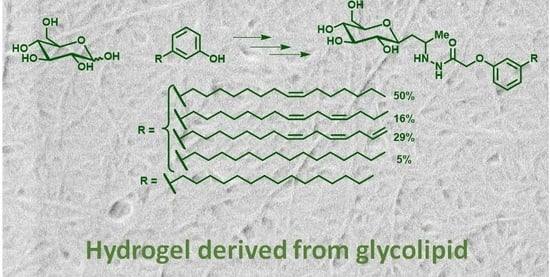
Supramolecular Gel Formation Based on Glycolipids Derived from Renewable Resources
Abstract
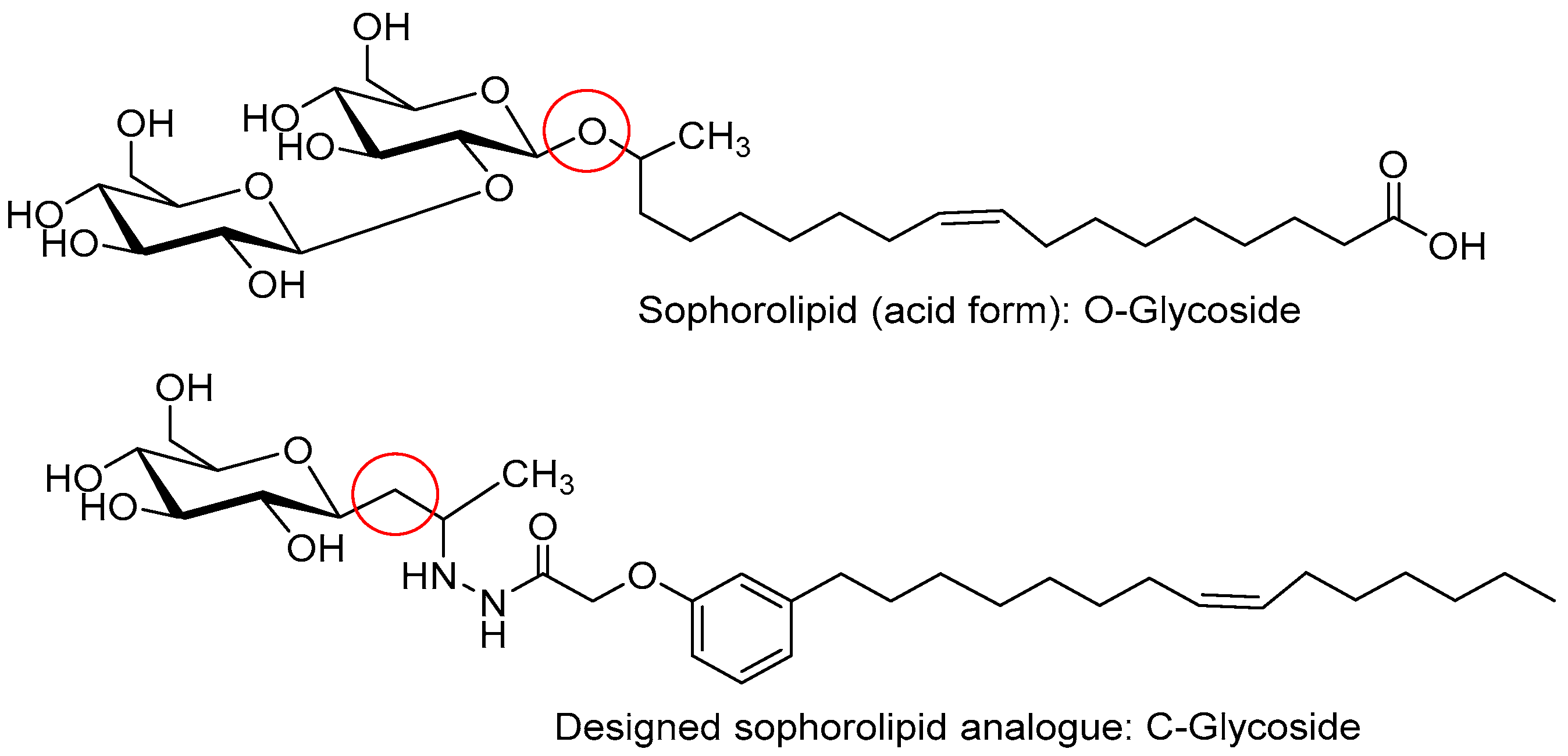
:1. Introduction
2. Results and Discussion
2.1. Gelation Studies
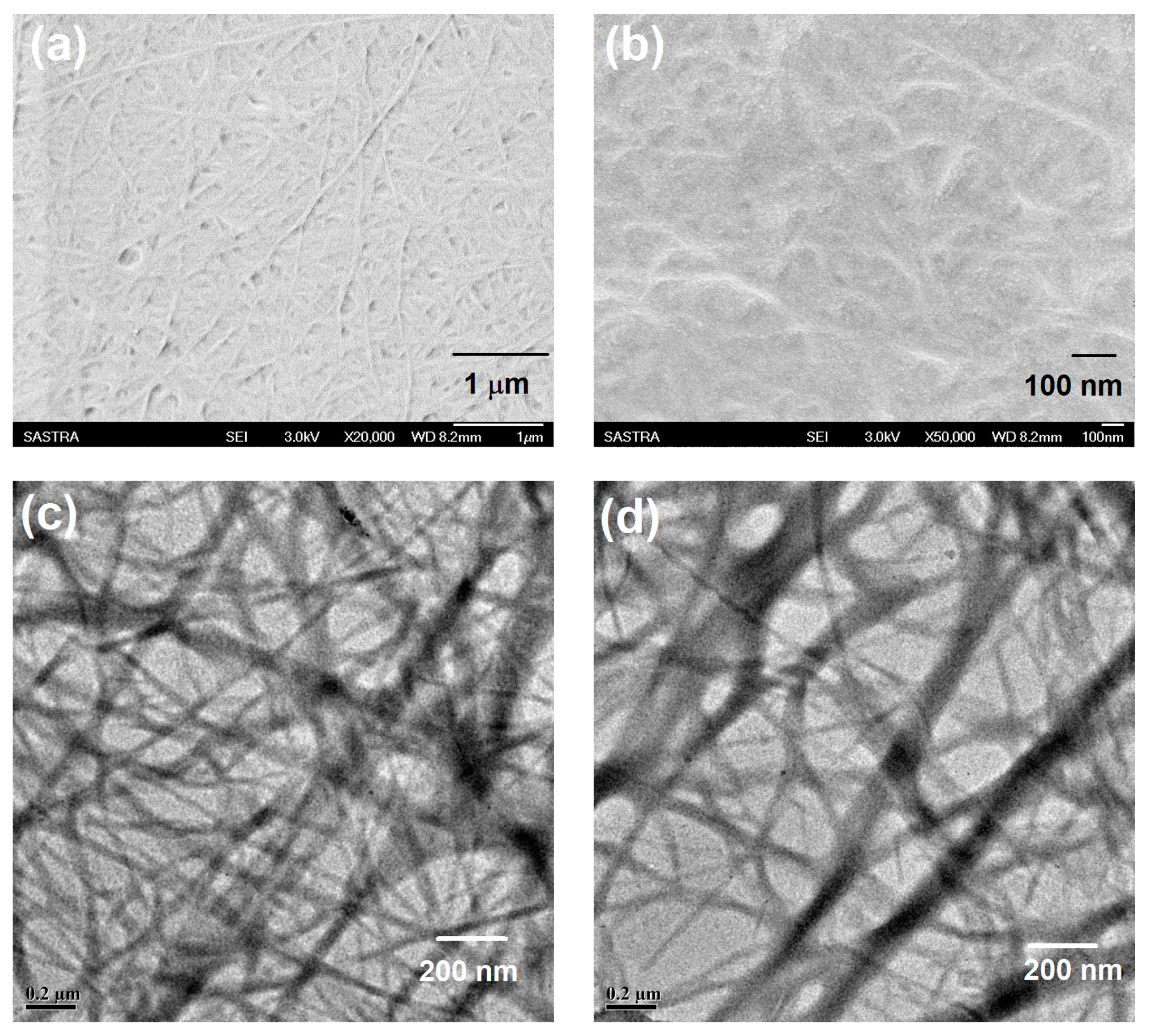
2.2. Morphological Analysis of the Gel
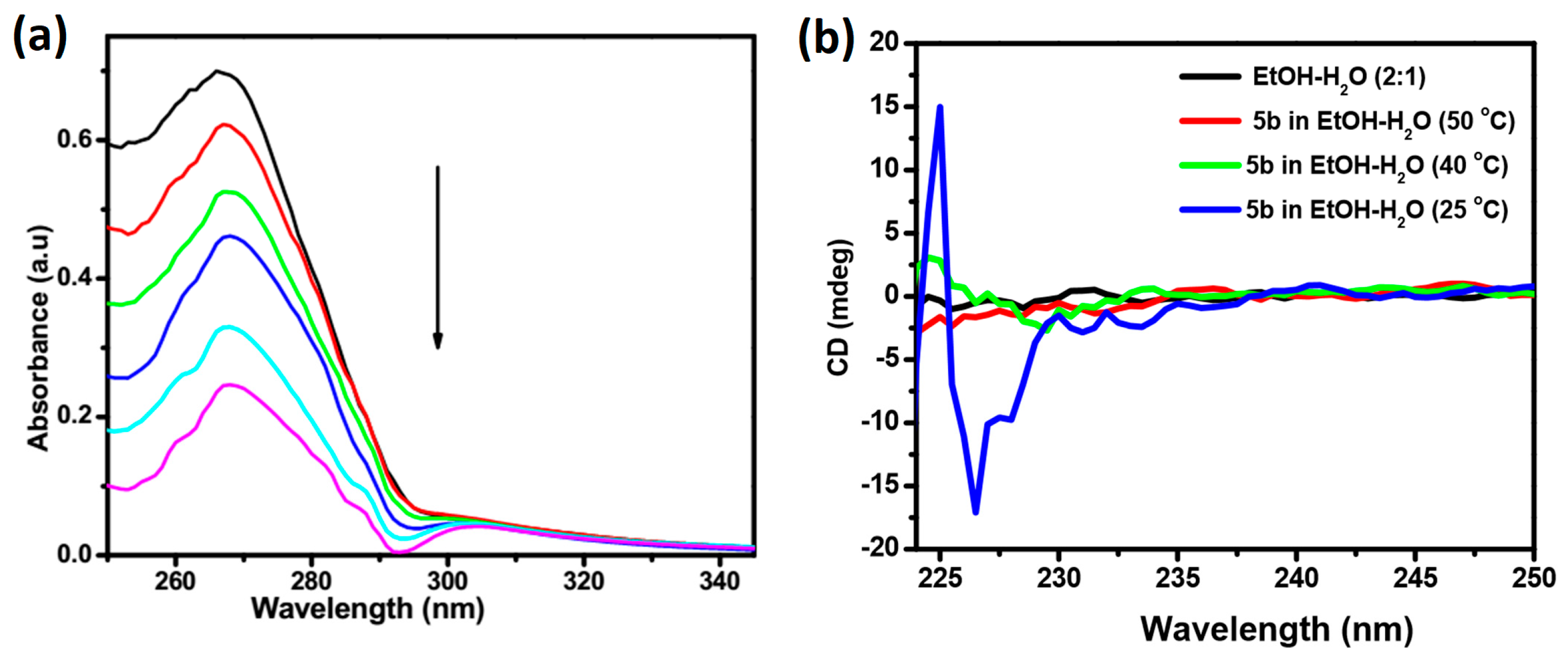
2.3. Absorption and CD Spectral Studies
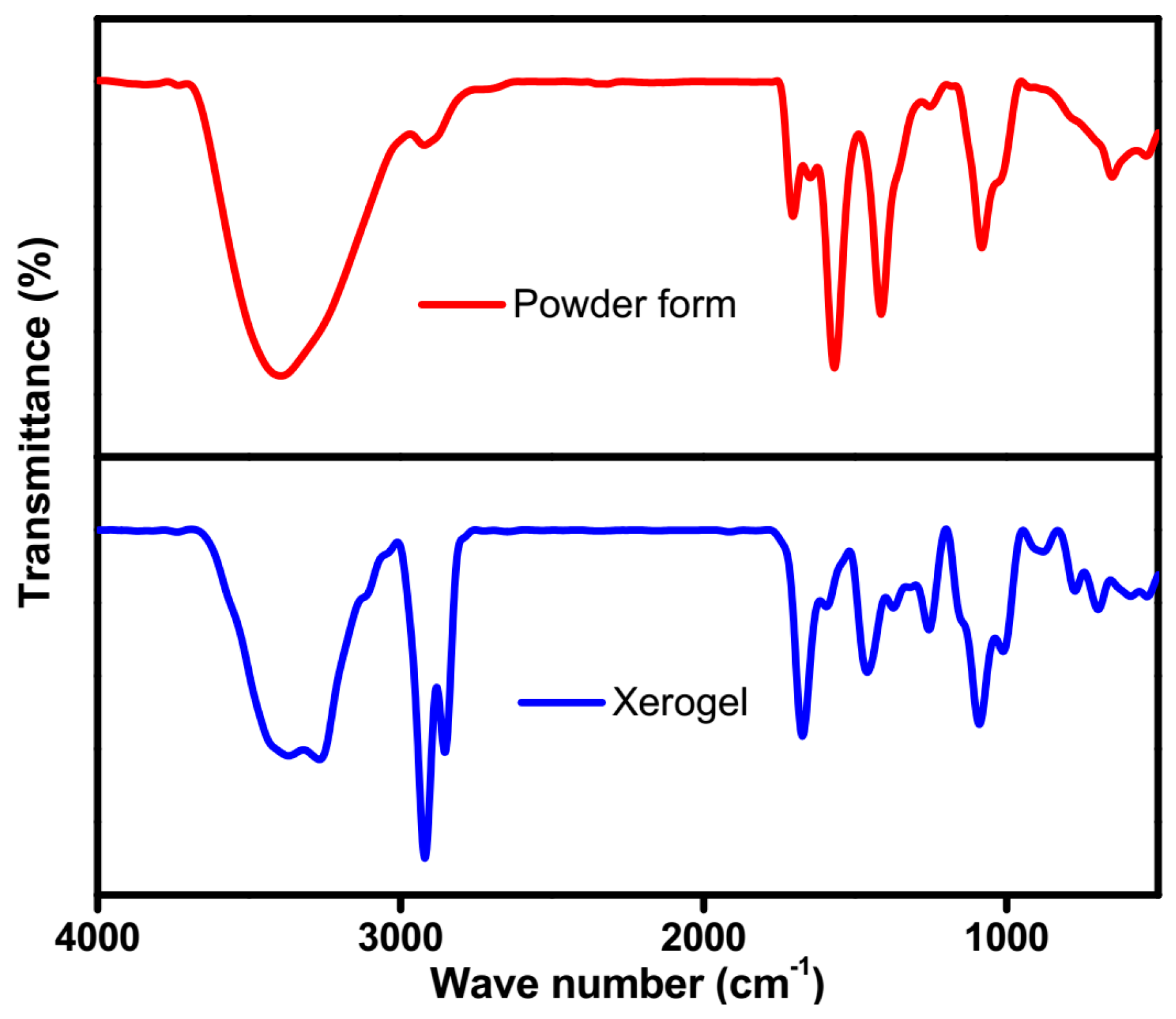
2.4. FTIR Studies
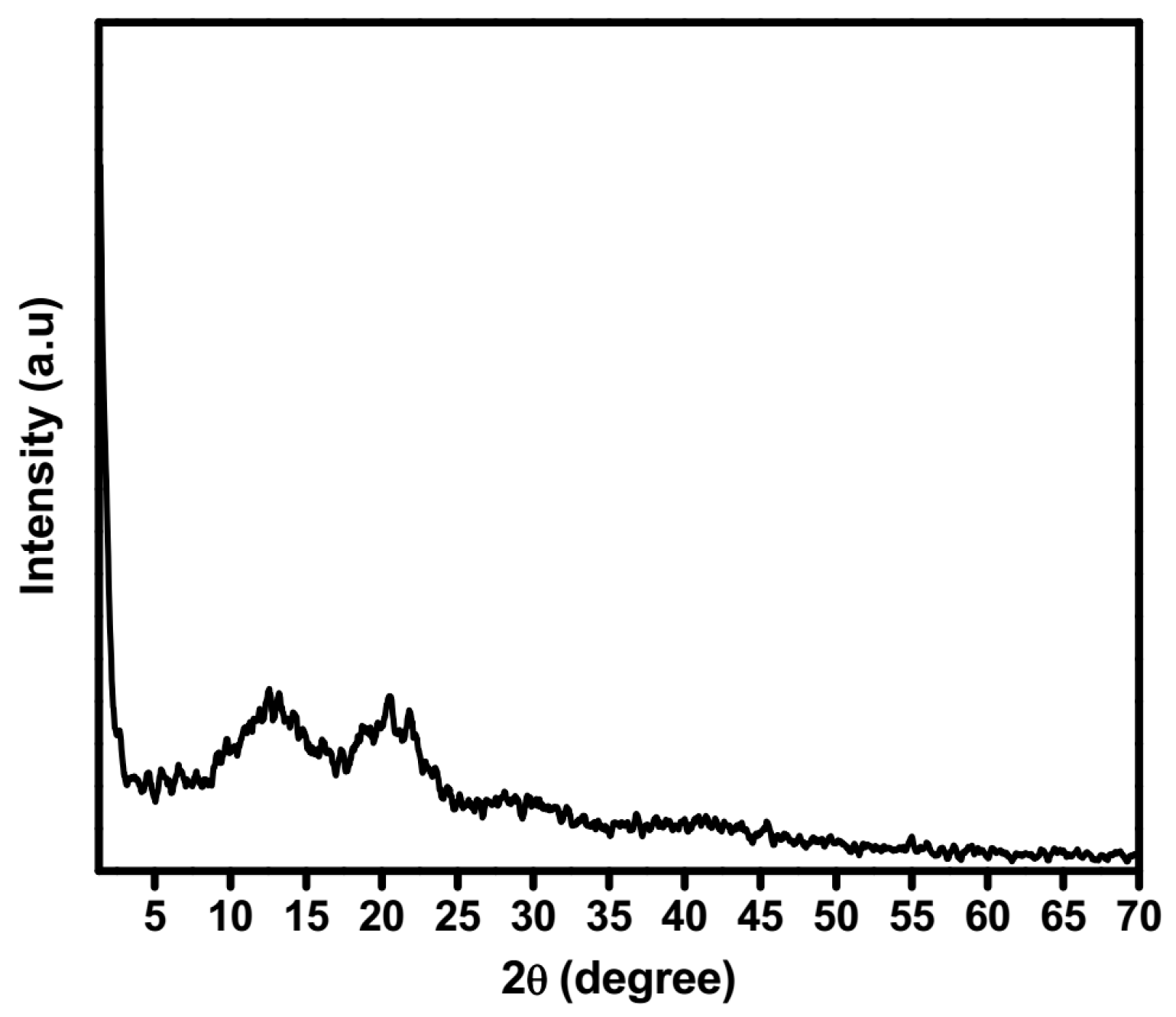
2.5. XRD Studies
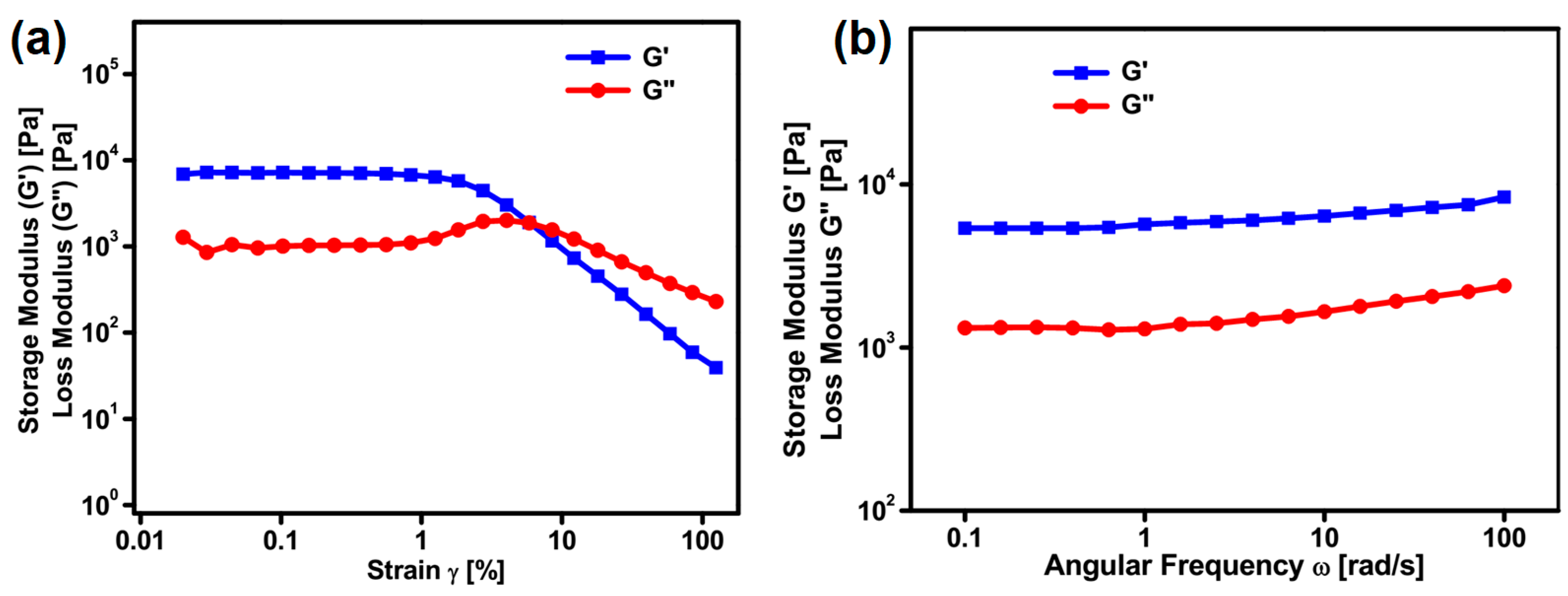
2.6. Rheological Studies
3. Conclusions
4. Materials and Methods
4.1. General Materials and Methods
4.2. Characterization Methods
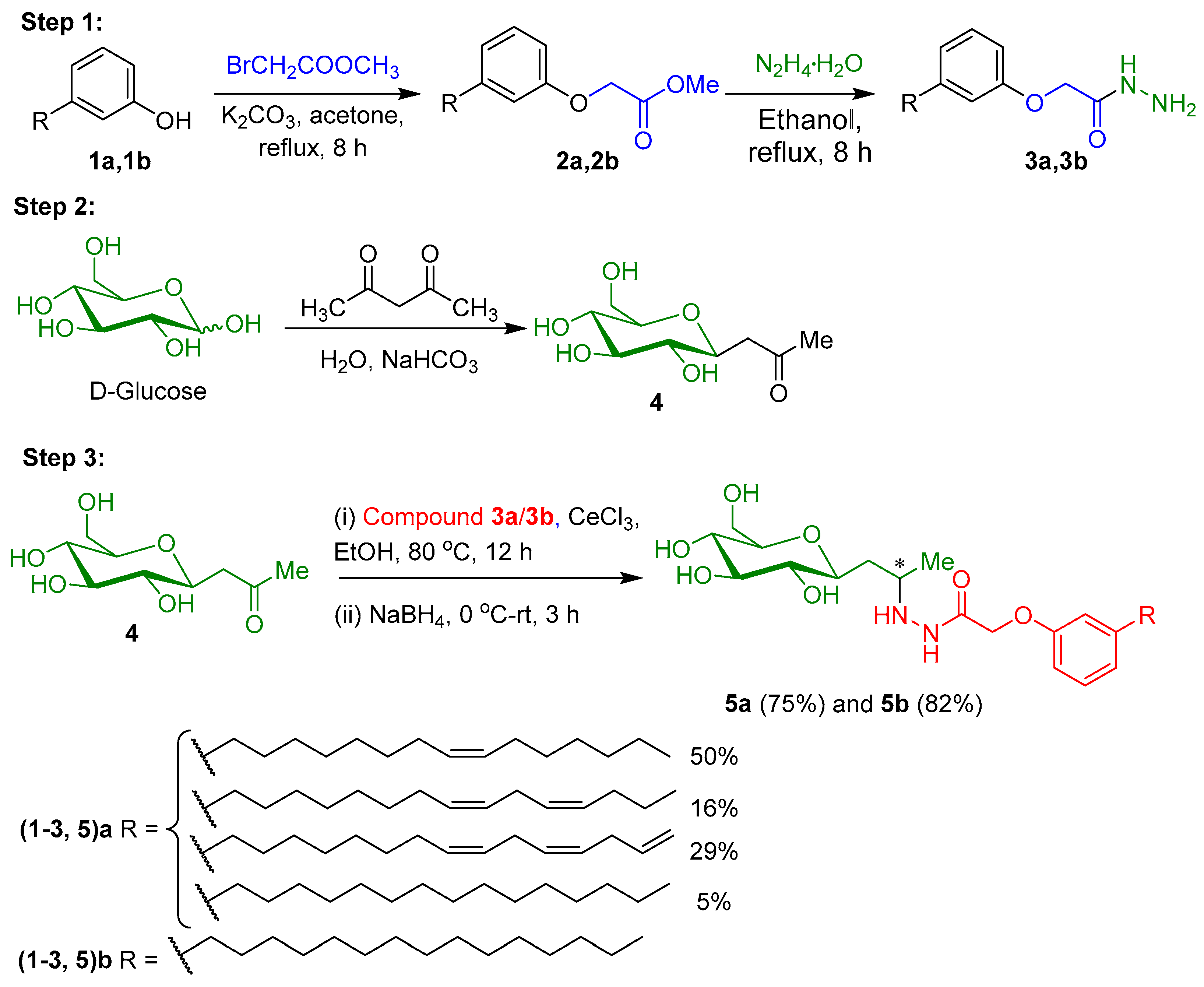
4.3. Synthesis
4.3.1. Synthesis of 2-(3-Alkylphenoxy)acetohydrazides, 3a and 3b
4.3.2. Synthesis of 1-(α-d-Glucopyranosyl)-propan-2-one 4
4.3.3. Synthesis of Glycolipids 5a and 5b
4.4. Gelation Method
4.5. UV-Visible and Circular Dichroism (CD) Spectral Analysis
4.6. X-ray Diffraction Studies
4.7. Morphological Analysis
4.8. Rheological Measurements
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lehn, J.-M. Supramolecular chemistry: Where from? Where to? Chem. Soc. Rev. 2017, 46, 2378–2379. [Google Scholar] [CrossRef] [PubMed]
- Amabilino, D.B.; Smith, D.K.; Steed, J.W. Supramolecular materials. Chem. Soc. Rev. 2017, 46, 2404–2420. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.C.; Parisi, J. Bottom-Up Self-Organization in Supramolecular Soft Matter: Principles and Prototypical Examples of Recent Advances; Springer: New York, NY, USA, 2015. [Google Scholar]
- Ciferri, A. Translation of molecular order to the macroscopic level. Chem. Rev. 2016, 116, 1353–1374. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Mai, Y.; Wu, D.; Zhang, F.; Feng, X. Two-dimensional soft nanomaterials: A fascinating world of materials. Adv. Mater. 2015, 403–427. [Google Scholar] [CrossRef] [PubMed]
- Vemula, P.K.; John, G. Crops: A Green Approach toward Self-Assembled Soft Materials. Acc. Chem. Res. 2008, 41, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Lehn, J.M. Perspectives in chemistry—Aspects of adaptive chemistry and materials. Angew. Chem. Int. Ed. 2015, 54, 3276–3289. [Google Scholar] [CrossRef] [PubMed]
- Okesola, B.O.; Smith, D.K. Applying low-molecular weight supramolecular gelators in an environmental setting—Self-Assembled gels as smart materials for pollutant removal. Chem. Soc. Rev. 2016, 45, 4226–4251. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.D.; Steed, J.W. Gels with sense: Supramolecular materials that respond to heat, light and sound. Chem. Soc. Rev. 2016, 45, 6546–6596. [Google Scholar] [CrossRef] [PubMed]
- Uto, K.; DeForest, C.A.; Kim, D.H. Soft Shape-Memory materials. In Biomaterials Nanoarchitectonics; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 237–251. [Google Scholar]
- Dastidar, P.; Roy, R.; Parveen, R.; Ganguly, S.; Majumder, J.; Paul, M. Chapter 2. Designing soft supramolecular materials using intermolecular interactions. In Monographs in Supramolecular Chemistry; Royal Society of Chemistry, Thomas Graham House: Cambridge, UK, 2017; pp. 37–74. [Google Scholar]
- Ghosh, S.; Praveen, V.K.; Ajayaghosh, A. The chemistry and applications of π-gels. Annu. Rev. Mater. Res. 2016, 46, 235–262. [Google Scholar] [CrossRef]
- Yoza, K.; Amanokura, N.; Ono, Y.; Akao, T.; Shinmori, H.; Takeuchi, M.; Shinkai, S.; Reinhoudt, D.N. Sugar-integrated gelators of organic solvents-their remarkable diversity in gelation ability and aggregate structure. Chem. Eur. J. 1999, 5, 2722–2729. [Google Scholar] [CrossRef]
- Jung, J.H.; John, G.; Masuda, M.; Yoshida, K.; Shinkai, S.; Shimizu, T. Self-Assembly of a sugar-based gelator in water: Its remarkable diversity in gelation ability and aggregate structure. Langmuir 2001, 17, 7229–7232. [Google Scholar] [CrossRef]
- Vibhute, A.M.; Muvvala, V.; Sureshan, K.M. A Sugar-based gelator for marine oil-spill recovery. Angew. Chem. Int. Ed. 2016, 5, 7782–7785. [Google Scholar] [CrossRef] [PubMed]
- Sukegawa, H.; Nishimura, T.; Yoshio, M.; Kajiyama, S.; Kato, T. One-dimensional supramolecular hybrids: Self-assembled nanofibrous materials based on a sugar gelator and calcite developed along an unusual axis. CrystEngComm 2017, 19, 1580–1584. [Google Scholar] [CrossRef]
- John, G.; Jadhav, S.R.; Menon, V.M.; John, V.T. Flexible optics: Recent developments in molecular gels. Angew. Chem. Int. Ed. 2012, 51, 1760–1762. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.R.; Hwang, H.; Huang, Q.; John, G. Medium-chain sugar amphiphiles: A new family of healthy vegetable oil structuring agents. J. Agric. Food Chem. 2013, 61, 12005–12011. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Masuda, M.; Minamikawa, H. Supramolecular nanotube architectures based on amphiphilic molecules. Chem. Rev. 2005, 105, 1401–1443. [Google Scholar] [CrossRef] [PubMed]
- Dave, H.; Gao, F.; Lee, J.H.; Liberatore, M.; Ho, C.C.; Co, C.C. Self-assembly in sugar-oil complex glasses. Nat. Mater. 2007, 6, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Vemula, P.K.; Li, J.; John, G. Enzyme catalysis: Tool to make and break amygdalin hydrogelators from renewable resources: A delivery model for hydrophobic drugs. J. Am. Chem. Soc. 2006, 128, 8932–8938. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; Todd, K.; Sherbourne, E.; Gross, R.A. Fundamental characterization of the micellar self-assembly of sophorolipid esters. Langmuir 2017, 33, 5760–5768. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Xu, C.; Wang, J.; Gao, W.; Akhverdiyeva, R.; Shah, V.; Gross, R. Supramolecular assemblies of a naturally derived sophorolipid. Langmuir 2014, 20, 7926–7932. [Google Scholar] [CrossRef] [PubMed]
- Claus, S.; Van Bogaert, I.N.A. Sophorolipid production by yeasts: A critical review of the literature and suggestions for future research. Appl. Microbiol. Biotechnol. 2017, 101, 7811–7821. [Google Scholar] [CrossRef] [PubMed]
- Andrade, T.d.J.A.d.S.; Araújo, B.Q.; Citó, A.M.d.G.L.; Da Silva, J.; Saffi, J.; Richter, M.F.; Ferraz, A.d.B.F. Antioxidant properties and chemical composition of technical Cashew Nut Shell Liquid (tCNSL). Food Chem. 2011, 126, 1044–1048. [Google Scholar] [CrossRef]
- Patel, R.N.; Bandyopadhyay, S.; Ganesh, A. Extraction of cashew (Anacardium occidentale) nut shell liquid using supercritical carbon dioxide. Bioresour. Technol. 2006, 97, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Ryu, M.; Oda, Y.; Igarashi, K.; Nagatsuka, A.; Furuta, T.; Sugiura, M. Novel characteristics of sophorolipids, yeast glycolipid biosurfactants, as biodegradable low-foaming surfactants. J. Biosci. Bioeng. 2009, 108, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, K.; Jenifer, P.; Prasad, Y.S.; Muthusamy, K.; John, G.; Nagarajan, S. A self-assembled π-conjugated system as an anti-proliferative agent in prostate cancer cells and a probe for intra-cellular imaging. RSC Adv. 2014, 4, 48433–48437. [Google Scholar] [CrossRef]
- Faure, L.; Nagarajan, S.; Hwang, H.; Montgomery, C.L.; Khan, B.R.; John, G.; Koulen, P.; Blancaflor, E.B.; Chapman, K.D. Synthesis of phenoxyacyl-ethanolamides and their effects on fatty acid amide hydrolase activity. J. Biol. Chem. 2014, 289, 9340–9351. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, K.; Prasad, Y.S.; Maheswari, C.U.; Sridharan, V.; John, G.; Nagarajan, S. Stimuli responsive hydrogels derived from a renewable resource: Synthesis, self-assembly in water and application in drug delivery. J. Mater. Chem. B 2015, 3, 5560–5568. [Google Scholar] [CrossRef]
- Lalitha, K.; Nagarajan, S. Strongly fluorescent organogels and self-assembled nanostructures from pyrene coupled coumarin derivatives: Application in cell imaging. J. Mater. Chem. B 2015, 3, 5690–5701. [Google Scholar] [CrossRef]
- Lalitha, K.; Prasad, Y.S.; Sridharan, V.; Maheswari, C.U.; John, G.; Nagarajan, S. A renewable resource-derived thixotropic self-assembled supramolecular gel: Magnetic stimuli responsive and real-time self-healing behaviour. RSC Adv. 2015, 5, 77589–77594. [Google Scholar] [CrossRef]
- Lalitha, K.; Sridharan, V.; Maheswari, C.U.; Vemula, P.K.; Nagarajan, S. Morphology transition in helical tubules of a supramolecular gel driven by metal ions. Chem. Commun. 2017, 53, 1538–1541. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, K.; Sandeep, M.; Prasad, Y.S.; Sridharan, V.; Maheswari, C.U.; Srinandan, C.S.; Nagarajan, S. Intrinsic hydrophobic antibacterial thin film from renewable resources: Application in the development of anti-biofilm urinary catheters. ACS Sustain. Chem. Eng. 2017, 5, 436–449. [Google Scholar] [CrossRef]
- Prasad, Y.S.; Sandeep, M.; Lalitha, K.; Ranjitha, K.; Barbhaiwala, S.; Sridharan, V.; Maheswari, C.U.; Srinandan, C.S.; Nagarajan, S. Disassembly of bacterial biofilms by the self-assembled glycolipids derived from renewable resources. ACS Appl. Mater. Interfaces 2017, 9, 40047–40058. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, K.; Sridharan, V.; Maheswari, C.U.; Nagarajan, S. Lipase catalyzed synthesis of fluorescent glycolipids: Gelation studies and graphene incorporated self-assembled sheet formation for semiconductor applications. Green Chem. 2016, 18, 3722–3731. [Google Scholar] [CrossRef]
- Lalitha, K.; Muthusamy, K.; Prasad, Y.S.; Vemula, P.K.; Nagarajan, S. Recent developments in β-C-glycosides: Synthesis and applications. Carbohydr. Res. 2015, 402, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular hydrogelators and hydrogels: From soft matter to molecular biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, S.; Ma, M.; Yang, M.; Wang, Y.; Hao, A.; Xing, P. Tuning of gel morphology with supramolecular chirality amplification using a solvent strategy based on an fmoc-amino acid building block. New J. Chem. 2016, 40, 5568–5576. [Google Scholar] [CrossRef]
- Himabindu, M.; Palanisamy, A. Ultrasound- and temperature-induced gelation of gluconosemicarbazide gelator in DMSO and water mixtures. Gels 2017, 3, 12. [Google Scholar] [CrossRef]
- Bachl, J.; Sampedro, D.; Mayr, J.; Díaz Díaz, D. Ultrasonication-enhanced gelation properties of a versatile amphiphilic formamidine-based gelator exhibiting both organogelation and hydrogelation abilities. Phys. Chem. Chem. Phys. 2017, 19, 22981–22994. [Google Scholar] [CrossRef] [PubMed]
- Hashemnejad, S.M.; Kundu, S. Probing gelation and rheological behavior of a self-assembled molecular gel. Langmuir 2017, 33, 7769–7779. [Google Scholar] [CrossRef] [PubMed]
| S.No. | Solvent/Oil | Observation a (CGC % w/v) | |
|---|---|---|---|
| 5a | 5b | ||
| 1 | Cyclohexane | S | S |
| 2 | Toluene | S | S |
| 3 | 1,2-Dichlorobenzene | S | S |
| 4 | DMSO | S | S |
| 5 | DMF | S | S |
| 6 | DMSO + Water (1:5) | PG | G (0.67) |
| 7 | DMF + Water (1:5) | PG | G (1.5) |
| 8 | Heavy paraffin Oil | PG | PG |
| 9 | Heavy paraffin Oil | PG | PG |
| 10 | Hazelnut Oil | S | S |
| 11 | Linseed oil | PG | PG |
| 12 | Methanol | S | S |
| 13 | Methanol + water (1:5) | S | G (0.67) |
| 14 | Ethanol | S | S |
| 15 | Ethanol + water (1:5) | PG | G (0.29) |
| 16 | Water | I | I |
| 17 | Decanol | PG | PG |
| 18 | Dodecanol | PG | PG |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lalitha, K.; Gayathri, K.; Prasad, Y.S.; Saritha, R.; Thamizhanban, A.; Maheswari, C.U.; Sridharan, V.; Nagarajan, S. Supramolecular Gel Formation Based on Glycolipids Derived from Renewable Resources. Gels 2018, 4, 1. https://doi.org/10.3390/gels4010001
Lalitha K, Gayathri K, Prasad YS, Saritha R, Thamizhanban A, Maheswari CU, Sridharan V, Nagarajan S. Supramolecular Gel Formation Based on Glycolipids Derived from Renewable Resources. Gels. 2018; 4(1):1. https://doi.org/10.3390/gels4010001
Chicago/Turabian StyleLalitha, Krishnamoorthy, Kandasamy Gayathri, Yadavali Siva Prasad, Rajendhiran Saritha, A. Thamizhanban, C. Uma Maheswari, Vellaisamy Sridharan, and Subbiah Nagarajan. 2018. "Supramolecular Gel Formation Based on Glycolipids Derived from Renewable Resources" Gels 4, no. 1: 1. https://doi.org/10.3390/gels4010001