Detrimental Impact of Energy Drink Compounds on Developing Oligodendrocytes and Neurons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs
2.2. Primary Cell Culture
2.2.1. Oligodendrocytes
2.2.2. Hippocampal Neurons
2.3. Western-Blot Analysis
2.4. Immunocytochemistry
2.5. Confocal Microscopy and Quantitative Analysis
2.6. Statistical Analysis
3. Results
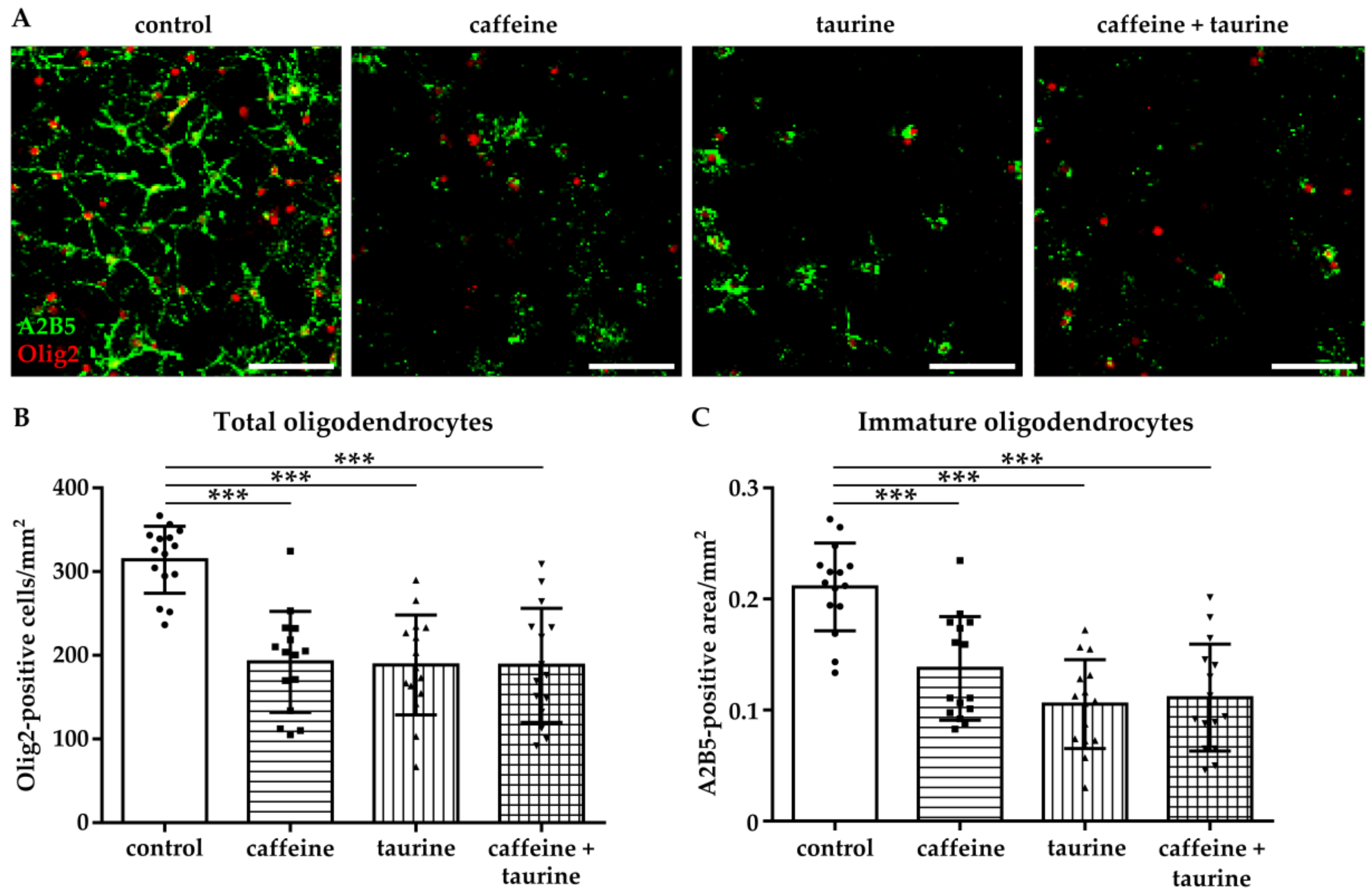
3.1. The Main Compounds of Energy Drinks Caffeine and Taurine Induce Cell Degeneration and Inhibit Proliferation of Immature Oligodendrocytes
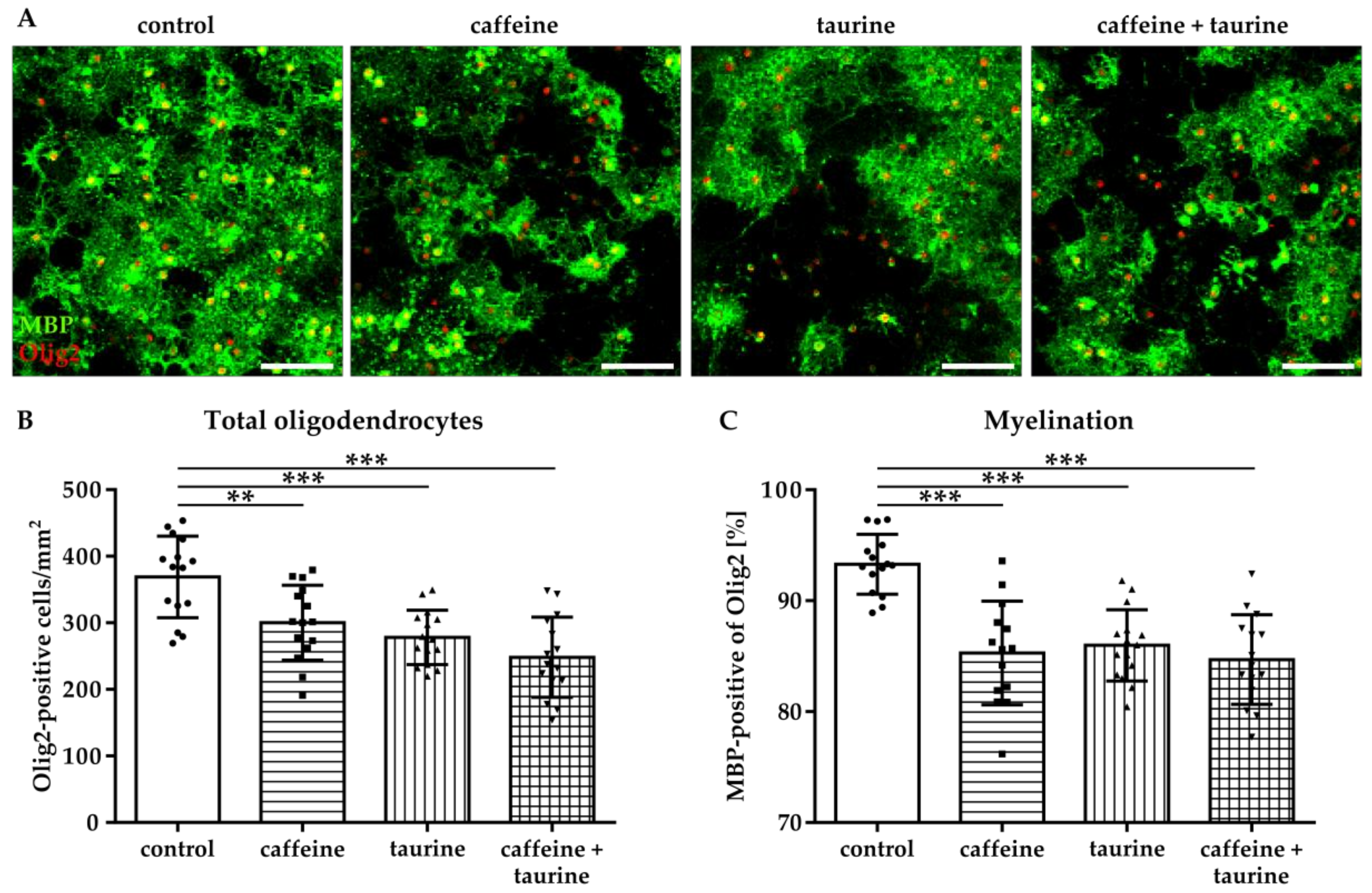
3.2. Differentiation Capacity of Oligodendrocytes is Reduced by Caffeine and Taurine
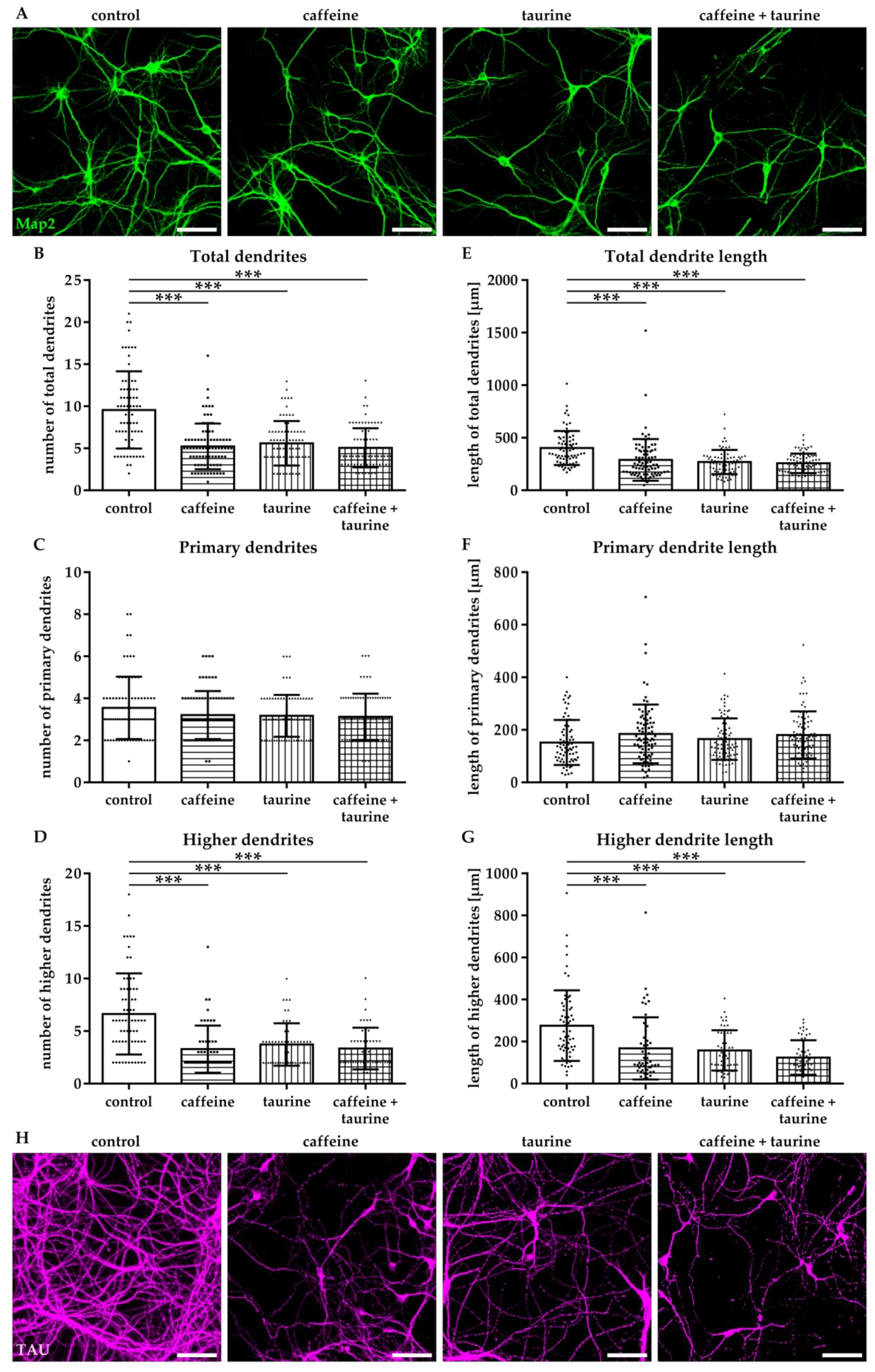
3.3. Caffeine and Taurine Reduce Dendrite Branching Immediately after Treatment
3.4. Prolonged Disturbance of Dendrite and Axonal Morphology Following Short-Term Caffeine and Taurine Treatment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gallimberti, L.; Buja, A.; Chindamo, S.; Vinelli, A.; Lazzarin, G.; Terraneo, A.; Scafato, E.; Baldo, V. Energy drink consumption in children and early adolescents. Eur. J. Pediatr. 2013, 172, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Seifert, S.M.; Schaechter, J.L.; Hershorin, E.R.; Lipshultz, S.E. Health effects of energy drinks on children, adolescents, and young adults. Pediatrics 2011, 127, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, A.; Marakis, G.; Lampen, A.; Hirsch-Ernst, K.I. Risk assessment of energy drinks with focus on cardiovascular parameters and energy drink consumption in Europe. Food Chem. Toxicol. 2019, 130, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Al-Basher, G.I.; Aljabal, H.; Almeer, R.S.; Allam, A.A.; Mahmoud, A.M. Perinatal exposure to energy drink induces oxidative damage in the liver, kidney and brain, and behavioral alterations in mice offspring. Biomed. Pharmacother. 2018, 102, 798–811. [Google Scholar] [CrossRef] [PubMed]
- Grasser, E.K.; Yepuri, G.; Dulloo, A.G.; Montani, J.P. Cardio- and cerebrovascular responses to the energy drink Red Bull in young adults: A randomized cross-over study. Eur. J. Nutr. 2014, 53, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Holubcikova, J.; Kolarcik, P.; Madarasova Geckova, A.; Reijneveld, S.A.; van Dijk, J.P. Regular energy drink consumption is associated with the risk of health and behavioural problems in adolescents. Eur. J. Pediatr. 2017, 176, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Hammond, D.; Reid, J.L.; Zukowski, S. Adverse effects of caffeinated energy drinks among youth and young adults in Canada: A Web-based survey. CMAJ Open 2018, 6, e19–e25. [Google Scholar] [CrossRef]
- Giedd, J.N.; Blumenthal, J.; Jeffries, N.O.; Castellanos, F.X.; Liu, H.; Zijdenbos, A.; Paus, T.; Evans, A.C.; Rapoport, J.L. Brain development during childhood and adolescence: A longitudinal MRI study. Nat. Neurosci. 1999, 2, 861–863. [Google Scholar] [CrossRef]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, 106, 1–16. [Google Scholar] [CrossRef]
- Geeraert, B.L.; Lebel, R.M.; Lebel, C. A multiparametric analysis of white matter maturation during late childhood and adolescence. Hum. Brain Mapp. 2019, 40, 4345–4356. [Google Scholar] [CrossRef] [Green Version]
- Reis, R.; Charehsaz, M.; Sipahi, H.; Ekici, A.I.; Macit, C.; Akkaya, H.; Aydin, A. Energy Drink Induced Lipid Peroxidation and Oxidative Damage in Rat Liver and Brain When Used Alone or Combined with Alcohol. J. Food Sci. 2017, 82, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Zeidan-Chulia, F.; Gelain, D.P.; Kolling, E.A.; Rybarczyk-Filho, J.L.; Ambrosi, P.; Terra, S.R.; Pires, A.S.; da Rocha, J.B.; Behr, G.A.; Moreira, J.C. Major components of energy drinks (caffeine, taurine, and guarana) exert cytotoxic effects on human neuronal SH-SY5Y cells by decreasing reactive oxygen species production. Oxid. Med. Cell. Longev. 2013, 2013, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Brehmer, F.; Bendix, I.; Prager, S.; van de Looij, Y.; Reinboth, B.S.; Zimmermanns, J.; Schlager, G.W.; Brait, D.; Sifringer, M.; Endesfelder, S.; et al. Interaction of inflammation and hyperoxia in a rat model of neonatal white matter damage. PLoS ONE 2012, 7, e49023. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, K.D.; de Vellis, J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol. 1980, 85, 890–902. [Google Scholar] [CrossRef]
- Serdar, M.; Herz, J.; Kempe, K.; Lumpe, K.; Reinboth, B.S.; Sizonenko, S.V.; Hou, X.; Herrmann, R.; Hadamitzky, M.; Heumann, R.; et al. Fingolimod protects against neonatal white matter damage and long-term cognitive deficits caused by hyperoxia. Brain Behav. Immun. 2016, 52, 106–119. [Google Scholar] [CrossRef] [Green Version]
- Janowska, J.; Ziemka-Nalecz, M.; Sypecka, J. The Differentiation of Rat Oligodendroglial Cells Is Highly Influenced by the Oxygen Tension: In Vitro Model Mimicking Physiologically Normoxic Conditions. Int. J. Mol. Sci. 2018, 19, 331. [Google Scholar] [CrossRef]
- Pfeiffer, S.E.; Warrington, A.E.; Bansal, R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 1993, 3, 191–197. [Google Scholar] [CrossRef]
- Stuchlik, A. Dynamic learning and memory, synaptic plasticity and neurogenesis: An update. Front. Behav. Neurosci. 2014, 8, 106. [Google Scholar] [CrossRef]
- Persad, L.A.B. Energy drinks and the neurophysiological impact of caffeine. Front. Neurosci. 2011, 5, 116. [Google Scholar] [CrossRef]
- Beaudoin, G.M., III; Lee, S.H.; Singh, D.; Yuan, Y.; Ng, Y.G.; Reichardt, L.F.; Arikkath, J. Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nat. Protoc. 2012, 7, 1741–1754. [Google Scholar] [CrossRef]
- Meijering, E.; Jacob, M.; Sarria, J.-C.; Steiner, P.; Hirling, H.; Unser, M.; Sarria, J. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry 2004, 58, 167–176. [Google Scholar] [CrossRef]
- Zucconi, S.; Volpato, C.; Adinolfi, F.; Gandini, E.; Gentile, E.; Loi, A.; Fioriti, L. Gathering consumption data on specific consumer groups of energy drinks. EFSA Support. Publ. 2013, 10. [Google Scholar] [CrossRef] [Green Version]
- Crowley, L.C.; Waterhouse, N.J. Detecting Cleaved Caspase-3 in Apoptotic Cells by Flow Cytometry. Cold Spring Harb. Protoc. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Stokin, G.B.; Lillo, C.; Falzone, T.L.; Brusch, R.G.; Rockenstein, E.; Mount, S.L.; Raman, R.; Davies, P.; Masliah, E.; Williams, D.S.; et al. Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science 2005, 307, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Kolahdouzan, M.; Hamadeh, M.J. The neuroprotective effects of caffeine in neurodegenerative diseases. CNS Neurosci. Ther. 2017, 23, 272–290. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Ma, T.; Xu, Y.; Tian, Y.; Cai, Q.; Li, B.; Li, H. Caffeine Treatment Promotes Differentiation and Maturation of Hypoxic Oligodendrocytes via Counterbalancing Adenosine 1 Adenosine Receptor-Induced Calcium Overload. Med. Sci. Monit. 2019, 25, 1729–1739. [Google Scholar] [CrossRef]
- Endesfelder, S.; Weichelt, U.; Strauß, E.; Schlör, A.; Sifringer, M.; Scheuer, T.; Bührer, C.; Schmitz, T. Neuroprotection by Caffeine in Hyperoxia-Induced Neonatal Brain Injury. Int. J. Mol. Sci. 2017, 18, 187. [Google Scholar] [CrossRef]
- Endesfelder, S.; Zaak, I.; Weichelt, U.; Bührer, C.; Schmitz, T. Caffeine protects neuronal cells against injury caused by hyperoxia in the immature brain. Free. Radic. Biol. Med. 2014, 67, 221–234. [Google Scholar] [CrossRef]
- He, Z.; Ma, W.-Y.; Hashimoto, T.; Bode, A.M.; Yang, C.S.; Dong, Z. Induction of apoptosis by caffeine is mediated by the p53, Bax, and caspase 3 pathways. Cancer Res. 2003, 63, 4396–4401. [Google Scholar]
- Jang, M.H.; Shin, M.C.; Kang, I.S.; Baik, H.H.; Cho, Y.H.; Chu, J.P.; Kim, E.H.; Kim, C.J. Caffeine induces apoptosis in human neuroblastoma cell line SK-N-MC. J. Korean Med. Sci. 2002, 17, 674–678. [Google Scholar] [CrossRef]
- Doyle, W.; Shide, E.; Thapa, S.; Chandrasekaran, V. The effects of energy beverages on cultured cells. Food Chem. Toxicol. 2012, 50, 3759–3768. [Google Scholar] [CrossRef] [PubMed]
- White, J.R.; Padowski, J.M.; Zhong, Y.; Chen, G.; Luo, S.; Lazarus, P.; Layton, M.E.; McPherson, S. Pharmacokinetic analysis and comparison of caffeine administered rapidly or slowly in coffee chilled or hot versus chilled energy drink in healthy young adults. Clin. Toxicol. 2016, 54, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.B.; Greenblatt, D.J.; Leduc, B.W.; Thompson, M.L.; Shader, R.I. Relationship of plasma and brain concentrations of caffeine and metabolites to benzodiazepine receptor binding and locomotor activity. J. Pharmacol. Exp. Ther. 1989, 248, 1078–1083. [Google Scholar] [PubMed]
- Abdel-Hady, H.; Nasef, N.; Shabaan, A.E.; Nour, I. Caffeine therapy in preterm infants. World J. Clin. Pediatr. 2015, 4, 81–93. [Google Scholar] [CrossRef]
- Curran, C.P.; Marczinski, C.A. Taurine, caffeine, and energy drinks: Reviewing the risks to the adolescent brain. Birth Defects Res. 2017, 109, 1640–1648. [Google Scholar] [CrossRef]
- Kilb, W. Putative Role of Taurine as Neurotransmitter During Perinatal Cortical Development. In Advances in Experimental Medicine and Biology; Lee, D.H., Schaffer, S.W., Eds.; Springer: Dordrecht, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Tu, S.; Zhang, X.L.; Wan, H.F.; Xia, Y.Q.; Liu, Z.Q.; Yang, X.H.; Wan, F.S. Effect of taurine on cell proliferation and apoptosis human lung cancer A549 cells. Oncol. Lett. 2018, 15, 5473–5480. [Google Scholar] [CrossRef] [Green Version]
- Baum, M.; Weiss, M. The influence of a taurine containing drink on cardiac parameters before and after exercise measured by echocardiography. Amino Acids 2001, 20, 75–82. [Google Scholar] [CrossRef]
- Imagawa, T.F.; Hirano, I.; Utsuki, K.; Horie, M.; Naka, A.; Matsumoto, K.; Imagawa, S. Caffeine and taurine enhance endurance performance. Int. J. Sports Med. 2009, 30, 485–488. [Google Scholar] [CrossRef]
- De Hoz, L.; Simons, M. The emerging functions of oligodendrocytes in regulating neuronal network behaviour. Bioessays 2015, 37, 60–69. [Google Scholar] [CrossRef]
- Wang, F.; Yang, Y.-J.; Yang, N.; Chen, X.-J.; Huang, N.-X.; Zhang, J.; Wu, Y.; Liu, Z.; Gao, X.; Li, T.; et al. Enhancing Oligodendrocyte Myelination Rescues Synaptic Loss and Improves Functional Recovery after Chronic Hypoxia. Neuron 2018, 99, 689–701. [Google Scholar] [CrossRef]
- Altman, J.; Das, G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965, 124, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Shors, T.J.; Miesegaes, G.; Beylin, A.; Zhao, M.; Rydel, T.; Gould, E. Neurogenesis in the adult is involved in the formation of trace memories. Nature 2001, 410, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Tavosanis, G. Dendritic structural plasticity. Dev. Neurobiol. 2012, 72, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Arikkath, J. Molecular mechanisms of dendrite morphogenesis. Front. Cell. Neurosci. 2012, 6, 61. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-C.; Koleske, A.J. Mechanisms of synapse and dendrite maintenance and their disruption in psychiatric and neurodegenerative disorders. Annu. Rev. Neurosci. 2010, 33, 349–378. [Google Scholar] [CrossRef]
- Shivaraj, M.C.; Marcy, G.; Low, G.; Ryu, J.R.; Zhao, X.; Rosales, F.J.; Goh, E.L.K. Taurine Induces Proliferation of Neural Stem Cells and Synapse Development in the Developing Mouse Brain. PLoS ONE 2012, 7, e42935. [Google Scholar] [CrossRef]
- Yu, N.Y.; Bieder, A.; Raman, A.; Mileti, E.; Katayama, S.; Einarsdottir, E.; Fredholm, B.B.; Falk, A.; Tapia-Páez, I.; Daub, C.O.; et al. Acute doses of caffeine shift nervous system cell expression profiles toward promotion of neuronal projection growth. Sci. Rep. 2017, 7, 11458. [Google Scholar] [CrossRef] [Green Version]
- Nusetti, S.; Obregón, F.; Quintal, M.; Benzo, Z.; Lima, L. Taurine and Zinc Modulate Outgrowth from Goldfish Retinal Explants. Neurochem. Res. 2005, 30, 1483–1492. [Google Scholar] [CrossRef]
- Beirowski, B. Concepts for regulation of axon integrity by enwrapping glia. Front. Cell. Neurosci. 2013, 7, 256. [Google Scholar] [CrossRef]
- Catalán-Latorre, A.; Nacher, A.; Merino, V.; Diez, O.; Sanjuán, M.M. A preclinical study to model taurine pharmokinetics in the undernourished rat. Br. J. Nutr. 2018, 119, 826–835. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.-S.; Ohtsuki, S.; Takanaga, H.; Tomi, M.; Hosoya, K.-I.; Terasaki, T. Regulation of taurine transport at the blood-brain barrier by tumor necrosis factor-α, taurine and hypertonicity. J. Neurochem. 2002, 83, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Lachance, M.; Marlowe, C.; Waddell, W. Autoradiographic disposition of [1-methyl-14C]- and [2-14C] caffeine in mice. Toxicol. Appl. Pharmacol. 1983, 71, 237–241. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serdar, M.; Mordelt, A.; Müser, K.; Kempe, K.; Felderhoff-Müser, U.; Herz, J.; Bendix, I. Detrimental Impact of Energy Drink Compounds on Developing Oligodendrocytes and Neurons. Cells 2019, 8, 1381. https://doi.org/10.3390/cells8111381
Serdar M, Mordelt A, Müser K, Kempe K, Felderhoff-Müser U, Herz J, Bendix I. Detrimental Impact of Energy Drink Compounds on Developing Oligodendrocytes and Neurons. Cells. 2019; 8(11):1381. https://doi.org/10.3390/cells8111381
Chicago/Turabian StyleSerdar, Meray, Annika Mordelt, Katharina Müser, Karina Kempe, Ursula Felderhoff-Müser, Josephine Herz, and Ivo Bendix. 2019. "Detrimental Impact of Energy Drink Compounds on Developing Oligodendrocytes and Neurons" Cells 8, no. 11: 1381. https://doi.org/10.3390/cells8111381




