Characterization of La/Fe/TiO2 and Its Photocatalytic Performance in Ammonia Nitrogen Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Modification Methods
2.3. Characterization
2.4. Photocatalytic Removal Experiments and Analytical Methods
3. Results and Discussion
3.1. Characterization
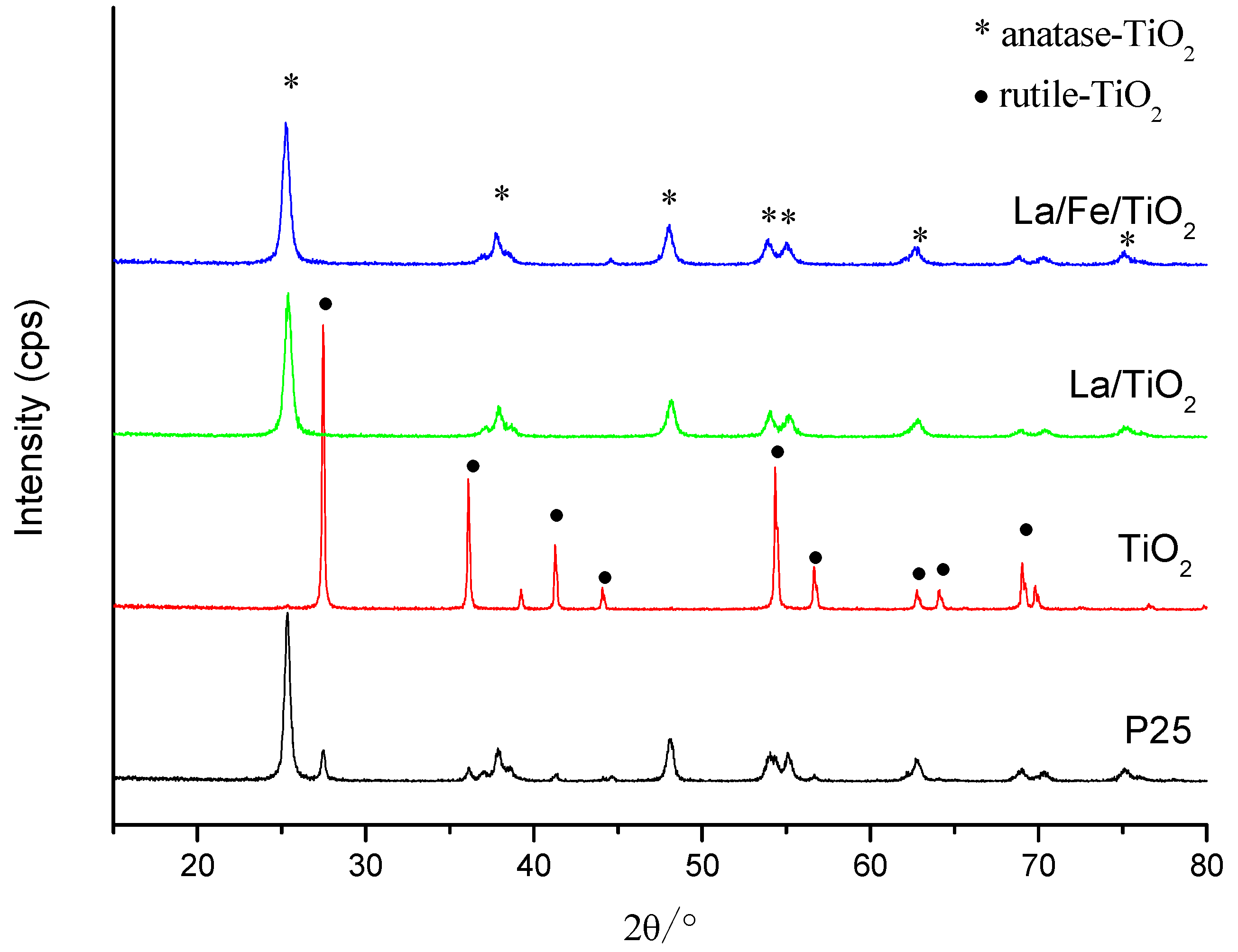
3.1.1. XRD
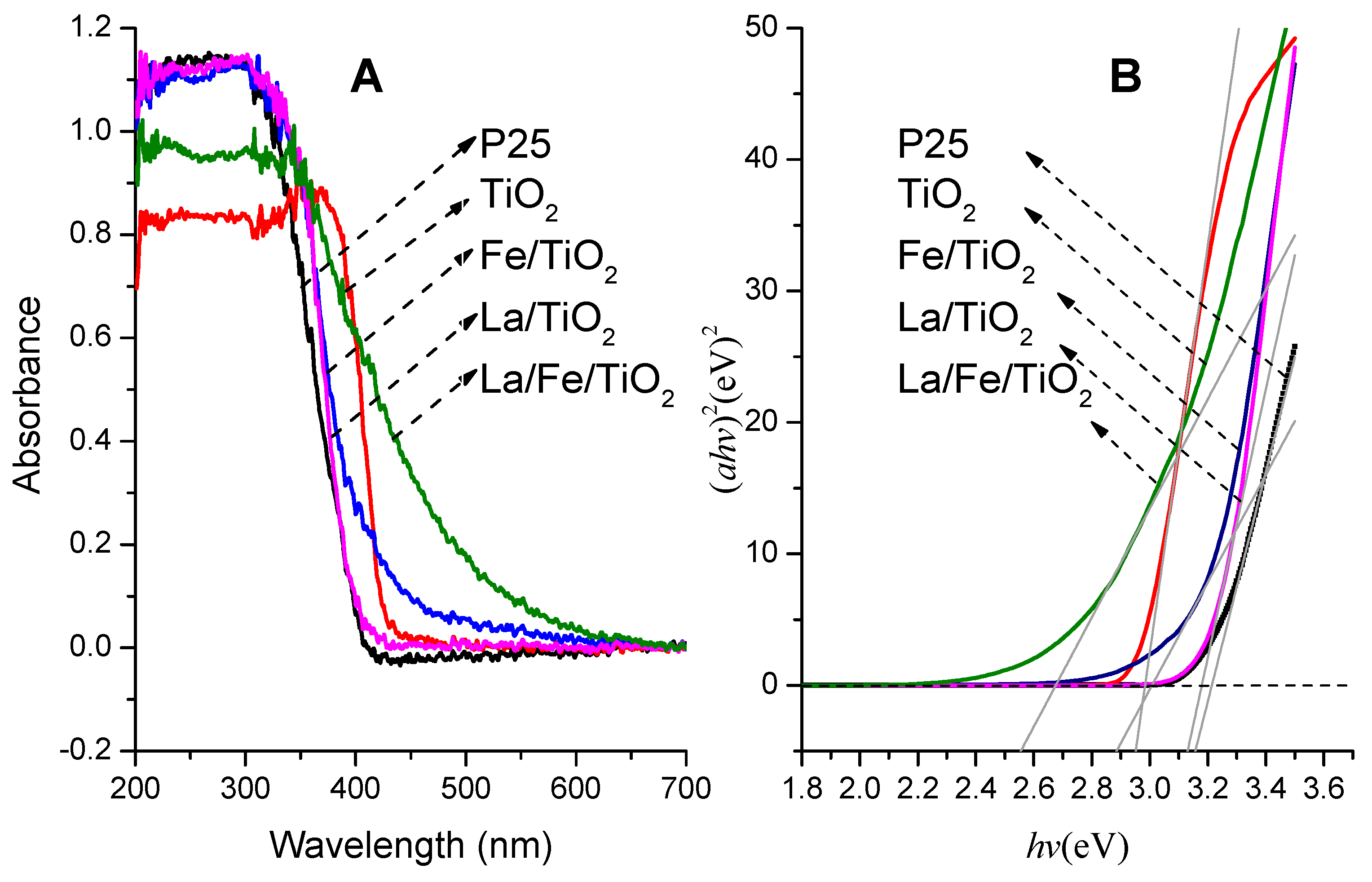
3.1.2. UV-Vis DRS
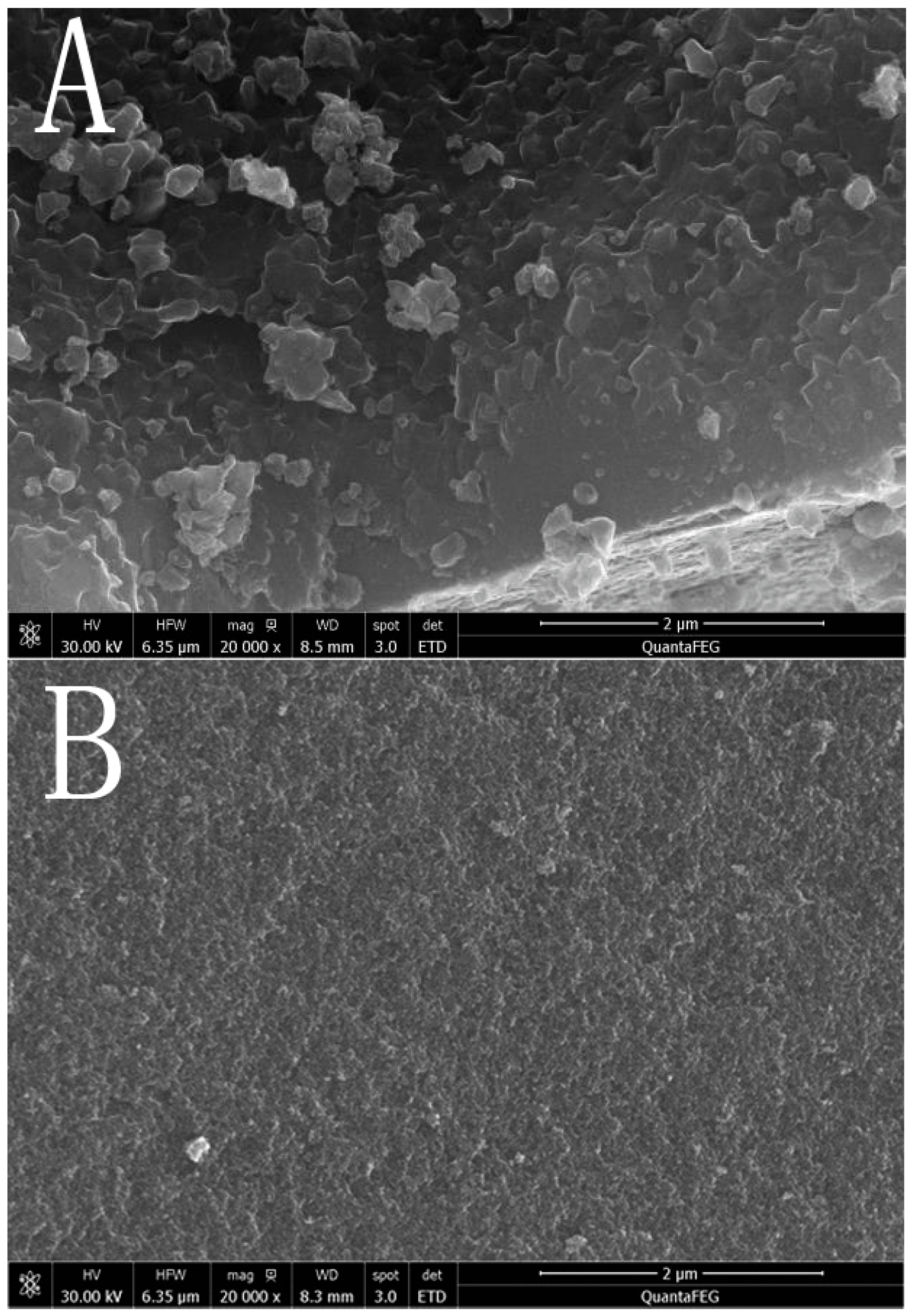
3.1.3. Surface Morphology Analysis
3.1.4. Specific Surface Area Analysis
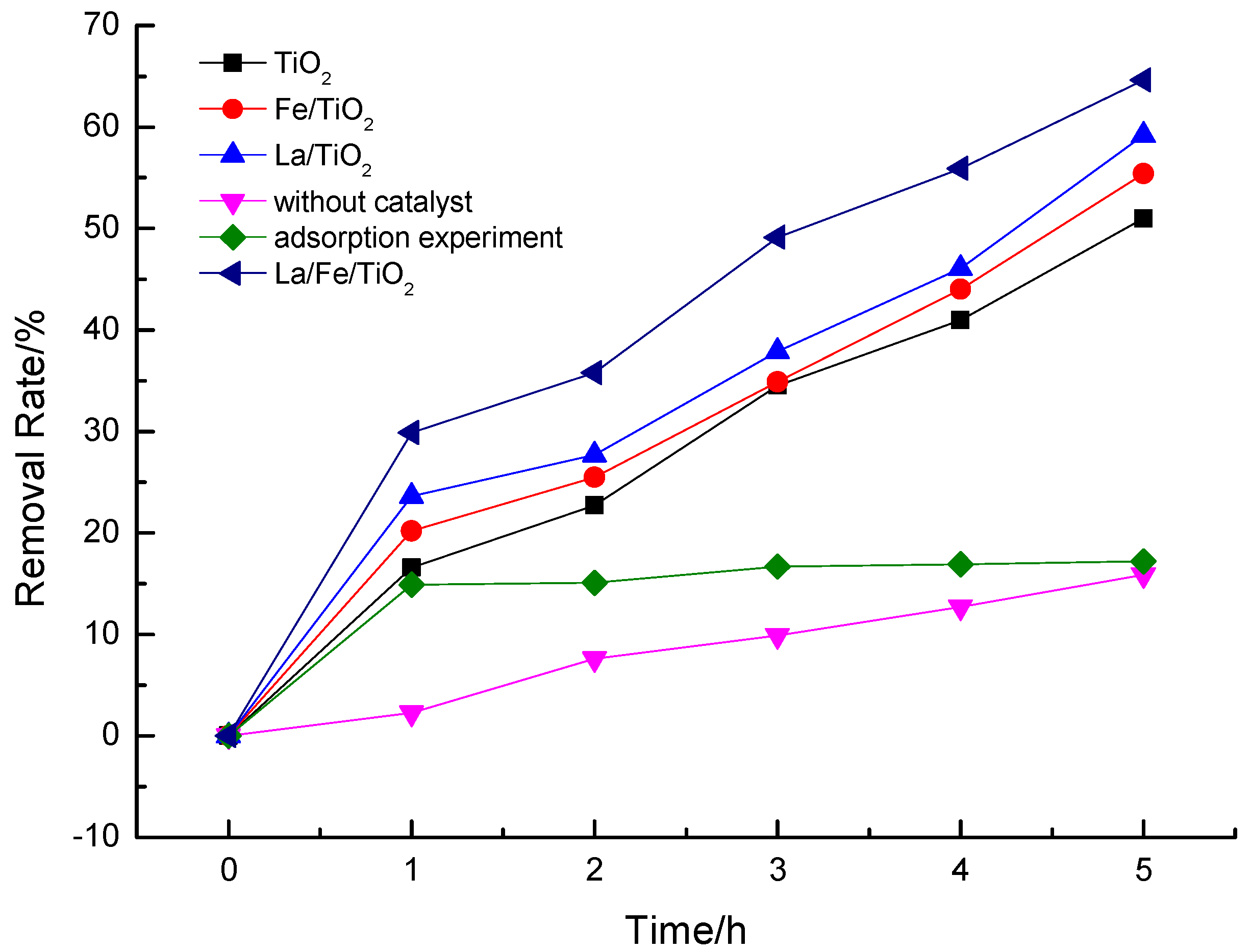
3.2. Degradation Performance of Ammonia Nitrogen Wastewater
| Catalyst | k | R2 |
|---|---|---|
| TiO2 | 0.137 | 0.990 |
| Fe/TiO2 | 0.150 | 0.988 |
| La/TiO2 | 0.163 | 0.976 |
| La/Fe/TiO2 | 0.196 | 0.984 |
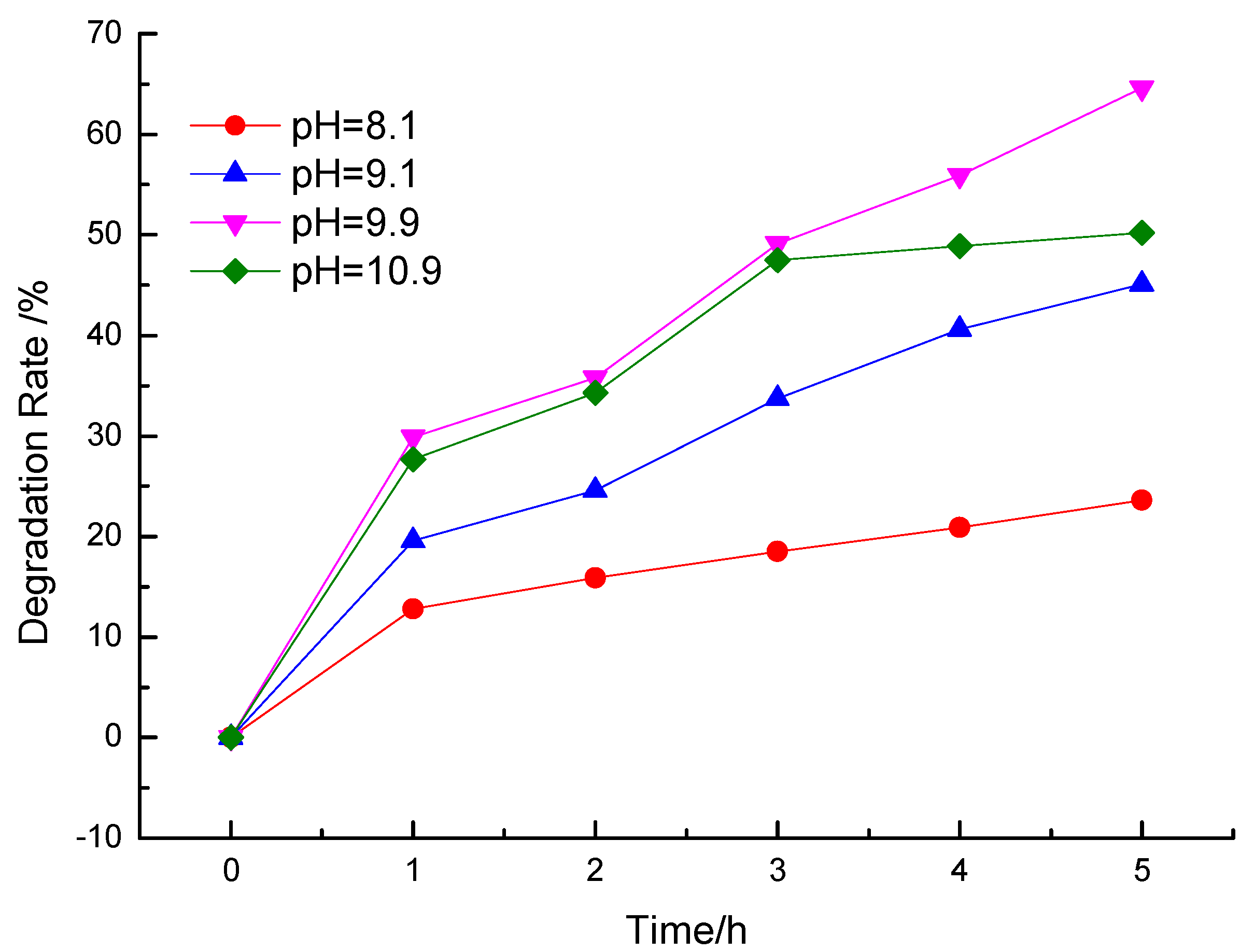
3.2.1. Effect of Different pH
3.2.2. Effect of H2O2

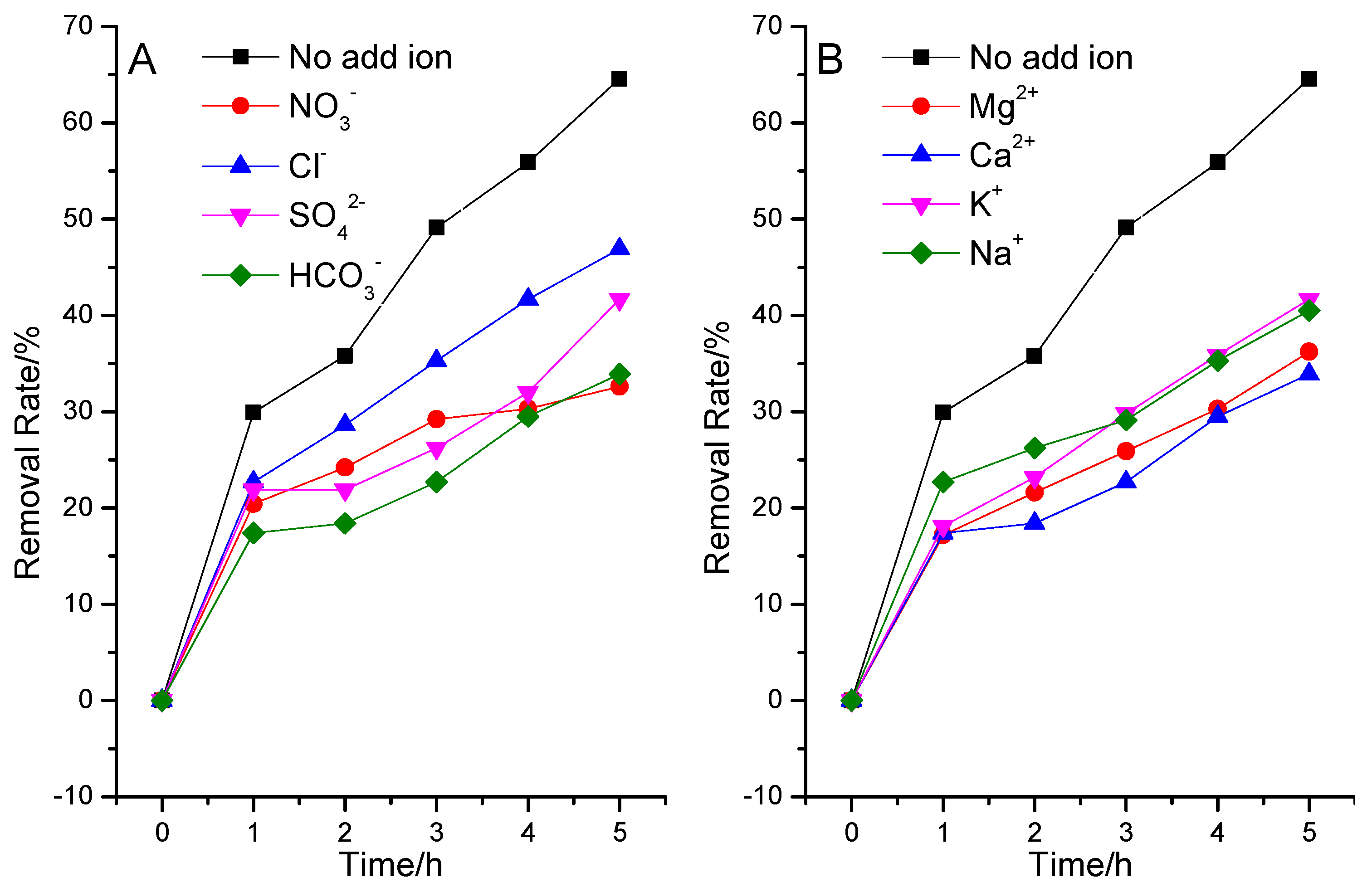
3.2.3. Effects of Inorganic Ions
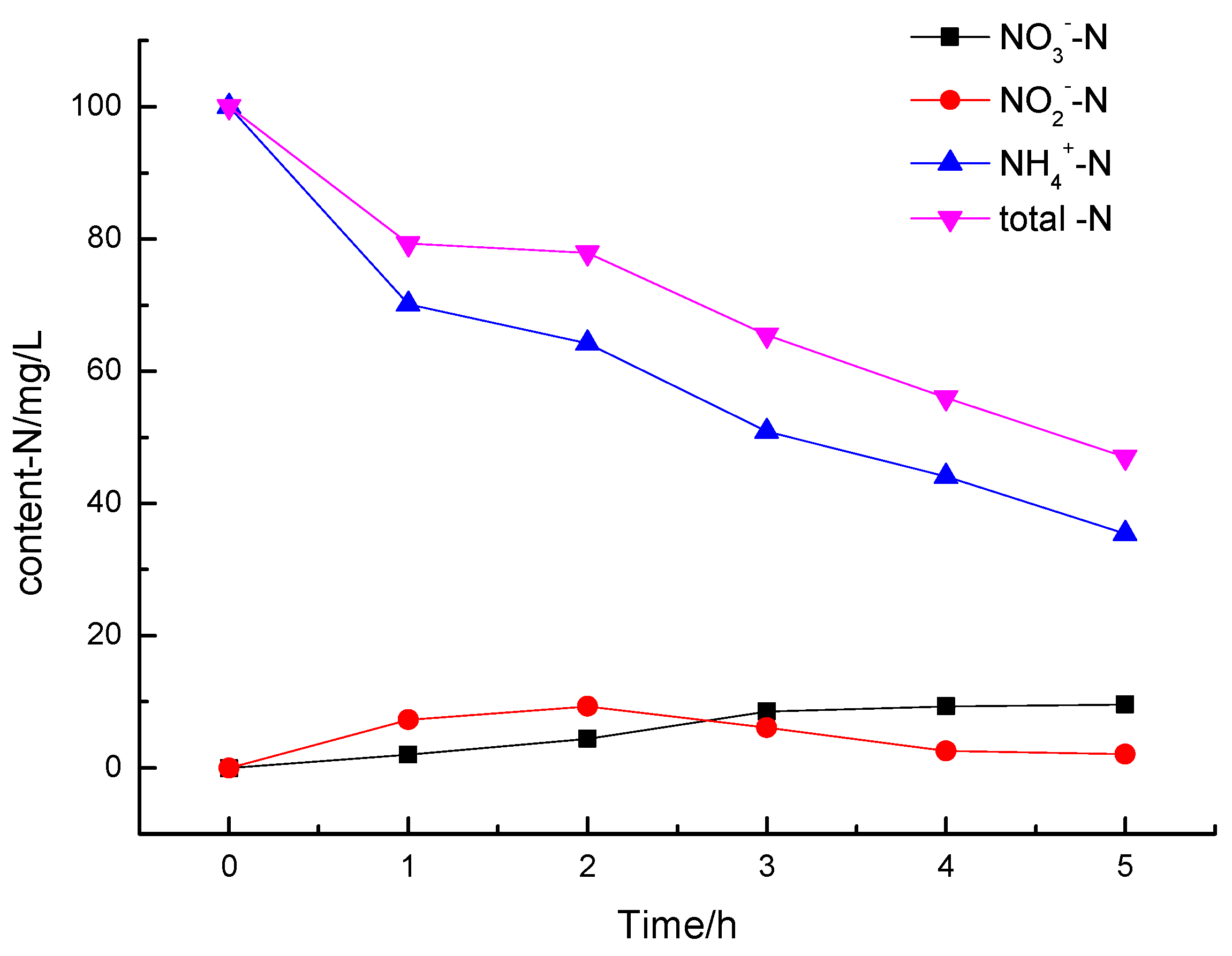
3.2.4. Analysis of Degradation Products of Ammonia Nitrogen
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yu, H.; Xu, L.; Wang, P.; Wang, X.; Yu, J. Enhanced photoinduced stability and photocatalytic activity of AgBr photocatalyst by surface modification of Fe(III) cocatalyst. Appl. Catal. B Environ. 2014, 144, 75–82. [Google Scholar] [CrossRef]
- Alshameri, A.; Ibrahim, A.; Assabri, A.M.; Lei, X.; Wang, H.; Yan, C. The investigation into the ammonium removal performance of Yemeni natural zeolite: Modification, ion exchange mechanism, and thermodynamics. Powder Technol. 2014, 258, 20–31. [Google Scholar] [CrossRef]
- Qu, D.; Sun, D.; Wang, H.; Yun, Y. Experimental study of ammonia removal from water by modified direct contact membrane distillation. Desalination 2013, 326, 135–140. [Google Scholar] [CrossRef]
- Iwata, R.; Yamauchi, T.; Hirota, Y.; Aoki, M.; Shimazu, T. Reaction kinetics of ammonia absorption/desorption of metal salts. Appl. Therm. Eng. 2014, 72, 244–249. [Google Scholar] [CrossRef]
- Altomare, M.; Selli, E. Effects of metal nanoparticles deposition on the photocatalytic oxidation of ammonia in TiO2 aqueous suspensions. Catal. Today 2013, 209, 127–133. [Google Scholar] [CrossRef]
- Seyedjamali, H.; Pirisedigh, A. In situ sol-gel fabrication of new poly(amide-ether-imide)/titania (TiO2) nanocomposite thin films containing l-leucine moieties. Colloid Polym. Sci. 2011, 289, 15–20. [Google Scholar] [CrossRef]
- Gomez, S.; Marchena, C.L.; Pizzio, L.; Pierella, L. Preparation and characterization of TiO2/HZSM-11 zeolite for photodegradation of dichlorvos in aqueous solution. J. Hazard. Mater. 2013, 258–259, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Četojević-Simin, D.D.; Armaković, S.J.; Šojić, D.V.; Abramović, B.F. Toxicity assessment of metoprolol and its photodegradation mixtures obtained by using different type of TiO2 catalysts in the mammalian cell lines. Sci. Total Environ. 2013, 463–464, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Daels, N.; Radoicic, M.; Radetic, M.; van Hulle, S.W.H.; de Clerck, K. Functionalisation of electrospun polymer nanofibre membranes with TiO2 nanoparticles in view of dissolved organic matter photodegradation. Sep. Purif. Technol. 2014, 133, 282–290. [Google Scholar] [CrossRef]
- Zhou, W.; Pan, K.; Qu, Y.; Sun, F.; Tian, C.; Ren, Z.; Tian, G.; Fu, H. Photodegradation of organic contamination in wastewaters by bonding TiO2/single-walled carbon nanotube composites with enhanced photocatalytic activity. Chemosphere 2010, 81, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Sun, D.; Sun, W.; Yang, W.; Li, Q.; Shang, J.K. Efficient photocatalytic removal of aqueous NH4+–NH3 by palladium-modified nitrogen-doped titanium oxide nanoparticles under visible light illumination, even in weak alkaline solutions. Chem. Eng. J. 2015, 264, 728–734. [Google Scholar] [CrossRef]
- Glaze, W.H.; Kang, J.-W.; Chapin, D.H. The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet Radiation. Ozone Sci. Eng. 1987, 9, 335–352. [Google Scholar] [CrossRef]
- Ohko, Y.; Ando, I.; Niwa, C. Degradation of bisphenol A in water by TiO2 photocatalyst. Environ. Sci. Technol. 2001, 35, 2365–2368. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, E.; Reszczyńska, J.; Zaleska, A. Mechanism of phenol photodegradation in the presence of pure and modified-TiO2: A review. Water Res. 2012, 46, 5453–5471. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, T.; Fujishima, A. Photoelectrochemical properties of TiO2 photocatalyst and its applications for environmental purification. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 247–262. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for environmental photocatalytic applications: A review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.C.; Zhang, L.; Zheng, Z.; Zhao, J. Synthesis and characterization of phosphated mesoporous titanium dioxide with high photocatalytic activity. Chem. Mater. 2003, 15, 2280–2286. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated Titanium Dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.G.; Devi, L.G. Review on modified TiO2 photocatalysis under UV/Visible light: Selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef] [PubMed]
- Obata, K.; Kishishita, K.; Okemoto, A.; Taniya, K.; Ichihashi, Y.; Nishiyama, S. Photocatalytic Decomposition of NH3 over TiO2 Catalysts Doped with Fe. Appl. Catal. B Environ. 2014, 160–161, 200–203. [Google Scholar] [CrossRef]
- Reli, M.; Ambrožová, N.; Šihor, M.; Matějová, L.; Čapek, L.; Obalová, L.; Matěj, Z.; Kotarba, A.; Kočí, K. Novel cerium doped titania catalysts for photocatalytic decomposition of ammonia. Appl. Catal. B Environ. 2015, 178, 108–116. [Google Scholar] [CrossRef]
- Altomare, M.; Dozzi, M.V.; Chiarello, G.L.; di Paola, A.; Palmisano, L.; Selli, E. High activity of brookite TiO2 nanoparticles in the photocatalytic abatement of ammonia in water. Catal. Today 2015, 252, 184–189. [Google Scholar] [CrossRef]
- Liu, J.; Liu, B.; Ni, Z.; Deng, Y.; Zhong, C.; Hu, W. Improved catalytic performance of Pt/TiO2 nanotubes electrode for ammonia oxidation under UV-light illumination. Electrochim. Acta 2014, 150, 146–150. [Google Scholar] [CrossRef]
- Shavisi, Y.; Sharifnia, S.; Hosseini, S.N.; Khadivi, M.A. Application of TiO2/perlite photocatalysis for degradation of ammonia in wastewater. J. Ind. Eng. Chem. 2014, 20, 278–283. [Google Scholar] [CrossRef]
- Administration, S.E.P. Water Quality-Determination of Nitrate-Spectrophotometric Method with Phenol Disulfonic Acid, 1st ed.; China Standard Press: Bejing, China, 1987; p. 4. [Google Scholar]
- Administration, S.E.P. Water Quality-Determination of Nitrogen (Nitrite)-Spectrophotometric Method, 1st ed.; China Standard Press: Beijing, China, 1987; p. 4. [Google Scholar]
- Jing, J.; Zhang, Y.; Li, W.; Yu, W.W. Visible light driven photodegradation of quinoline over TiO2/graphene oxide nanocomposites. J. Catal. 2014, 316, 174–181. [Google Scholar] [CrossRef]
- Ghobadi, N.; Moradian, R. Strong localization of the charge carriers in CdSe nanostructural films. Int. Nano Lett. 2013, 3, 1–5. [Google Scholar] [CrossRef]
- Wang, X.H.; Li, J.G.; Kamiyama, H.; Katada, M.; Ohashi, N.; Moriyoshi, Y.; Ishigaki, T. Pyrogenic Iron(III)-doped TiO2 nanopowders synthesized in RF thermal plasma: Phase formation, defect structure, band gap, and magnetic properties. J. Am. Chem. Soc. 2005, 127, 10982–10990. [Google Scholar] [CrossRef] [PubMed]
- Parshetti, G.K.; Doong, R.-A. Dechlorination and photodegradation of trichloroethylene by Fe/TiO2 nanocomposites in the presence of nickel ions under anoxic conditions. Appl. Catal. B Environ. 2010, 100, 116–123. [Google Scholar] [CrossRef]
- Anandan, S.; Ikuma, Y.; Murugesan, V. Highly active rare-earth-metal La-doped photocatalysts: Fabrication, characterization, and their photocatalytic activity. Int. J. Photoenergy 2012, 2012. [Google Scholar] [CrossRef]
- Xiaodong, W.; Zhichun, S.; Guo, L.; Duan, W.; Ziran, M. Effects of cerium and vanadium on the activity and selectivity of MnOx-TiO2 catalyst for low-temperature NH3-SCR. J. Rare Earths 2011, 29, 64–68. [Google Scholar] [CrossRef]
- Poulios, I.; Micropoulou, E.; Panou, R.; Kostopoulou, E. Photooxidation of eosin Y in the presence of semiconducting oxides. Appl. Catal. B Environ. 2003, 41, 345–355. [Google Scholar] [CrossRef]
- Dasary, S.S.R.; Saloni, J.; Fletcher, A.; Anjaneyulu, Y.; Yu, H. Photodegradation of Selected PCBs in the Presence of Nano-TiO2 as Catalyst and H2O2 as an Oxidant. Int. J. Environ. Res. Public Health 2010, 7, 3987–4001. [Google Scholar] [CrossRef] [PubMed]
- Sörensen, M.; Frimmel, F.H. Photochemical degradation of hydrophilic xenobiotics in the UVH2O2 process: Influence of nitrate on the degradation rate of EDTA, 2-amino-1-naphthalenesulfonate, diphenyl-4-sulfonate and 4,4′-diaminostilbene-2,2′-disulfonate. Water Res. 1997, 31, 2885–2891. [Google Scholar] [CrossRef]
- Dai, S. Environmental Chemistry, 2nd ed.; Higher Education Press: Beijing, China, 2006; p. 147. [Google Scholar]
- Kim, D.H.; Anderson, M.A. Solution factors affecting the photocatalytic and photoelectrocatalytic degradation of formic acid using supported TiO2 thin films. J. Photochem. Photobiol. A Chem. 1996, 94, 221–229. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, L.; Wei, M.; Chen, P.; Shan, G. Photolytic reaction mechanism and impacts of coexisting substances on photodegradation of bisphenol A by Bi2WO6 in water. Water Res. 2012, 46, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Liu, H.; Zhang, C.; Cheng, T.; Zhang, G.; Wu, F.; Sun, S. Effects of inorganic cations on photocatalytic reduction of chromium (VI) over TiO2 thin films. Tech. Equip. Environ. Pollut. Control 2010, 2, 288–292. [Google Scholar]
- Jie, W.; Guo, C.J. Photocatalysis degradation of inorganic nitrogen in Water. Sci.-Tech. Inf. Dev. Econ. 2005, 15, 173–175. [Google Scholar]
- Lee, J.; Park, H.; Choi, W. Selective photocatalytic oxidation of NH3 to N2 on platinized TiO2 in water. Environ. Sci. Technol. 2002, 36, 5462–5468. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Chen, C.; Yang, J.; Wang, J.; Yan, Q.; Shi, H.; Wang, C. Characterization of La/Fe/TiO2 and Its Photocatalytic Performance in Ammonia Nitrogen Wastewater. Int. J. Environ. Res. Public Health 2015, 12, 14626-14639. https://doi.org/10.3390/ijerph121114626
Luo X, Chen C, Yang J, Wang J, Yan Q, Shi H, Wang C. Characterization of La/Fe/TiO2 and Its Photocatalytic Performance in Ammonia Nitrogen Wastewater. International Journal of Environmental Research and Public Health. 2015; 12(11):14626-14639. https://doi.org/10.3390/ijerph121114626
Chicago/Turabian StyleLuo, Xianping, Chunfei Chen, Jing Yang, Junyu Wang, Qun Yan, Huquan Shi, and Chunying Wang. 2015. "Characterization of La/Fe/TiO2 and Its Photocatalytic Performance in Ammonia Nitrogen Wastewater" International Journal of Environmental Research and Public Health 12, no. 11: 14626-14639. https://doi.org/10.3390/ijerph121114626






