter jejuni after artificial inoculation onto chicken skin as a function of temperature and packaging conditions. J. Food Prot. 1998, 61, 1609–1614. [Google Scholar] [PubMed]
- Burnette, C.N.; Yoon, K. Comparison of growth and survival kinetics of Salmonella typhimurium and Campylobacter jejuni on cooked chicken breast stored under aerobic conditions at various temperatures. Food Sci. Biotechnol. 2004, 13, 796–800. [Google Scholar]
- Yoon, K.; Burnette, C.; Oscar, T. Development of predictive models for the survival of Campylobacter jejuni (ATCC 43051) on cooked chicken breast patties and in broth as a function of temperature. J. Food Prot. 2004, 67, 64–70. [Google Scholar] [PubMed]
- González, M.; Skandamis, P.N.; Hänninen, M.-L. A modified Weibull model for describing the survival of Campylobacter jejuni in minced chicken meat. Int. J. Food Microbiol. 2009, 136, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Oyarzabal, O.A.; Oscar, T.P.; Speegle, L.; Nyati, H. Survival of Campylobacter jejuni and Campylobacter coli on retail broiler meat stored at −20, 4, or 12 °C and development of Weibull models for survival. J. Food Prot. 2010, 73, 1438–1446. [Google Scholar] [PubMed]
- González, M.; Hänninen, M.L. Effect of temperature and antimicrobial resistance on survival of Campylobacter jejuni in well water: Application of the Weibull model. J. Appl. Microbiol. 2012, 113, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Park, N.Y.; Ro, E.Y.; Jo, H.J.; Park, K.S.; Yoon, K.S. Effect of packaging and temperature on survival kinetics of Campylobacter jejuni on precooked chicken breast. J. Food Saf. 2014, 34, 371–379. [Google Scholar] [CrossRef]
- Joint Institute for Food Safety and Applied Nutrition (JIFSAN) and Risk Sciences International (RSI). Food and Drug Administration Center for Food Safety and Applied Nutrition (FDA/CFSAN), FDA-iRISK® Version 2.0.; FDA/CFSAN: College Park, MD, USA, 2015. [Google Scholar]
- Chen, Y.; Dennis, S.B.; Hartnett, E.; Paoli, G.; Pouillot, R.; Ruthman, T.; Wilson, M. FDA-iRISK—A comparative risk assessment system for evaluating and ranking food-hazard pairs: Case studies on microbial hazards. J. Food Prot. 2013, 76, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Geeraerd, A.H.; Valdramidis, V.P.; Van Impe, J.F. GInaFiT, a freeware tool to assess non-log-linear microbial survivor curves. Int. J. Food Microbiol. 2005, 102, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Albert, I.; Mafart, P. A modified Weibull model for bacterial inactivation. Int. J. Food Microbiol. 2005, 100, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Davey, K. Applicability of the Davey (linear Arrhenius) predictive model to the lag phase of microbial growth. J. Appl. Bacteriol. 1991, 70, 253–257. [Google Scholar] [CrossRef]
- Mataragas, M.; Skandamis, P.; Drosinos, E. Risk profiles of pork and poultry meat and risk ratings of various pathogen/product combinations. Int. J. Food Microbiol. 2008, 126, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health and Welfare. Korea Health Statistics 2011: Korea National Health and Nutrition EXAMINATION survey (KNHANES. V-2); Ministry of Health and Welfare: Sejong, Korea.
- Teunis, P.; Van den Brandhof, W.; Nauta, M.; Wagenaar, J.; Van den Kerkhof, H.; Van Pelt, W. A reconsideration of the Campylobacter dose–response relation. Epidemiol. Infect. 2005, 133, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Kemmeren, J.M.; Mangen, M.; Van Duynhoven, Y.; Havelaar, A. Priority Setting of Foodborne Pathogens: Disease Burden and Costs of Selected Enteric Pathogens. Available online: http://rivm.openrepository.com/rivm/bitstream/10029/7316/1/330080001.pdf (accessed on 18 August 2015).
- Bover-Cid, S.; Belletti, N.; Garriga, M.; Aymerich, T. Model for Listeria monocytogenes inactivation on dry-cured ham by high hydrostatic pressure processing. Food Microbiol. 2011, 28, 804–809. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Microbiological Specifications for Foods (ICMSF). Microorganisms in Foods 5: Characteristics of Microbial Pathogens; Springer: Berlin, Germany; Heidelberg, Germany, 1996; Volume 5. [Google Scholar]
- Murphy, C.; Carroll, C.; Jordan, K.N. Environmental survival mechanisms of the foodborne pathogen Campylobacter jejuni. J. Appl. Microbiol. 2006, 100, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Stintzi, A.; Whitworth, L. Investigation of the Campylobacter jejuni cold-shock response by global transcript profiling. Genome Lett. 2003, 2, 18–27. [Google Scholar]
- Hazeleger, W.C.; Wouters, J.A.; Rombouts, F.M.; Abee, T. Physiological activity of Campylobacter jejuni far below the minimal growth temperature. Appl. Environ. Microbiol. 1998, 64, 3917–3922. [Google Scholar] [PubMed]
- Blankenship, L.; Craven, S. Campylobacter jejuni survival in chicken meat as a function of temperature. Appl. Environ. Microbiol. 1982, 44, 88–92. [Google Scholar] [PubMed]
- Jackson, A.L.; Kulchaiyawat, C.; Sullivan, G.A.; Sebranek, J.G.; Dickson, J.S. Use of natural ingredients to control growth of Clostridium perfringens in naturally cured frankfurters and hams. J. Food Prot. 2011, 74, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Uradziński, J.; Szteyn, J. Effect of preservatives on survival of Campylobacter jejuni in ground pork meat. Rocz. Panstwowego Zakl. Hig. 1992, 44, 395–402. [Google Scholar]
- Sellars, M.J.; Hall, S.J.; Kelly, D.J. Growth of Campylobacter jejuni supported by respiration of fumarate, nitrate, nitrite, trimethylamine-N-oxide, or dimethyl sulfoxide requires oxygen. J. Bacteriol. 2002, 184, 4187–4196. [Google Scholar] [CrossRef] [PubMed]
- Pittman, M.S.; Elvers, K.T.; Lee, L.; Jones, M.A.; Poole, R.K.; Park, S.F.; Kelly, D.J. Growth of Campylobacter jejuni on nitrate and nitrite: Electron transport to NapA and NrfA via NrfH and distinct roles for NrfA and the globin Cgb in protection against nitrosative stress. Mol. Microbiol. 2007, 63, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Berwal, J. Sensitivity of food pathogens to garlic (Allium sativum). J. Appl. Microbiol. 1998, 84, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Cottrell, S.; Plummer, S.; Lloyd, D. Antimicrobial properties of Allium sativum (garlic). Appl. Microbiol. Biotechnol. 2001, 57, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Ross, Z.; O’Gara, E.A.; Hill, D.J.; Sleightholme, H.; Maslin, D.J. Antimicrobial properties of garlic oil against human enteric bacteria: Evaluation of methodologies and comparisons with garlic oil sulfides and garlic powder. Appl. Environ. Microbiol. 2001, 67, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Leuschner, R.G.; Zamparini, J. Effects of spices on growth and survival of Escherichia coli 0157 and Salmonella enterica serovar Enteritidis in broth model systems and mayonnaise. Food Control 2002, 13, 399–404. [Google Scholar] [CrossRef]
- El Astal, Z. The inhibitory action of aqueous garlic extract on the growth of certain pathogenic bacteria. Eur. Food Res. Technol. 2004, 218, 460–464. [Google Scholar] [CrossRef]
- Benkeblia, N. Antimicrobial activity of essential oil extracts of various onions Allium cepa and garlic Allium sativum. LWT Food Sci. Technol. 2004, 37, 263–268. [Google Scholar] [CrossRef]
- Cutler, R.; Wilson, P. Antibacterial activity of a new, stable, aqueous extract of allicin against methicillin-resistant Staphylococcus aureus. Br. J. Biomed. Sci. 2004, 61, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Iwalokun, B.; Ogunledun, A.; Ogbolu, D.; Bamiro, S.; Jimi-Omojola, J. In vitro antimicrobial properties of aqueous garlic extract against multidrug-resistant bacteria and Candida species from Nigeria. J. Med. Food 2004, 7, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Sallam, K.I.; Ishioroshi, M.; Samejima, K. Antioxidant and antimicrobial effects of garlic in chicken sausage. LWT Food Sci. Technol. 2004, 37, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.W.; Son, S.K.; Lee, G.R.; Kim, G.H.; Kim, Y.H. Antibacterial effects of garlic extract against pathogenic bacteria. Korean J. Vet. Serv. 2011, 34, 167–178. [Google Scholar] [CrossRef]
- Lu, X.; Rasco, B.A.; Jabal, J.M.; Aston, D.E.; Lin, M.; Konkel, M.E. Investigating antibacterial effects of garlic (Allium sativum) concentrate and garlic-derived organosulfur compounds on Campylobacter jejuni by using Fourier transform infrared spectroscopy, Raman spectroscopy, and electron microscopy. Appl. Environ. Microbiol. 2011, 77, 5257–5269. [Google Scholar] [CrossRef] [PubMed]

| Element of Risk Scenario | C. jejuni in Processed Meat Products, Total Population | ||
|---|---|---|---|
| Input Parameter, iRISK Template | Model Input | References | |
| Food | Processed meat products | Description | |
| Hazard | C. jejuni | Description | |
| Process model | Initial prevalence | 0.001 | Mataragas et al. (2008) [31] |
| Initial concentration | A: Uniform (5.75, 5.88/6.00, 6.00) log CFU B: Uniform (5.85, 5.98/6.06, 6.06) log CFU C: Uniform (5.86, 5.88/6.14, 6.15) log CFU D: Uniform (5.99, 6.02/6.05, 6.13) log CFU E: Uniform (5.92, 6.02/6.15, 6.24) log CFU F: Uniform (5.62, 6.04/5.29, 5.72) log CFU | ||
| Initial unit mass | A: 100 g, B: 250 g, C: 210 g, D: 250 g, E: 160 g, F: 350 g | ||
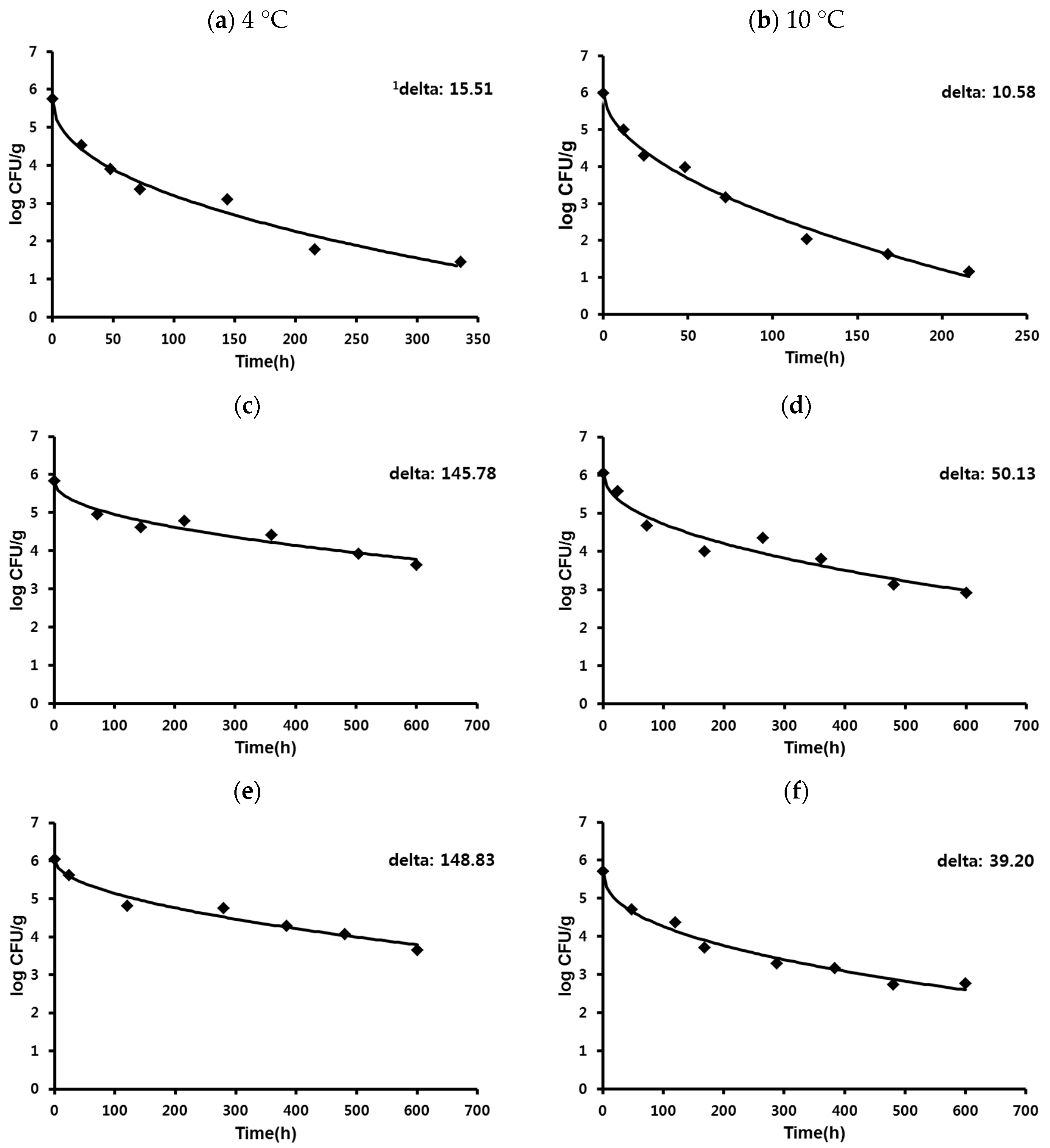
| Process stage 1: storage at 4 and 10 °C, decrease | (a): Uniform (4.30, 4.88/4.85, 5.00) log CFU (b): Uniform (2.17, 2.22/3.15, 3.31) log CFU (c): Uniform (1.95, 2.04/2.94, 3.16) log CFU (d): Uniform (1.68, 1.88/2.59, 2.62) log CFU (e): Uniform (1.67, 1.94/2.83, 2.97) log CFU (f): Uniform (2.32, 2.38/2.68, 2.94) log CFU | ||
| Consumption model | Grams per eating occasion | A: 12 g/B,C,D,E,F: 30 g | |
| Eating occasions per year | A: 3.3/B,C,D,E,F: 33.6 | Ministry of Health and Welfare and Centers for Disease Control and Prevention (2011) [34] | |
| Dose-response model | Beta-Poisson model | α = 0.024; β = 0.011 | Teunis et al. (2005) [35] |
| Health effects | DALY template (Campylobacteriosis) | 0.03 DALYs per case | Kemmeren et al. (2006) [36] |
| Sample | Dry-Cured Ham | Round Ham with Sodium Nitrite | Garlic Seasoning Ham with Sodium Nitrite | Round ham without Sodium Nitrite | Garlic Seasoning Ham without Sodium Nitrite | Sausage without Sodium Nitrite |
|---|---|---|---|---|---|---|
| Aw | 0.852 ± 0.0022 | 0.972 ± 0.0029 | 0.980 ± 0.0017 | 0.983 ± 0.0026 | 0.984 ± 0.0024 | 0.992 ± 0.0017 |
| Salinity (%) | 6.70 ± 0.38 | 1.35 ± 0.30 | 1.10 ± 0.20 | 1.70 ± 0.12 | 1.40 ± 0.23 | 1.28 ± 0.15 |
| pH | 5.96 ± 0.010 | 6.68 ± 0.013 | 6.53 ± 0.021 | 6.38 ± 0.014 | 6.48 ± 0.013 | 6.36 ± 0.013 |
| Sample | Delta 1 (Hour) | |||||
|---|---|---|---|---|---|---|
| 4 °C | 10 °C | 17 °C | 24 °C | 30 °C | 36 °C | |
| Dry-cured ham | B 15.51 a | E 10.58 a,b | C 3.84 b,c | B 1.20 c | B 0.89 c | D 0.31 c |
| Round ham with sodium nitrite | A 145.78 a | C 50.13 b | B,C 14.27 c | A 13.21 c | B 0.91 c | C,D 0.65 c |
| Garlic seasoning ham with sodium nitrite | A 179.20 a | B 65.14 b | A,B,C 20.26 c | A 14.54 c,d | A 5.13 d,e | A,B 1.99 e |
| Round ham without sodium nitrite | A 137.70 a | B 65.25 b | A,B 21.82 c | A 13.79 c | A 4.84 c | A 2.81 c |
| Garlic seasoning ham without sodium nitrite | A 181.12 a | A 81.03 b | A 34.49 c | A 14.08 d | A 5.81 d | A,B,C 1.71 d |
| Sausage without sodium nitrite | A 148.83 a | D 39.20 b | B,C 13.53 b,c | B 2.65 c | B 1.52 c | B,C,D 1.35 c |
| Sample | Total No. of Illnesses | Mean Risk of Illness | Per Eating Occasions or Consumer | Total DALYs per Year |
|---|---|---|---|---|
| Dry-cured ham | 0.000692 (0.000671) 1 | 2.10 × 10−4 (2.03 × 10−4) | 6.29 × 10−6 (6.10 × 10−6) | 0.0000208 (0.0000201) |
| Sausage without sodium nitrite | 0.0107 (0.00965) | 3.18 × 10−4 (2.87 × 10−4) | 9.53 × 10−6 (8.62 × 10−6) | 0.000320 (0.000290) |
| Round ham with sodium nitrite | 0.0110 (0.00983) | 3.27 × 10−4 (2.93 × 10−4) | 9.80 × 10−6 (8.78 × 10−6) | 0.000329 (0.000295) |
| Garlic seasoning ham with sodium nitrite | 0.0112 (0.0102) | 3.32 × 10−4 (3.03 × 10−4) | 9.97 × 10−6 (9.09 × 10−6) | 0.000335 (0.000305) |
| Round ham without sodium nitrite | 0.0116 (0.0107) | 3.45 × 10−4 (3.18 × 10−4) | 1.04 × 10−5 (9.53 × 10−6) | 0.000348 (0.000320) |
| Garlic seasoning ham without sodium nitrite | 0.0115 (0.0104) | 3.43 × 10−4 (3.11 × 10−4) | 1.03 × 10−5 (9.32 × 10−6) | 0.000346 (0.000313) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.H.; Kim, H.S.; Yoon, K.S. Survival and Risk Comparison of Campylobacter jejuni on Various Processed Meat Products. Int. J. Environ. Res. Public Health 2016, 13, 580. https://doi.org/10.3390/ijerph13060580
Hong SH, Kim HS, Yoon KS. Survival and Risk Comparison of Campylobacter jejuni on Various Processed Meat Products. International Journal of Environmental Research and Public Health. 2016; 13(6):580. https://doi.org/10.3390/ijerph13060580
Chicago/Turabian StyleHong, Soo Hyeon, Han Sol Kim, and Ki Sun Yoon. 2016. "Survival and Risk Comparison of Campylobacter jejuni on Various Processed Meat Products" International Journal of Environmental Research and Public Health 13, no. 6: 580. https://doi.org/10.3390/ijerph13060580






