Radon Concentrations in Drinking Water in Beijing City, China and Contribution to Radiation Dose
Abstract
:1. Introduction
2. Experimental Section
2.1. Water Sampling
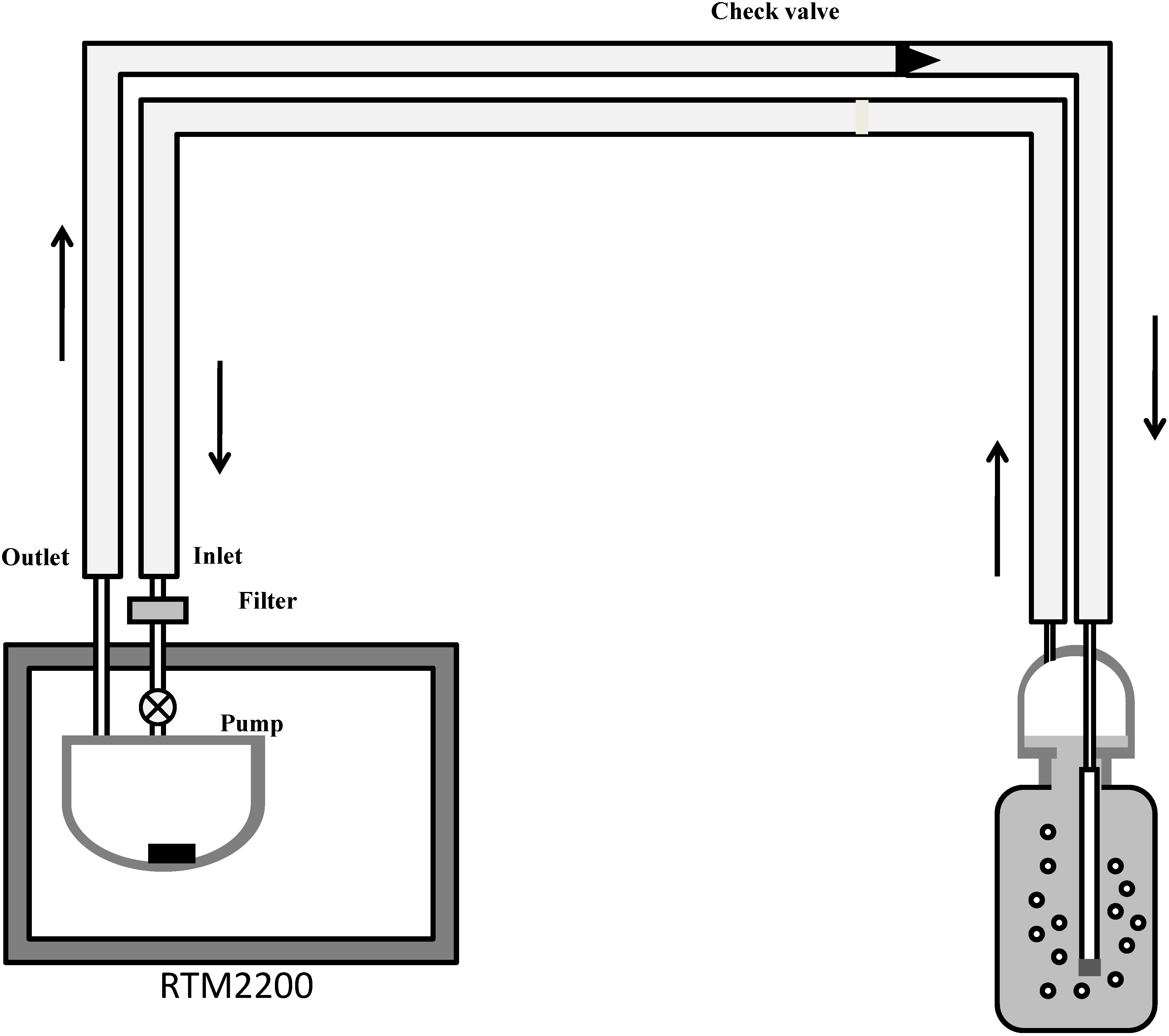
2.2. Method of Measurements

3. Results and Discussion
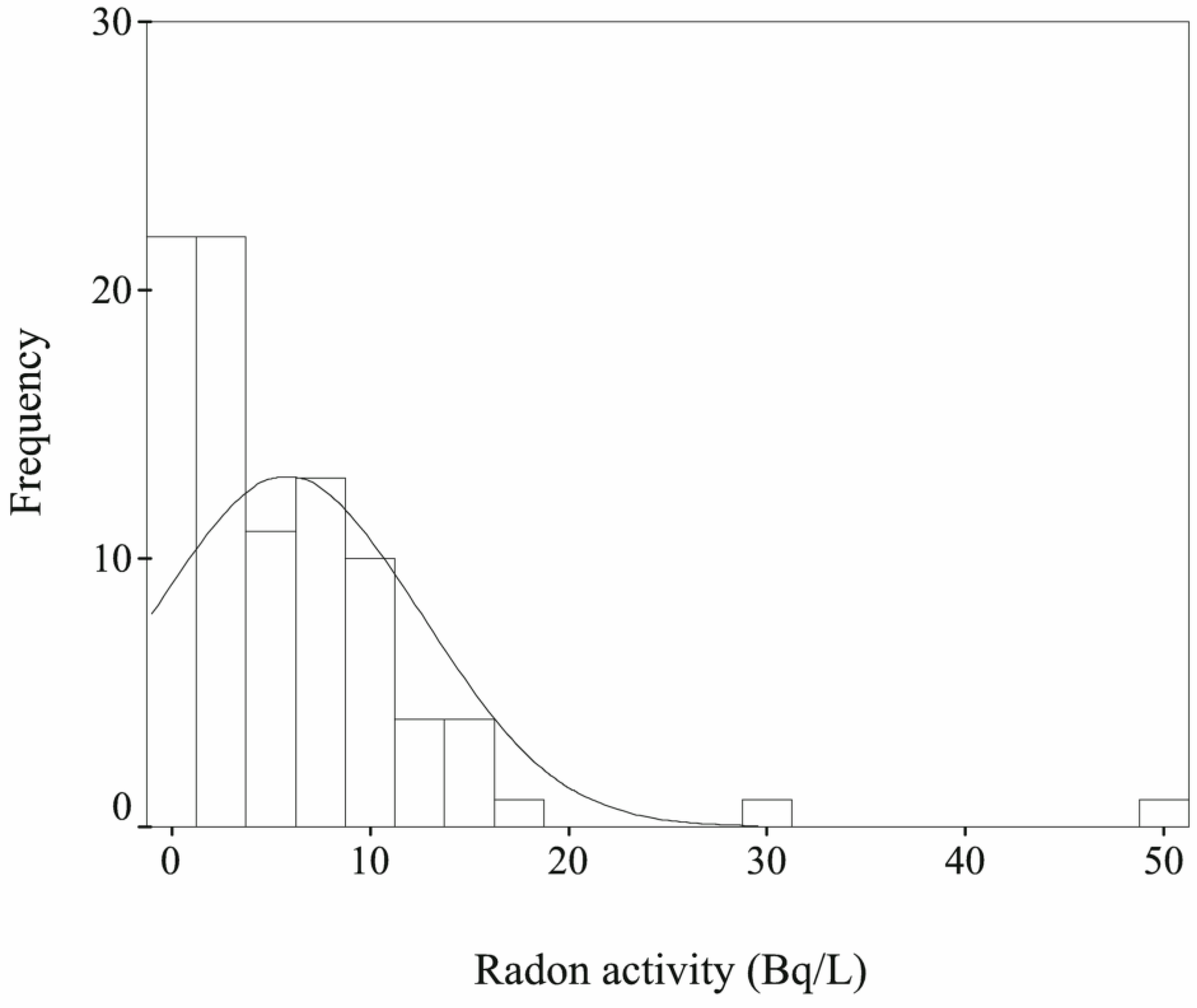
3.1. Radon Concentrations
| Water Type | N | Mean(Bq/L) | Standard Deviation | Range(Bq/L) |
|---|---|---|---|---|
| Public water | 73 | 4.63 | 4.75 | LLD-29.00 |
| Well | 16 | 11.41 | 11.00 | 1.45-49.00 |
| Water Type | N | Mean(Bq/L) | Range(Bq/L) | Country | Reference |
|---|---|---|---|---|---|
| Groundwater | 260 | 2.63 | 0.9–5.1 | India | [9] |
| Well | 70 | - | Background–3.8 | Mexico | [10] |
| Well | 27 | 9.28 | 1.42–53.64 | Turkey | [11] |
| Tap | 19 | 5.65 | 0.91–12.58 | Turkey | [11] |
| Groundwater | 89 | 1.92 | 0.42–10.52 | Poland | [12] |
| Surface water | 1511 | 15.4 | 0.5–129.3 | Romaina | [13] |
| Groundwater | |||||
| Well | |||||
| Spring water |
| No. | Water Type | Before Treatment (Bq/L) | After Treatment (Bq/L) | Consumers’ Tap (Bq/L) |
|---|---|---|---|---|
| WS1 | Public water | 32.63 ± 1.75 | 28.53 ± 2.23 | 29.00 ± 0.22 |
| WS2 | Public water | 12.19 ± 0.57 | 7.87 ± 1.12 | 9.50 ± 1.12 |
| WS3 | Public water | 12.39 ± 0.93 | 11.23 ± 0.66 | 11.37 ± 1.23 |
| WS4 | Public water | 7.59 ± 1.36 | 3.56 ± 0.86 a | 3.68 ± 0.81 |
| WS5 | Public water | 15.68 ± 1.52 | 3.56 ± 0.46 b | - |
| WS6 | Public water | 11.72 ± 1.61 | 10.80 ± 1.33 | - |
| WS7 | Public water | 11.72 ± 1.61 | 11.12 ± 0.87 | - |
| WS8 | Public water | 13.19 ± 1.32 | 11.12 ± 0.87 | - |
| WS9 | Public water | 19.44 ± 1.11 | 19.53 ± 1.38 | - |
| WS10 | Public water | 14.45 ± 1.85 | 4.61 ± 0.36 b | - |
| WS11 | Public water | 16.58 ± 0.54 | 4.56 ± 0.42 b | - |
| WS12 | Public water | 18.19 ± 2.28 | 5.59 ± 0.93 b | - |
| WS13 | Public water | 26.19 ± 1.60 | 24.98 ± 1.37 | - |
| WS14 | Well | 14.71 ± 0.97 | - | 14.69 ± 0.39 |
| WS15 | Well | 7.48 ± 0.43 | - | 8.23 ± 1.15 |
| WS16 | Well | 14.28 ± 0.82 | - | 17.94 ± 0.67 |
| WS17 | Well | 49.00 ±2.84 | - | 52.85 ± 2.08 |
3.2. Effective Dose Calculation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kitto, M.E.; Kuhland, M.K.; Dansereau, R.E. Direct comparison of three methods for the determination of radon in well water. Health Phys. 1996, 70, 358–362. [Google Scholar]
- National Research Council. Risk Assessment of Radon in Drinking Water; National Academy Press, National Research Council: Washington, DC, USA, 1999. [Google Scholar]
- National Primary Drinking Water Regulation; Environmental Protection Agency: Washington, DC, USA, 1991.
- Report to Congress: Radon in Drinking Water Regulations. Available online: http://water.epa.gov/lawsregs/rulesregs/sdwa/radon/upload/epa815r12002.pdf (accessed on 30 May 2014).
- Guidelines for drinking-water quality. In Radiological Aspects, 4th ed.; World Health Organization: Geneva, Switzerland, 2011; Chapter 9.
- European Union Commission Recommendation on the Protection of the Public against Exposure to Radon in Drinking Water Supplies; Official Journal of the European Communities: Brussels, Belgium, 2001.
- Synnott, H.; Fenton, D. An Evaluation of Radon Reference Levels and Radon Measurement Techniques and Protocols in European Countries; Radiological Protection Institute of Ireland: Dublin, Ireland, 2005. [Google Scholar]
- Todorovic, N.; Nikolov, J.; Forkapic, S.; Bikit, I.; Mrdja, D.; Krmar, M. Public exposure to radon in drinking water in Serbia. Appl. Radia. Isot. 2012, 70, 543–549. [Google Scholar]
- Duggal, V.; Mehra, R.; Rani, A. Determination of 222Rn level in groundwater using a RAD7 detector in the Bathinda district of Punjab, India. Radiat. Prot. Dosim. 2013, 156, 239–245. [Google Scholar]
- Vázquez-López, C.; Zendejas-Leal, B.E.; Golzarri, J.I.; Espinosa, G. A survey of 222Rn in drinking water in Mexico City. Radiat. Prot. Dosim. 2011, 145, 320–324. [Google Scholar]
- Akar, T.U.; Gurler, O.; Akkaya, G.; Kilic, N.; Yalcin, S.; Kaynak, G.; Gundogdu, O. Evaluation of radon concentration in well and tap waters in Bursa, Turkey. Radiat. Prot. Dosim. 2012, 150, 207–212. [Google Scholar]
- Bem, H.; Plota, U.; Staniszewska, M.; Bem, E.M.; Mazurek, D. Radon (222Rn) in underground drinking water supplies of the southern greater Poland region. J. Radioanal. Nucl. Chem. 2013, 299, 1307–1312. [Google Scholar]
- Cosma, C.; Moldovan, M.; Dicu, T.; Kovacs, T. Radon in water from Transylvania (Romania). Radiat. Meas. 2008, 43, 1423–1428. [Google Scholar]
- Weigel, V.F. Radon. Chem. Ztg. 1978, 102, 287–299. [Google Scholar]
- Lu, X.W. Radon concentration in drinking water and its equivalent dose to the dweller in main city of northwestern china. Environ. Chem. 2004, 23, 345–348. (in Chinese). [Google Scholar]
- Chen, Y.B.; Chen, D.F.; Zhang, B.; Zeng, D.C.; He, S.Y. Radon content in drinking water in partial city in China. Chin. J. Radiol. Med. Prot. 1994, 14, 366–369. (in Chinese). [Google Scholar]
- Gruber, V.; Maringer, F.J.; Landstetter, C. Radon and other natural radionuclides in drinking water in Austria: Measurement and assessment. Appl. Radia. Isot. 2009, 67, 913–917. [Google Scholar]
- Basic Safety Standards for Protection against Ionizing Radiation and for Safety of Radiation Sources; International Atomic Energy Agency: Vienna, Austria, 1996.
- Kendall, G.M.; Smith, T.J. Doses to organs and tissues from radon and its decay products. J. Radiol. Prot. 2002, 22, 389–406. [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and Effects of Ionizing Radiation; United Nations: New York, NY, USA, 2000. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-Y.; Ma, Y.-Z.; Cui, H.-X.; Liu, J.-X.; Sun, Y.-R.; Shang, B.; Su, X. Radon Concentrations in Drinking Water in Beijing City, China and Contribution to Radiation Dose. Int. J. Environ. Res. Public Health 2014, 11, 11121-11131. https://doi.org/10.3390/ijerph111111121
Wu Y-Y, Ma Y-Z, Cui H-X, Liu J-X, Sun Y-R, Shang B, Su X. Radon Concentrations in Drinking Water in Beijing City, China and Contribution to Radiation Dose. International Journal of Environmental Research and Public Health. 2014; 11(11):11121-11131. https://doi.org/10.3390/ijerph111111121
Chicago/Turabian StyleWu, Yun-Yun, Yong-Zhong Ma, Hong-Xing Cui, Jian-Xiang Liu, Ya-Ru Sun, Bing Shang, and Xu Su. 2014. "Radon Concentrations in Drinking Water in Beijing City, China and Contribution to Radiation Dose" International Journal of Environmental Research and Public Health 11, no. 11: 11121-11131. https://doi.org/10.3390/ijerph111111121




