Physiological and Psychological Effects of a Walk in Urban Parks in Fall
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Sites
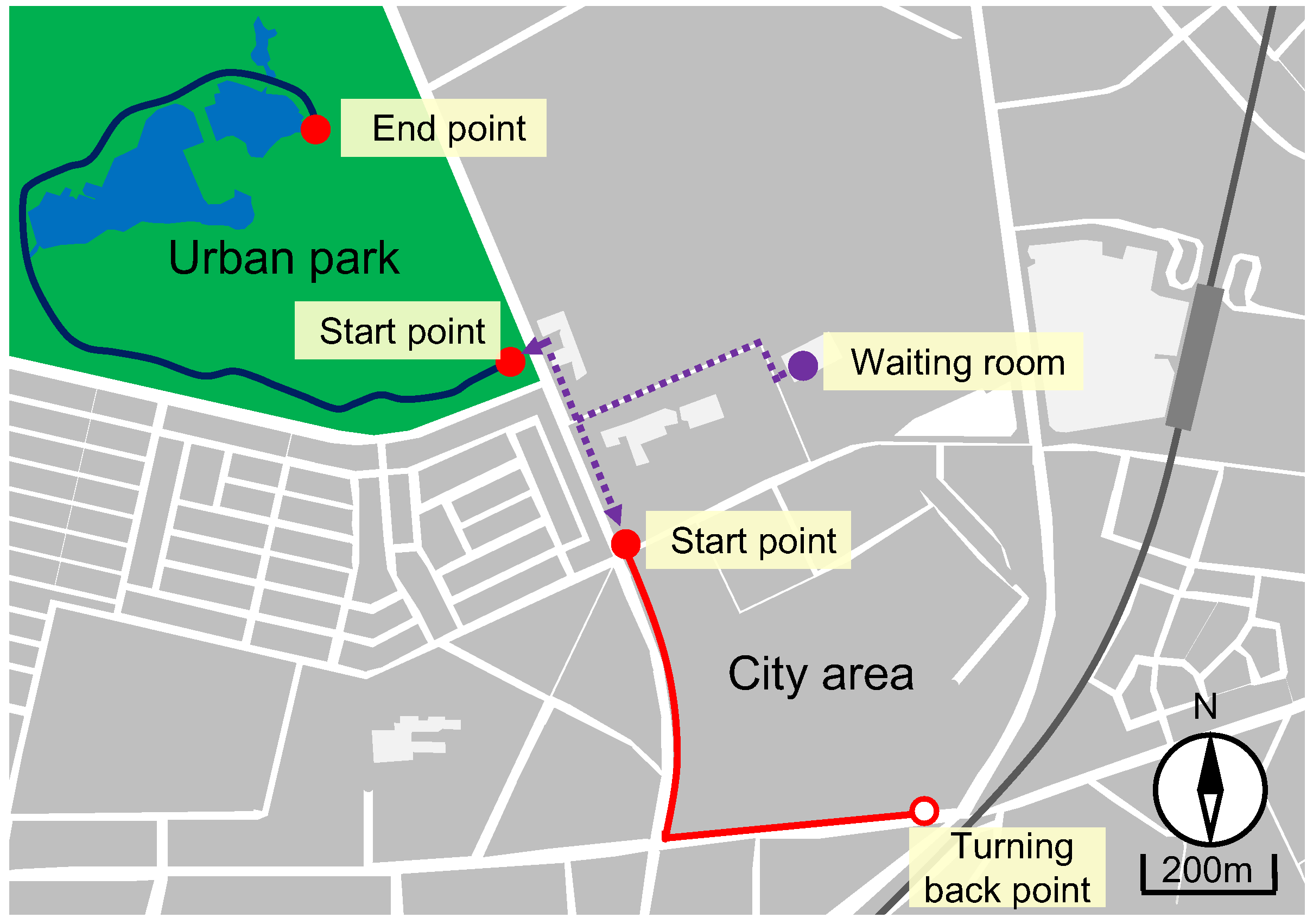
| Value (Mean ± SD) | P Value | ||
|---|---|---|---|
| Urban Park | City Area | ||
| Temperature (°C) | 18.0 ± 1.7 | 19.2 ± 1.9 | 0.258 |
| Relative humidity (%) | 71.5 ± 10.1 | 64.7 ± 8.7 | 0.244 |
| Intensity of illumination (lx) | 24,230 ± 11,220 | 38,870 ± 22,330 | 0.192 |
2.2. Participants
| Parameter | Value (Mean ± SD) |
|---|---|
| Total sample size | 23 |
| Sex | Male |
| Age (years) | 22.3 ± 1.2 |
| Height (cm) | 171.1 ± 4.7 |
| Weight (kg) | 63.4 ± 8.1 |
| BMI (kg/m2) | 21.5 ± 2.1 |
2.3. Experimental Design

2.4. Physiological Indices
2.5. Psychological Indices
2.6. Statistical Analyses
3. Results
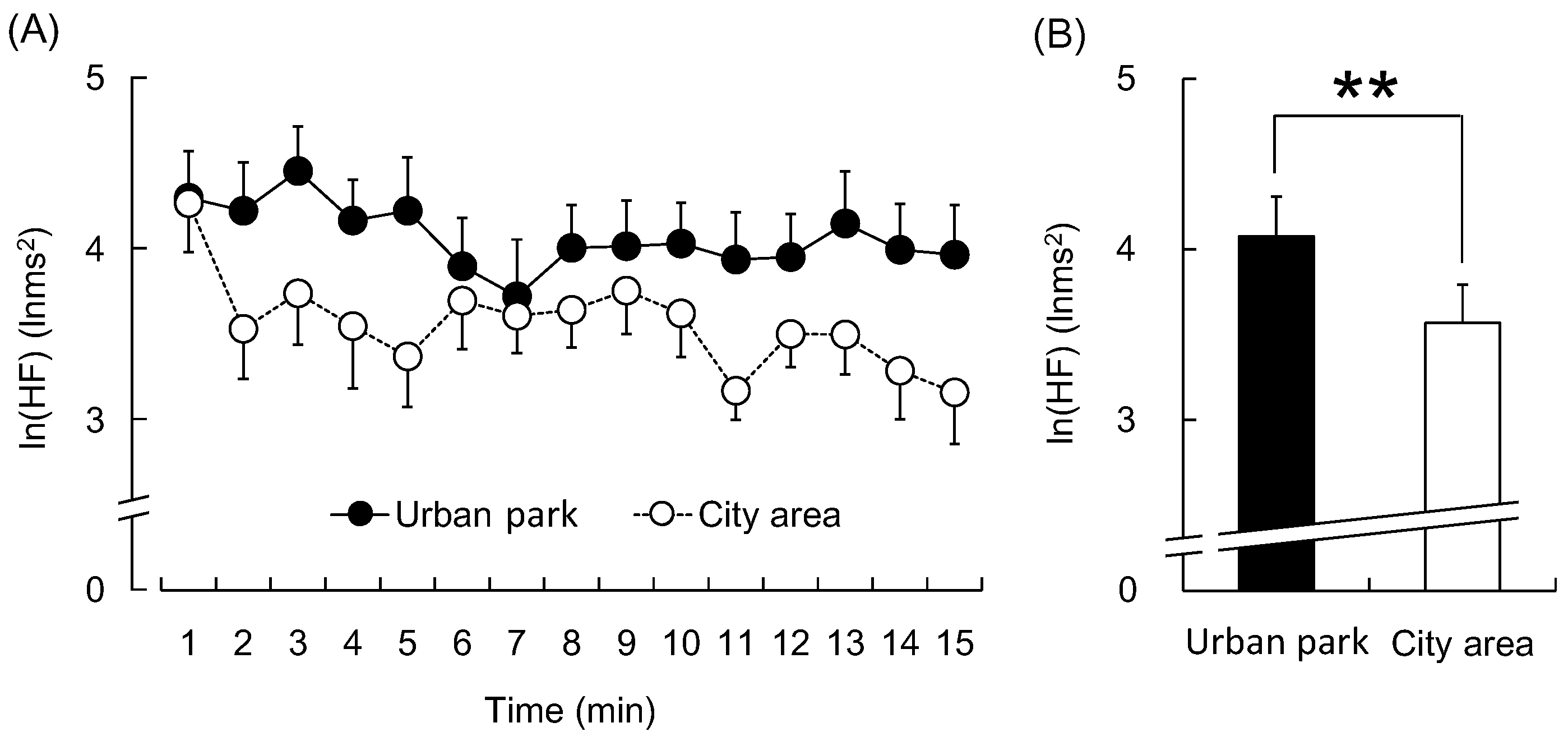
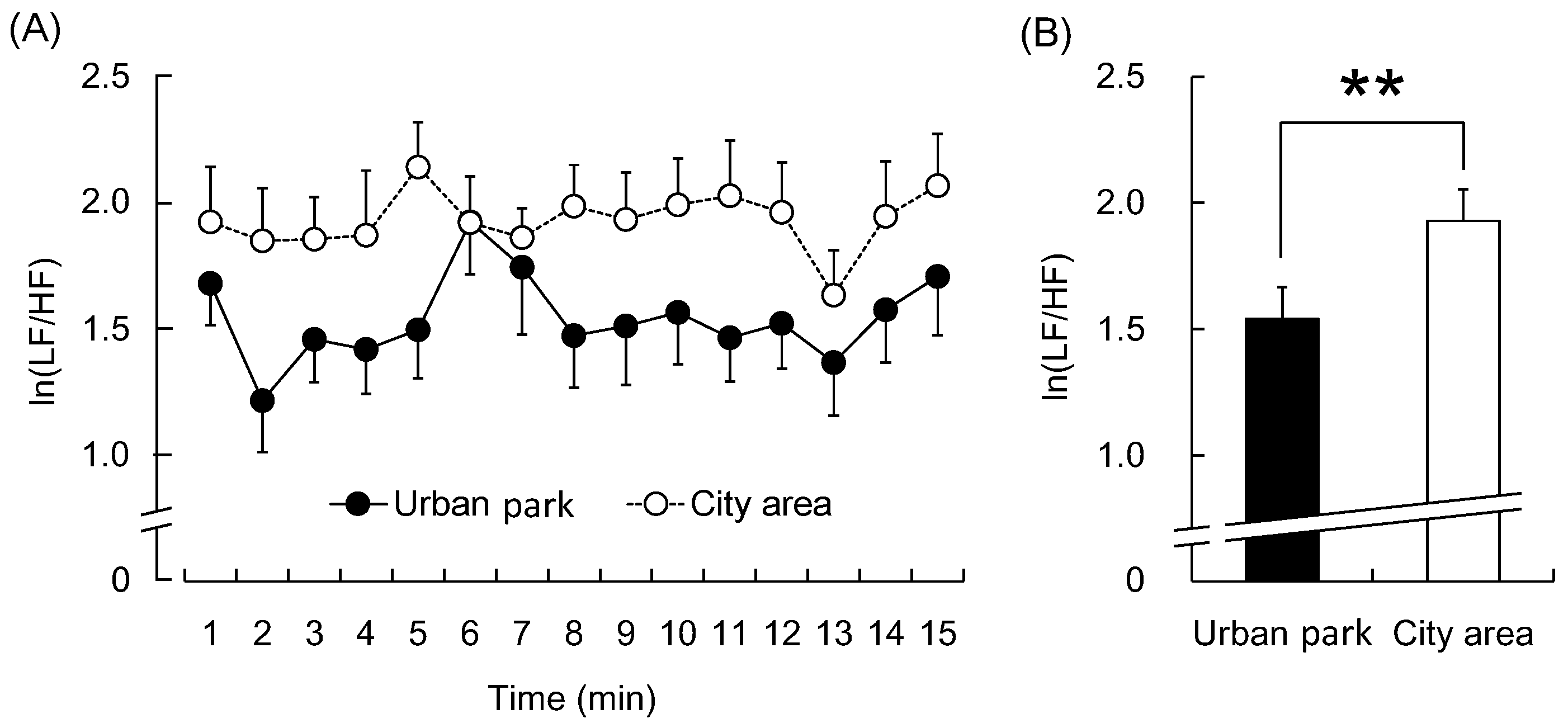
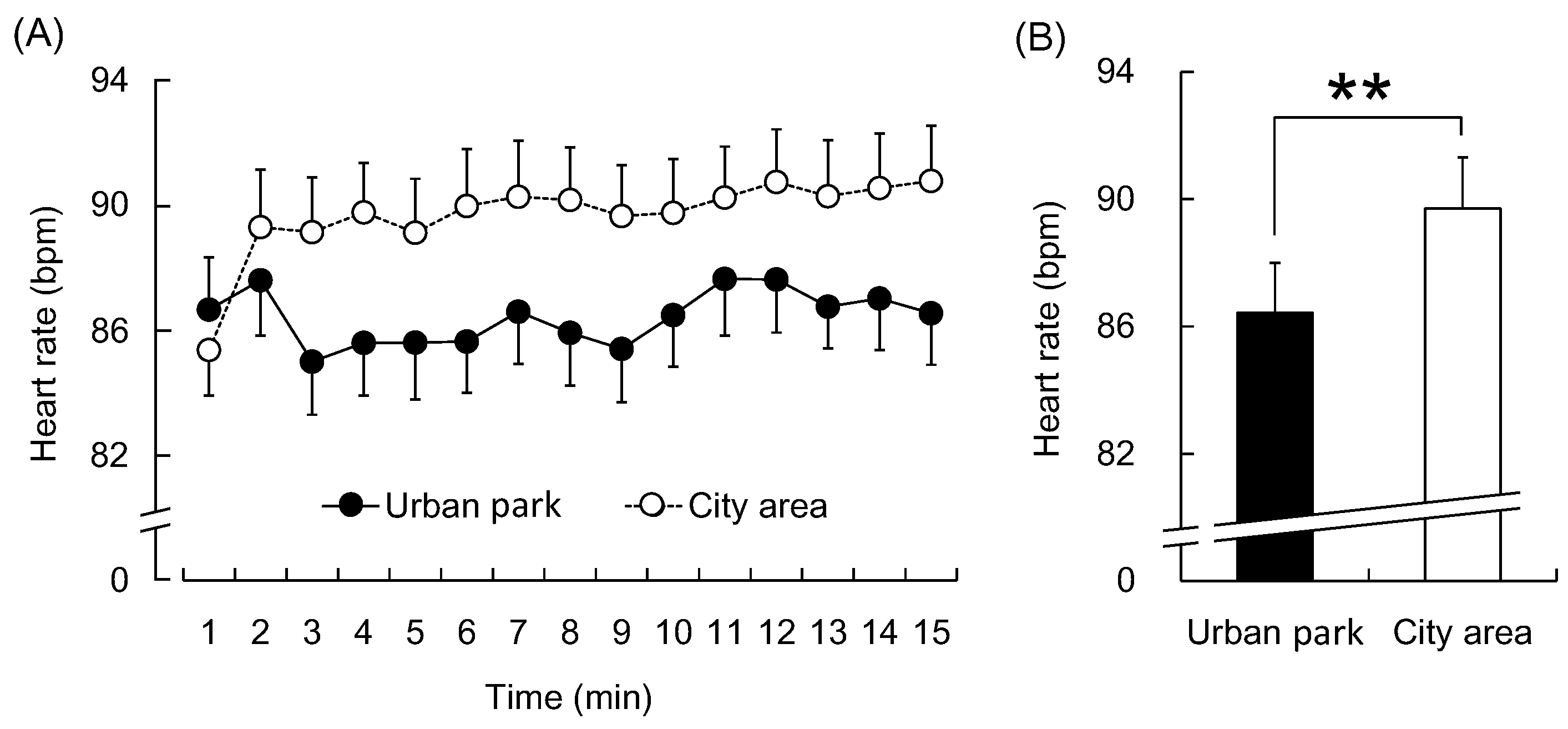
4. Discussion
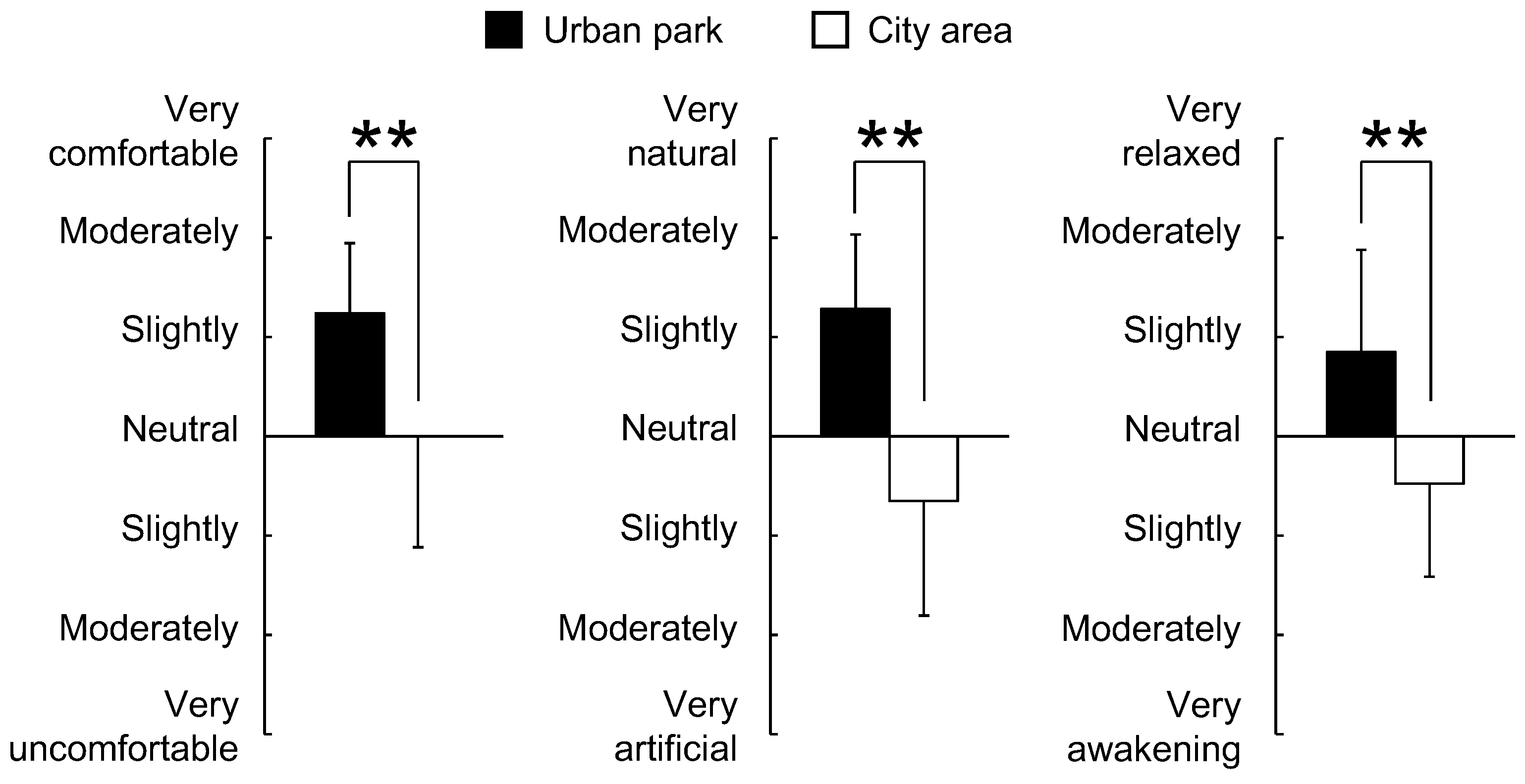
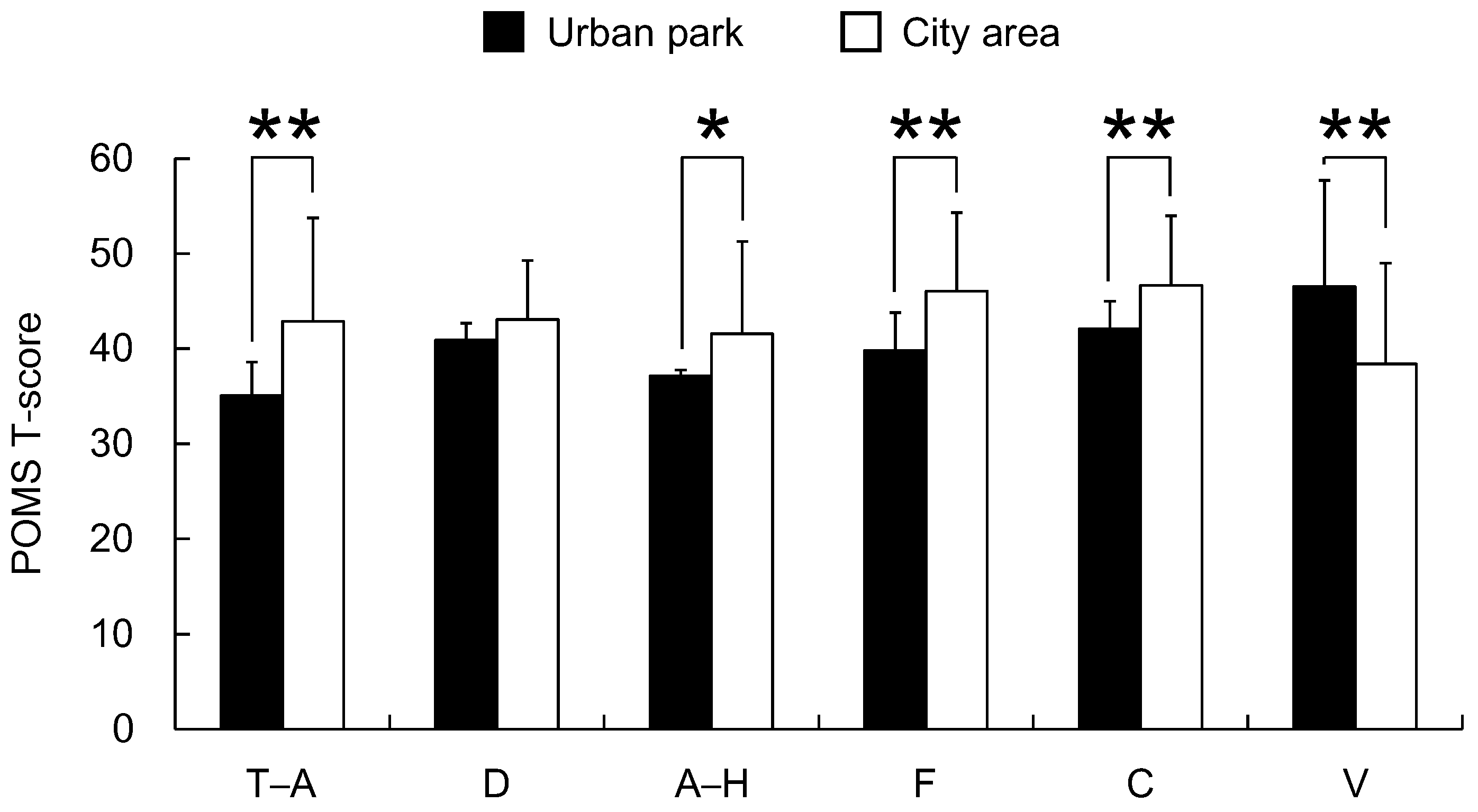
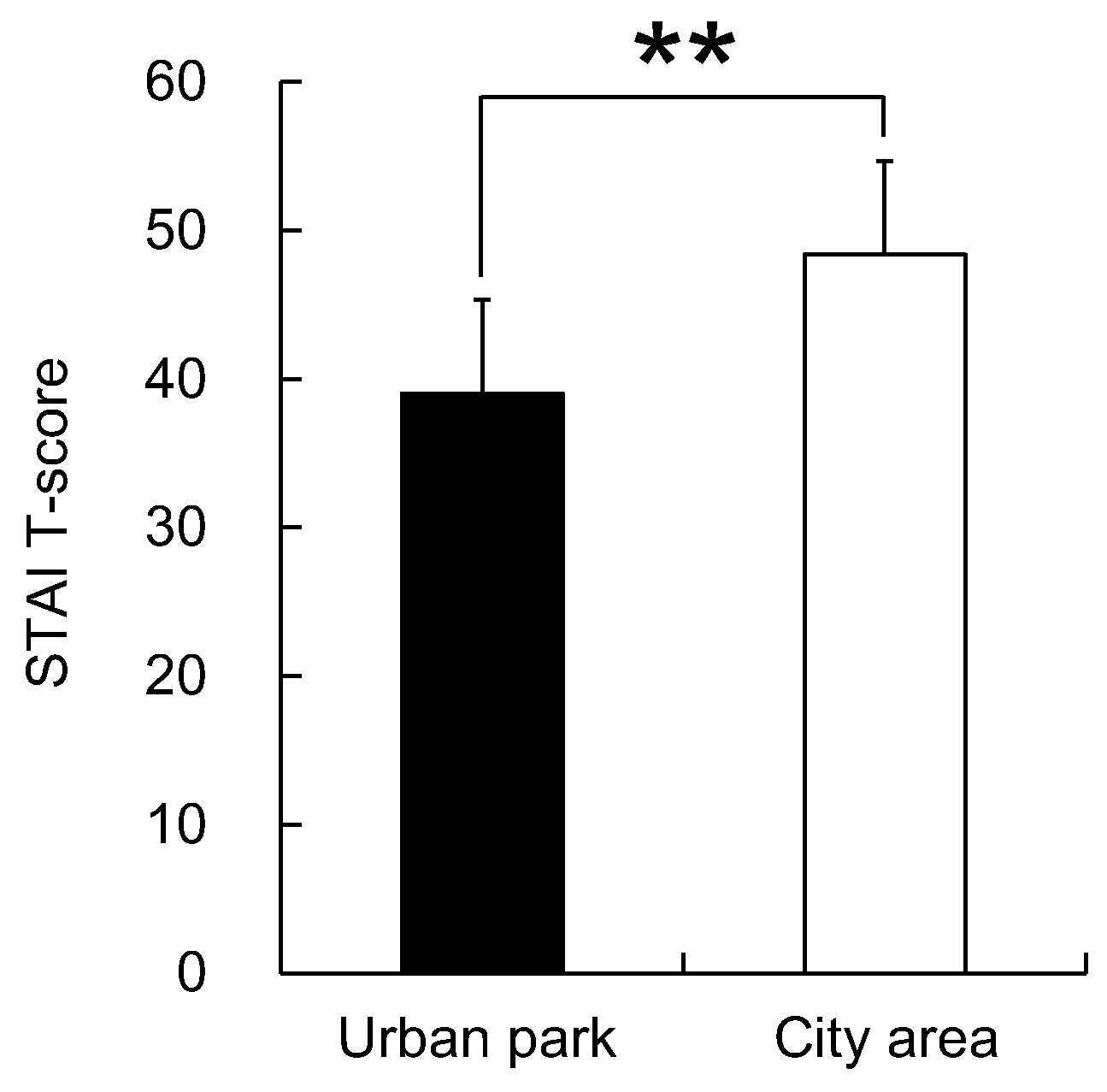
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dye, C. Health and urban living. Science 2008, 319, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Vlahov, D.; Freudenberg, N.; Proietti, F.; Ompad, D.; Quinn, A.; Nandi, V.; Galea, S. Urban as a determinant of health. J. Urban Health 2007, 84, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, M.K. Modern Environments and Human Health: Revisiting the Second Epidemiological Transition; Wiley-Blackwell: Hoboken, NJ, USA, 2014. [Google Scholar]
- Srakash, P. Nutrition transition and its health outcomes. Indian J. Pediatr. 2013, 80, S21–S27. [Google Scholar] [PubMed]
- Wagner, K.H.; Brath, H. A global view on the development of non communicable diseases. Prev. Med. 2012, 54, S38–S41. [Google Scholar] [CrossRef] [PubMed]
- Pronczuk, J.; Surdu, S. Children’s environmental health in the twenty-first century. Ann. NY Acad. Sci. 2008, 1140, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Patz, J.A.; Campbell, L.D.; Holloway, T.; Foley, J.A. Impact of regional climate change on human health. Nature 2005, 438, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Basara, J.B.; Basara, H.G.; Illston, B.G.; Crawford, K.C. The impact of the urban heat island during an intense heat wave in Oklahoma City. Adv. Meteorol. 2010, 2010, 1–9. [Google Scholar] [CrossRef]
- Changnon, S.A.; Kunkel, K.E.; Reinke, B.C. Impacts and responses to the 1995 heat wave: A call to action. Bull. Am. Meteorol. Soc. 1996, 77, 1497–1506. [Google Scholar] [CrossRef]
- Kosatsky, T. The 2003 European heat waves. Euro. Surveill. 2005, 10, 148–149. [Google Scholar] [PubMed]
- Kovats, R.S.; Hajat, S. Heat stress and public health: A critical review. Annu. Rev. Public Health 2008, 29, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Ghany, A.M.; Al-Helal, I.M.; Shady, M.R. Human thermal comfort and heat stress in an outdoor urban arid environment: A case study. Adv. Meteorol. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Brunekreef, B.; Holgate, S.T. Air pollution and health. Lancet 2002, 360, 1233–1242. [Google Scholar] [CrossRef]
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T.; Lancet Physical Activity Series Working Group. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef]
- Kohl, H.W.; Craig, C.L.; Lambert, E.V.; Inoue, S.; Alkandari, J.R.; Leetongin, G.; Kahlmeier, S.; Lancet Physical Activity Series Working Group. The pandemic of physical inactivity: Global action for public health. Lancet 2012, 380, 294–305. [Google Scholar] [CrossRef]
- Craig, B. Technostress: The Human Cost of the Computer Revolution; Addison-Wesley Publishing Company: Boston, MA, USA, 1984. [Google Scholar]
- Salanova, M.; Llorens, S.; Cifre, E. The dark side of technologies: Technostress among users of information and communication technologies. Int. J. Psychol. 2013, 48, 422–436. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Stokols, D. Psychological and health outcomes of perceived information overload. Environ. Behav. 2012, 44, 737–759. [Google Scholar] [CrossRef]
- Herbert, T.B.; Cohen, S. Stress and immunity in humans: A meta-analytic review. Psychosom. Med. 1993, 55, 364–379. [Google Scholar] [CrossRef] [PubMed]
- Gémes, K.; Ahnve, S.; Janszky, I. Inflammation a possible link between economical stress and coronary heart disease. Eur. J. Epidemiol. 2008, 23, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Lederbogen, F.; Kirsch, P.; Haddad, L.; Streit, F.; Tost, H.; Schuch, P.; Wüst, S.; Pruessner, J.C.; Rietschel, M.; Deuschle, M.; et al. City living and urban upbringing affect neural social stress processing in humans. Nature 2011, 474, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.F.; McPherson, E.G.; Schroeder, H.W.; Rowntree, R.A. Assessing the benefits and costs of the urban forest. J. Arboric. 1992, 18, 227–234. [Google Scholar]
- Saaroni, H.; Ziv, B. The impact of a small lake on heat stress in a Mediterranean urban park: The case of Tel Aviv, Israel. Int. J. Biometeorol. 2003, 47, 156–165. [Google Scholar] [PubMed]
- Cohen, P.; Potchter, O.; Schnell, I. The impact of an urban park on air pollution and noise levels in the Mediterranean city of Tel-Aviv, Israel. Environ. Pollut. 2014, 195, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Tse, M.S.; Chau, C.K.; Choy, Y.S.; Tsui, W.K.; Chan, C.N.; Tang, S.K. Perception of urban park soundscape. J. Acoust. Soc. Am. 2012, 131, 2762–2771. [Google Scholar] [CrossRef] [PubMed]
- Chiesura, A. The role of urban parks for the sustainable city. Landsc. Urban Plan. 2004, 68, 129–138. [Google Scholar] [CrossRef]
- Nutsford, D.; Pearson, A.L.; Kingham, S. An ecological study investigating the association between access to urban green space and mental health. Public Health 2013, 127, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Maas, J.; Verheij, R.A.; Groenewegen, P.P.; Vries, S.D.; Spreeuwenberg, P. Green space, urbanity, and health: How strong is the relation? J. Epidemiol. Community Health 2006, 60, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.; Popham, F. Effect of exposure to natural environment on health inequalities: An observational population study. Lancet 2008, 372, 1655–1660. [Google Scholar] [CrossRef]
- Takano, T.; Nakamura, K.; Watanabe, M. Urban residential environments and senior citizens’ longevity in megacity areas: The importance of walkable green spaces. J. Epidemiol. Community Health 2002, 56, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Ikei, H.; Igarashi, M.; Miwa, M.; Takagaki, M.; Miyazaki, Y. Physiological and psychological responses of young males during spring-time walks in urban parks. J. Physiol. Anthropol. 2014, 33. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Joung, D.; Ikei, H.; Igarashi, M.; Aga, M.; Park, B.J.; Miwa, M.; Takagaki, M.; Miyazaki, Y. Physiological and psychological effects of walking on young males in urban parks in winter. J. Physiol. Anthropol. 2013, 32. [Google Scholar] [CrossRef] [PubMed]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef] [PubMed]
- Pagani, M.; Lombardi, F.; Guzzetti, S.; Rimoldi, O.; Furlan, R.; Pizzinelli, P.; Sandrone, G.; Malfatto, G.; Dell’Orto, S.; Piccaluga, E.; et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ. Res. 1986, 59, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Park, B.J.; Miyazaki, Y. Normative references of heart rate variability and salivary alpha-amylase in a healthy young male population. J. Physiol. Anthropol. 2012, 31. [Google Scholar] [CrossRef] [PubMed]
- Osgood, C.E.; Suci, G.J.; Tannenbaum, P. The Measurement of Meaning; University of Illinois Press: Urbana, IL, USA, 1957. [Google Scholar]
- McNair, D.M.; Lorr, M. An analysis of mood in neurotics. J. Abnorm. Psychol. 1964, 69, 620–627. [Google Scholar] [CrossRef] [PubMed]
- McNair, D.M.; Lorr, M.; Droppleman, L. Profile of Mood States Manual; Educational and Industrial Testing Services: San Diego, CA, USA, 1992. [Google Scholar]
- Yokoyama, K. POMS Shortened Version-Manual and Commentary on Cases; Kaneko Syoboh: Tokyo, Japan, 2005. [Google Scholar]
- Spielberger, C.D. Manual for the State-Trait Anxiety Inventory: STAI (Form Y); Consulting Psychologist Press: Palo Alto, CA, USA, 1983. [Google Scholar]
- Hidano, N.; Fukuhara, M.; Iwawaki, M.; Soga, S.; Spielberger, C.D. State-Trait Anxiety Inventory—Form JYZ; Jitsumu-Kyoiku Syuppan: Tokyo, Japan, 2000. [Google Scholar]
- Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Morikawa, T.; Kagawa, T.; Miyazaki, Y. Physiological effects of forest recreation in a young conifer forest in Hinokage town, Japan. Silva Fenn. 2009, 43, 291–301. [Google Scholar] [CrossRef]
- Lee, J.; Park, B.J.; Tsunetsugu, Y.; Kagawa, T.; Miyazaki, Y. Restorative effects of viewing real forest landscapes, based on a comparison with urban landscapes. Scand. J. For. Res. 2009, 24, 227–234. [Google Scholar] [CrossRef]
- Tsunetsugu, Y.; Park, B.J.; Ishii, H.; Hirano, H.; Kagawa, T.; Miyazaki, Y. Physiological effects of Shinrin-yoku (taking in the atmosphere of the forest) in an old-growth broadleaf forest in Yamagata prefecture, Japan. J. Physiol. Anthropol. 2007, 26, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Tsunetsugu, Y.; Lee, J.; Park, B.J.; Tyrväinend, L.; Kagawa, T.; Miyazaki, Y. Physiological and psychological effects of viewing urban forest landscapes assessed by multiple measurement. Landsc. Urban Plan. 2013, 113, 90–93. [Google Scholar] [CrossRef]
- Park, B.J.; Tsunetsugu, Y.; Lee, J.; Kagawa, T.; Miyazaki, Y. Effect of the forest environment on physiological relaxation using the results of field tests at 35 sites throughout Japan. In Forest Medicine; Li, Q., Ed.; Nova Science Publishers: New York, NY, USA, 2012; pp. 55–65. [Google Scholar]
- Park, B.J.; Tsunetsugu, Y.; Ishii, H.; Furuhashi, S.; Hirano, H.; Kagawa, T.; Miyazaki, Y. Physiological effects of Shinrin-yoku (taking in the atmosphere of the forest) in a mixed forest in Shinano Town, Japan. Scand. J. For. Res. 2008, 23, 278–283. [Google Scholar] [CrossRef]
- Lee, J.; Park, B.J.; Tsunetsugu, Y.; Ohira, T.; Kagawa, T.; Miyazaki, Y. Effect of forest bathing on physiological and psychological responses in young Japanese male subjects. Public Health 2011, 125, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Tsunetsugu, Y.; Takayama, N.; Park, B.J.; Li, Q.; Song, C.; Komatsu, M.; Ikei, H.; Tyrväinen, L.; Kagawa, T.; et al. Influence of forest therapy on cardiovascular relaxation in young adults. Evid.-Based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Hirano, H.; Kagawa, T.; Sato, M.; Miyazaki, Y. Physiological effects of Shinrin-yoku (taking in the atmosphere of the forest)—Using salivary cortisol and cerebral activity as indicators. J. Physiol. Anthropol. 2007, 26, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Bratman, G.N.; Hamilton, J.P.; Hahn, K.S.; Daily, G.C.; Gross, J.J. Nature experience reduces rumination and subgenual prefrontal cortex activation. Proc. Natl. Acad. Sci. USA 2015, 112, 8567–8572. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, K.; Murray, A.L.; Booth, T. Do urban environments increase the risk of anxiety, depression and psychosis? An epidemiological study. J. Affect. Disord. 2013, 150, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, C.; Ikei, H.; Igarashi, M.; Takagaki, M.; Miyazaki, Y. Physiological and Psychological Effects of a Walk in Urban Parks in Fall. Int. J. Environ. Res. Public Health 2015, 12, 14216-14228. https://doi.org/10.3390/ijerph121114216
Song C, Ikei H, Igarashi M, Takagaki M, Miyazaki Y. Physiological and Psychological Effects of a Walk in Urban Parks in Fall. International Journal of Environmental Research and Public Health. 2015; 12(11):14216-14228. https://doi.org/10.3390/ijerph121114216
Chicago/Turabian StyleSong, Chorong, Harumi Ikei, Miho Igarashi, Michiko Takagaki, and Yoshifumi Miyazaki. 2015. "Physiological and Psychological Effects of a Walk in Urban Parks in Fall" International Journal of Environmental Research and Public Health 12, no. 11: 14216-14228. https://doi.org/10.3390/ijerph121114216







