Is Left Ventricular Global Longitudinal Strain by Two-Dimensional Speckle Tracking Echocardiography in Sepsis Cardiomyopathy Ready for Prime Time Use in the ICU?
Abstract
:1. Introduction
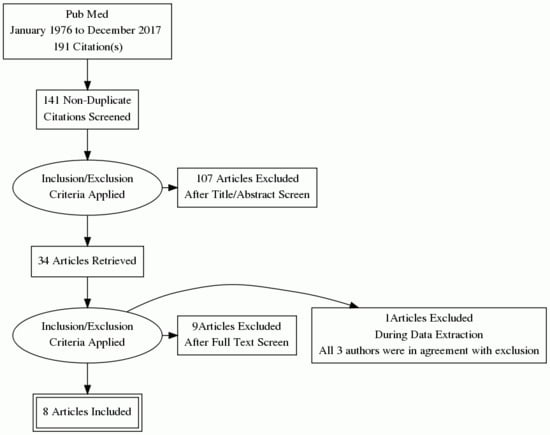
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sevilla Berrios, R.A.; O’Horo, J.C.; Velagapudi, V.; Pulido, J.N. Correlation of left ventricular systolic dysfunction determined by low ejection fraction and 30-day mortality in patients with severe sepsis and septic shock: A systematic review and meta-analysis. J. Crit. Care 2014, 29, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.M.; Shelhamer, J.H.; Bacharach, S.L.; Green, M.V.; Natanson, C.; Frederick, T.M.; Damske, B.A.; Parrillo, J.E. Profound but reversible myocardial depression in patients with septic shock. Ann. Intern. Med. 1984, 100, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W.J.; Tschope, C.; Sanderson, J.E.; Rusconi, C.; Flachskampf, F.A.; Rademakers, F.E.; Marino, P.; Smiseth, O.A.; De Keulenaer, G.; Leite-Moreira, A.F.; et al. How to diagnose diastolic heart failure: A consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur. Heart J. 2007, 28, 2539–2550. [Google Scholar] [CrossRef] [PubMed]
- Vignon, P.; Huang, S.J. Global longitudinal strain in septic cardiomyopathy: The hidden part of the iceberg? Intensive Care Med. 2015, 41, 1851–1853. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.H.; Cleland, J.G. Reliability of reporting left ventricular systolic function by echocardiography: A systematic review of 3 methods. Am. Heart J. 2003, 146, 388–397. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Grant, A.D.; Negishi, T.; Plana, J.C.; Popovic, Z.B.; Marwick, T.H. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: Application to patients undergoing cancer chemotherapy. J. Am. Coll. Cardiol. 2013, 61, 77–84. [Google Scholar] [CrossRef]
- Wood, P.W.; Choy, J.B.; Nanda, N.C.; Becher, H. Left ventricular ejection fraction and volumes: It depends on the imaging method. Echocardiography 2014, 31, 87–100. [Google Scholar] [CrossRef]
- Collier, P.; Phelan, D.; Klein, A. A Test in Context: Myocardial Strain Measured by Speckle-Tracking Echocardiography. J. Am. Coll. Cardiol. 2017, 69, 1043–1056. [Google Scholar] [CrossRef]
- Kalam, K.; Otahal, P.; Marwick, T.H. Prognostic implications of global LV dysfunction: A systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 2014, 100, 1673–1680. [Google Scholar] [CrossRef]
- Yang, F.; Chen, Y.; Zheng, R.; Ma, Y.; Yu, H.; Zhang, W.; Zhang, Y. Two-dimensional speckle tracking imaging in assessing the left ventricular systolic function and its dynamic changes of patients with septic shock. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2017, 29, 721–725. [Google Scholar] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.L.; Ramsay, G.; et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 2003, 31, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Lanspa, M.J.; Shahul, S.; Hersh, A.; Wilson, E.L.; Olsen, T.D.; Hirshberg, E.L.; Grissom, C.K.; Brown, S.M. Associations among left ventricular systolic function, tachycardia, and cardiac preload in septic patients. Ann. Intensive Care 2017, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Zaky, A.; Gill, E.A.; Lin, C.P.; Paul, C.P.; Bendjelid, K.; Treggiari, M.M. Characteristics of sepsis-induced cardiac dysfunction using speckle-tracking echocardiography: A feasibility study. Anaesth. Intensive Care 2016, 44, 65–76. [Google Scholar] [PubMed]
- Chang, W.T.; Lee, W.H.; Lee, W.T.; Chen, P.S.; Su, Y.R.; Liu, P.Y.; Liu, Y.W.; Tsai, W.C. Left ventricular global longitudinal strain is independently associated with mortality in septic shock patients. Intensive Care Med. 2015, 41, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- De Geer, L.; Engvall, J.; Oscarsson, A. Strain echocardiography in septic shock—A comparison with systolic and diastolic function parameters, cardiac biomarkers and outcome. Crit. Care 2015, 19, 122. [Google Scholar] [CrossRef]
- Landesberg, G.; Jaffe, A.S.; Gilon, D.; Levin, P.D.; Goodman, S.; Abu-Baih, A.; Beeri, R.; Weissman, C.; Sprung, C.L.; Landesberg, A. Troponin elevation in severe sepsis and septic shock: The role of left ventricular diastolic dysfunction and right ventricular dilatation*. Crit. Care Med. 2014, 42, 790–800. [Google Scholar] [CrossRef]
- Orde, S.R.; Pulido, J.N.; Masaki, M.; Gillespie, S.; Spoon, J.N.; Kane, G.C.; Oh, J.K. Outcome prediction in sepsis: Speckle tracking echocardiography based assessment of myocardial function. Crit. Care 2014, 18, R149. [Google Scholar] [CrossRef]
- Palmieri, V.; Innocenti, F.; Guzzo, A.; Guerrini, E.; Vignaroli, D.; Pini, R. Left Ventricular Systolic Longitudinal Function as Predictor of Outcome in Patients With Sepsis. Circ. Cardiovasc. Imaging 2015, 8, e003865. [Google Scholar] [CrossRef]
- Sanfilippo, F.; Corredor, C.; Fletcher, N.; Tritapepe, L.; Lorini, F.L.; Arcadipane, A.; Vieillard-Baron, A.; Cecconi, M. Left ventricular systolic function evaluated by strain echocardiography and relationship with mortality in patients with severe sepsis or septic shock: A systematic review and meta-analysis. Crit. Care 2018, 22, 183. [Google Scholar] [CrossRef]
- Vallabhajosyula, S.; Jentzer, J.C. Global Longitudinal Strain Using Speckle-Tracking Echocardiography in Sepsis. J. Intensive Care Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Velagapudi, V.M.; Tighe, D. Data Abstraction Error in Systematic Review of Global Longitudinal Strain Using Speckle-Tracking Echocardiography as a Mortality Predictor in Sepsis. J. Intensive Care Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Takeuchi, M.; Mizukoshi, K.; Wu, V.C.; Lin, F.C.; Negishi, K.; Nakatani, S.; Otsuji, Y. Intervendor variability of two-dimensional strain using vendor-specific and vendor-independent software. J. Am. Soc. Echocardiogr. 2015, 28, 630–641. [Google Scholar] [CrossRef]
- Farsalinos, K.E.; Daraban, A.M.; Unlu, S.; Thomas, J.D.; Badano, L.P.; Voigt, J.U. Head-to-Head Comparison of Global Longitudinal Strain Measurements among Nine Different Vendors: The EACVI/ASE Inter-Vendor Comparison Study. J. Am. Soc. Echocardiogr. 2015, 28, 1171–1181.e1172. [Google Scholar] [CrossRef] [PubMed]
| Study | Setting | Geography | Study Design | Study Period | Total Patients | Excluded Pts | No. of Centers | Inclusion Criteria | Primary Outcome | Secondary Outcomes | Cut off Threshold GLS | Echo Machine | Software | Timing | Operator | r2 Intra | r2 Inter | Ventilator | Shock |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | |||||||||||||||||
| Chang et al., 2015 [16] | University Hospital ICU | Taiwan | Prospective observational | January 2011–June 2013 | 111 | 25 | 1 | Septic shock | ICU mortality | Hospital mortality | −13 | GE Vivid-I or Q | EchoPAC | <24 h | 2 blinded | 0.88 | 0.94 | ||
| De Geer et al., 2015 [17] | University Hospital mixed ICU | Sweden | Prospective observational | October 2012–September 2014 | 44 | 7 | 1 | Septic shock | ICU mortality | 30 days, 90 days mortality | −15 | GE Vivid E9 | EchoPAC 112 | <24 h | 1 | 0.92 | 84 | ||
| Landesberg et al., 2014 [18] | Tertiary academic institute | Israel | Prospective observational | April 2009–March 2011 | 106 | 14 | 1 | Severe sepsis and septic shock | hs-cardiac troponin elevation | Hospital mortality | Philips IE33 | Philips Qlab 8.1 | <24 h | 2 blinded | 100 | ||||
| Orde et al., 2014 [19] | Tertiary academic center | USA | Prospective observational | August 2007–January 2009 | 60 | 13 | 1 | Severe sepsis and septic shock | 30 days mortality | 6 months mortality | −17 | GE Vivid 7 | Syngo Velocity Vector | <24 h | 3 | 0.9 ± 0.9 | 0.8 ± 0.5 | 65 | |
| Palmeieri et al., 2015 [20] | ED-HDU academic center | Italy | Prospective observational | October 2012–April 2015 | 115 | 34 | 1 | Sepsis and septic shock | 28 days mortality | 7 days mortality | Philips IE33 | Philips Qlab 8.1 | <24 h | 3 blinded | 0.9 | 0.82 | 0 | 39 | |
| Zaky et al., 2016 [15] | Tertiary care center any ICU | USA | Retrospective observational | January 2008–December 2011 | 54 | 43 | 1 | Sepsis and/or septic shock | In-hospital mortality | Mechanical ventilation, ICU & hospital stay | −15 | Philips IE33 | Philips Qlab 4.1 | <7 days | 5 | 0.83 | 0.84 | ||
| Lanspa et al., 2017 [14] | Tertiary academic centers (2 hospitals, 3 ICUs) | USA | Prospective observational | October 2012–November 2015 | 298 | 154 | 2 | Severe sepsis or septic shock | In-hospital mortality, 28 days mortality | Organ failure free days | −17 | Philips IE33 or CX50 | Image Arena | <24 h | 31 | 39 | |||
| Yang et al., 2017 [11] | Academic center | China | prospective observational | January 2016–April 2017 | 58 | 7 | 1 | Septic shock per sepsis 3 | 28 days mortality | GE Vivid-Q | EchoPAC | <24 h, day 1,3,7,14 | 2 | 100 |
| Study | Exclusion Criteria |
|---|---|
| Chang et al., 2015 [16] | none |
| De Geer et al., 2015 [17] | death < 24 h, treatment limitations, no consent, Heart Failure, Ischemic Heart Disease |
| Landesberg et al., 2014 [18] | Moderate mitral/aortic disease, poor windows, Atrial Fibrillation, arrhythmia, Regional Wall Motion Anamoly |
| Orde et al., 2014 [19] | pregnancy, congenital Heart Disease, poor image quality, prosthetic valves, cardiomyopathy, moderate or severe valve disease |
| Palmeieri et al., 2015 [20] | poor windows, greater than moderate aortic or mitral valve disease |
| Zaky et al., 2016 [15] | Age < 18 years, Atrial Fibrillation, LVEF < 40%, valve disease, valve replacement, ICDs, poor Echo views |
| Lanspa et al., 2017 [14] | echo > 24 h, poor image quality |
| Yang et al., 2017 [11] | Myocardial Infraction, congenital, valvular heart disease, hospitalization < 24 h, malignancy, liver, kidney failure, pericardial effusion, advanced malignancy, poor image quality |
| Study | ICU Non Survivor GLS | ICU Survivor GLS | Hospital Non Survivor GLS | Hospital Survivor GLS | 28 Days Non Survivor GLS | 28 Days Survivor GLS | 30 Days Non Survivor GLS | 30 Days Survivor GLS | 90 Days Non Survivor GLS | 90 Days Survivor GLS | 6 Months Non Survivor GLS | 6 Months Survivor GLS | Abnormal GLS Hospital Mortality | Abnormal GLS Hospital Mortality | Normal GLS Hospital Mortality | Abnormal GLS 28 Days Mortality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD in % | Alive n (%) | Dead n (%) | Dead n (%) | Dead n (%) | ||||||||||||
| Chang et al., 2015 [16] | −11.8 ± 4.5 | −15 ± 3.6 | −12.4 ± 4.9 | −14.9 ± 3.4 | ||||||||||||
| De Geer et al., 2015 [17] | −15 (−19.7 to −11) | −17.2 (−20 to −13) | −14.7 (−19 to −10.6) | −17.4 (−20.5 to −13.6) | ||||||||||||
| Landesberg et al., 2014 [18] | −12.3 ± 3.6 | −13.7 ± 2.7 | ||||||||||||||
| Orde et al., 2014 [19] | −14.6 ± 4.3 | −13.92 ± 4.2 | −14.28 ± 4.6 | −14 ± 4 | ||||||||||||
| Palmeieri et al., 2015 [20] | −9.1 ± 3.6 | −10.8 ± 3.2 | ||||||||||||||
| Zaky et al., 2016 [15] | 24 (80) | 12 (66.7) | ||||||||||||||
| Lanspa et al., 2017 [14] | 47 (22) | 31 (17) | 54 (25) | |||||||||||||
| Yang et al., 2017 [11] | −15.98 ± 1.41 | −17.66 ± 1.22 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velagapudi, V.M.; Pidikiti, R.; Tighe, D.A. Is Left Ventricular Global Longitudinal Strain by Two-Dimensional Speckle Tracking Echocardiography in Sepsis Cardiomyopathy Ready for Prime Time Use in the ICU? Healthcare 2019, 7, 5. https://doi.org/10.3390/healthcare7010005
Velagapudi VM, Pidikiti R, Tighe DA. Is Left Ventricular Global Longitudinal Strain by Two-Dimensional Speckle Tracking Echocardiography in Sepsis Cardiomyopathy Ready for Prime Time Use in the ICU? Healthcare. 2019; 7(1):5. https://doi.org/10.3390/healthcare7010005
Chicago/Turabian StyleVelagapudi, Venu Madhav, Rahul Pidikiti, and Dennis A. Tighe. 2019. "Is Left Ventricular Global Longitudinal Strain by Two-Dimensional Speckle Tracking Echocardiography in Sepsis Cardiomyopathy Ready for Prime Time Use in the ICU?" Healthcare 7, no. 1: 5. https://doi.org/10.3390/healthcare7010005