Nephron Patterning: Lessons from Xenopus, Zebrafish, and Mouse Studies
Abstract
:1. Introduction
2. Kidney Morphogenesis in Non-Amniotes and Amniotes
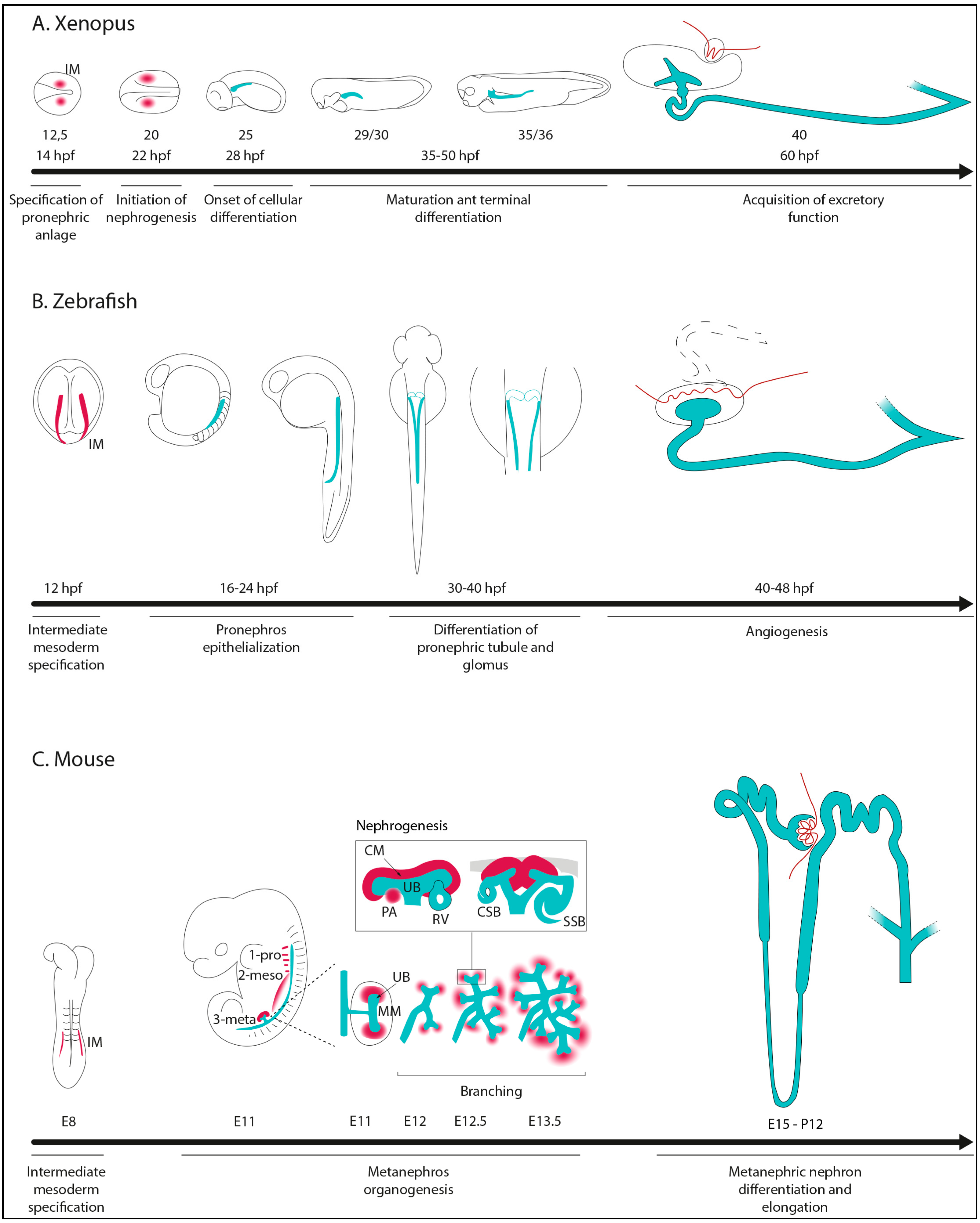
- Specification of IM cells and renal primordium formation.
- Epithelialization and differentiation of renal primordium.
- Patterning of nephrons to make functional and specialized segment tubules.
- Formation of glomerular capillaries for blood filtration.
3. Conservation of the Segmental Organization of Nephrons in Vertebrates

4. Nephron Patterning/Segmentation: Genetic and Transcriptional Regulation
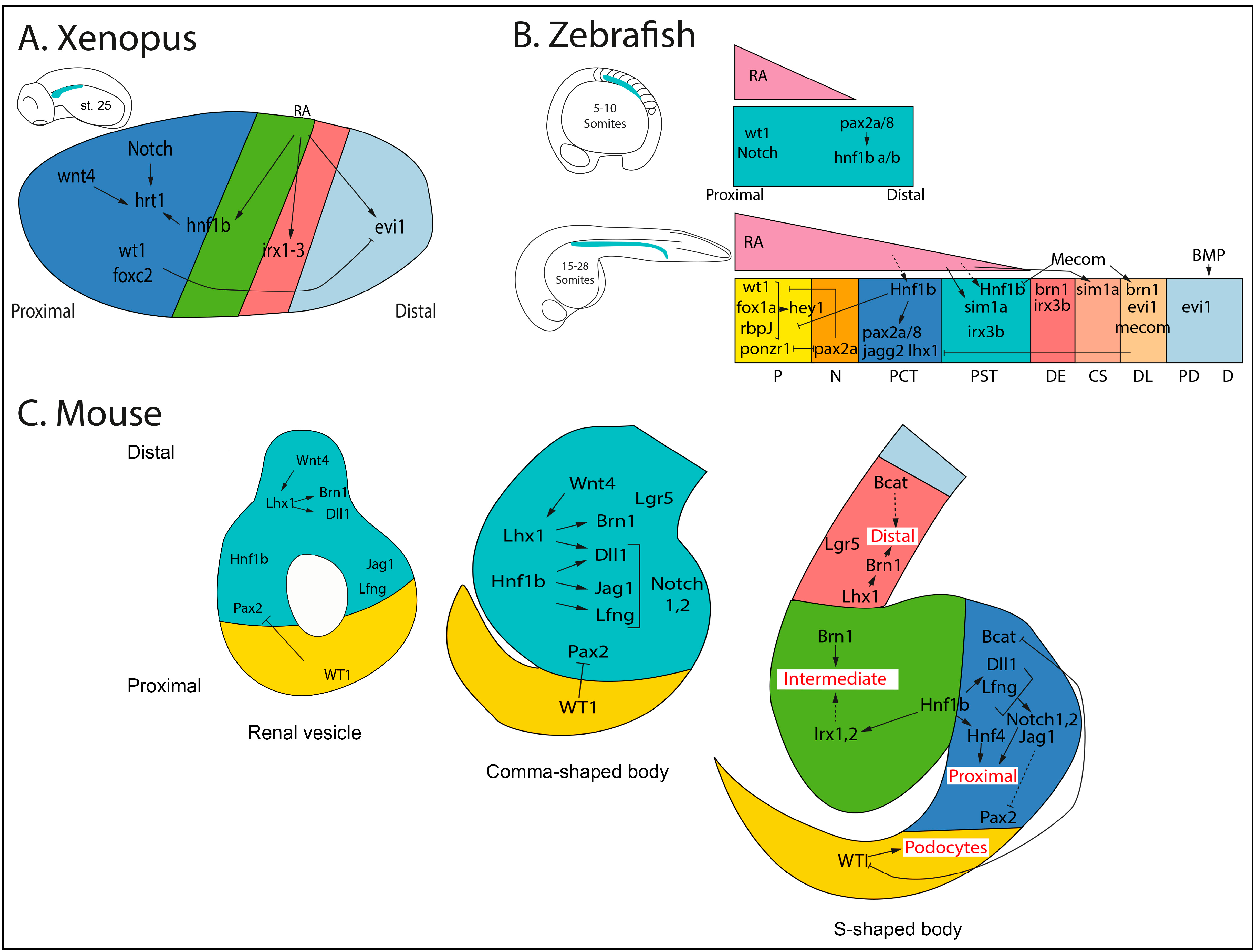
5. Conclusions and Future Prospects
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brändli, A.W. Towards a molecular anatomy of the Xenopus pronephric kidney. Int. J. Dev. Biol. 1999, 43, 381–395. [Google Scholar] [PubMed]
- Vize, P.; Seufert, D.; Carroll, T.; Wallingford, J. Model systems for the study of kidney development: Use of the pronephros in the analysis of organ induction and patterning. Dev. Biol. 1997, 188, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Vasilyev, A.; Liu, Y.; Mudumana, S.; Mangos, S.; Lam, P.Y.; Majumdar, A.; Zhao, J.; Poon, K.L.; Kondrychyn, I.; Korzh, V.; et al. Collective cell migration drives morphogenesis of the kidney nephron. PLoS Biol. 2009, 7, e9. [Google Scholar] [CrossRef] [PubMed]
- Drummond, I.; Davidson, A. Zebrafish kidney development. Methods Cell Biol. 2010, 100, 233–260. [Google Scholar] [PubMed]
- Serluca, F.C.; Fishman, M.C. Pre-pattern in the pronephric kidney field of zebrafish. Development 2001, 128, 2233–2241. [Google Scholar] [PubMed]
- Diep, C.Q.; Peng, Z.; Ukah, T.K.; Kelly, P.M.; Daigle, R.V.; Davidson, A.J. Development of the zebrafishmesonephros. Genesis 2015, 53, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Boucher, R.C.; Bollig, F.; Englert, C.; Hildebrandt, F. Characterization of mesonephric development and regeneration using transgenic zebrafish. Am. J. Physiol. Renal Physiol. 2010, 299, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Saxén, L.; Sariola, H. Early organogenesis of the kidney. Pediatr. Nephrol. 1987, 1, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Dressler, G. The cellular basis of kidney development. Annu. Rev. Cell Dev. Biol. 2006, 22, 509–529. [Google Scholar] [CrossRef] [PubMed]
- Orvis, G.D.; Behringer, R.R. Cellular mechanisms of Müllerian duct formation in the mouse. Dev. Biol. 2007, 306, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Costantini, F.; Kopan, R. Patterning a complex organ: Branching morphogenesis and nephron segmentation in kidney development. Dev. Cell 2010, 18, 698–712. [Google Scholar] [CrossRef] [PubMed]
- Hartman, H.A.; Lai, H.L.; Patterson, L.T. Cessation of renal morphogenesis in mice. Dev. Biol. 2007, 310, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, C. Using mouse models to understand normal and abnormal urogenital tract development. Organogenesis 2009, 5, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Raciti, D.; Reggiani, L.; Geffers, L.; Jiang, Q.; Bacchion, F.; Subrizi, A.E.; Clements, D.; Tindal, C.; Davidson, D.R.; Kaissling, B.; et al. Organization of the pronephric kidney revealed by large-scale gene expression mapping. Genome Biol. 2008, 9, R84. [Google Scholar] [CrossRef] [PubMed]
- Wingert, R.A.; Davidson, A.J. The zebrafish pronephros: A model to study nephron segmentation. Kidney Int. 2008, 73, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, R.; Georgas, K.; Rumballe, B.; Lesieur, E.; Chiu, H.; Taylor, D.; Tang, D.; Grimmond, S.; Little, M. Identification of anchor genes during kidney development defines ontological relationships, molecular subcompartments and regulatory pathways. PLoS ONE 2011, 6, e17286. [Google Scholar] [CrossRef]
- Yu, J.; Valerius, M.T.; Duah, M.; Staser, K.; Hansard, J.K.; Guo, J.J.; McMahon, J.; Vaughan, J.; Faria, D.; Georgas, K.; et al. Identification of molecular compartments and genetic circuitry in the developing mammalian kidney. Development 2012, 139, 1863–1873. [Google Scholar] [CrossRef] [PubMed]
- Denker, B.M.; Sabath, E. The biology of epithelial cell tight junctions in the kidney. J. Am. Soc. Nephrol. 2011, 22, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Møbjerg, N.; Larsen, E.H.; Jespersen, A. Morphology of the kidney in larvae of Bufo viridis (Amphibia, Anura, Bufonidae). J. Morphol. 2000, 245, 177–195. [Google Scholar] [CrossRef]
- Balinsky, J.B.; Shambaugh, G.E.; Cohen, P.P. Glutamate dehydrogenase biosynthesis in amphibian liver preparations. J. Biol. Chem. 1970, 245, 128–137. [Google Scholar] [PubMed]
- Vize, P.D. The chloride conductance channel ClC-K is a specific marker for the Xenopus pronephric distal tubule and duct. Gene Expr. Patterns 2003, 3, 347–350. [Google Scholar] [CrossRef]
- Zhou, X.; Vize, P. Proximo-distal specialization of epithelial transport processes within the Xenopus pronephric kidney tubules. Dev. Biol. 2004, 271, 322–338. [Google Scholar] [CrossRef] [PubMed]
- Van Campenhout, C.; Nichane, M.; Antoniou, A.; Pendeville, H.; Bronchain, O.J.; Marine, J.C.; Mazabraud, A.; Voz, M.L.; Bellefroid, E.J. Evi1 is specifically expressed in the distal tubule and duct of the Xenopus pronephros and plays a role in its formation. Dev. Biol. 2006, 294, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Nichane, M.; van Campenhout, C.; Pendeville, H.; Voz, M.L.; Bellefroid, E.J. The Na+/PO4 cotransporter SLC20A1 gene labels distinct restricted subdomains of the developing pronephros in Xenopus and zebrafish embryos. Gene Expr. Patterns 2006, 6, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Reggiani, L.; Raciti, D.; Airik, R.; Kispert, A.; Brandli, A.W. The prepattern transcription factor Irx3 directs nephron segment identity. Genes Dev. 2007, 21, 2358–2370. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M.; Turk, E. The sodium/glucose cotransport family SLC5. Pflugers Arch. 2004, 447, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Wingert, R.; Selleck, R.; Yu, J.; Song, H.D.; Chen, Z.; Song, A.; Zhou, Y.; Thisse, B.; Thisse, C.; McMahon, A.; et al. The cdx genes and retinoic acid control the positioning and segmentation of the zebrafish pronephros. PLoS Genet. 2007, 3, 1922–1938. [Google Scholar] [CrossRef] [PubMed]
- Kiener, T.K.; Sleptsova-Friedrich, I.; Hunziker, W. Identification, tissue distribution and developmental expression of tjp1/zo-1, tjp2/zo-2 and tjp3/zo-3 in the zebrafish, Danio rerio. Gene Expr. Patterns 2007, 7, 767–776. [Google Scholar] [CrossRef] [PubMed]
- McKee, R.; Gerlach, G.; Jou, J.; Cheng, C.; Wingert, R. Temporal and spatial expression of tight junction genes during zebrafish pronephros development. Gene Expr. Patterns 2014, 16, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, P.; Rodriguez-Seguel, E.; Fernandez-Gonzalez, A.; Rubio, R.; Gomez-Skarmeta, J.L. A dual requirement for Iroquois genes during Xenopus kidney development. Development 2008, 135, 3197–3207. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, K.A.; Rones, M.S.; Mercola, M. Notch regulates cell fate in the developing pronephros. Dev. Biol. 2000, 227, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.W.; Jones, E.A. Notch activates Wnt-4 signalling to control medio-lateral patterning of the pronephros. Development 2009, 136, 3585–3595. [Google Scholar] [CrossRef] [PubMed]
- Taelman, V.; van Campenhout, C.; Sölter, M.; Pieler, T.; Bellefroid, E.J. The Notch-effector HRT1 gene plays a role in glomerular development and patterning of the Xenopus pronephros anlagen. Development 2006, 133, 2961–2971. [Google Scholar] [CrossRef] [PubMed]
- White, J.T.; Zhang, B.; Cerqueira, D.M.; Tran, U.; Wessely, O. Notch signaling, wt1 and foxc2 are key regulators of the podocyte gene regulatory network in Xenopus. Development 2010, 137, 1863–1873. [Google Scholar] [CrossRef] [PubMed]
- Cartry, J.; Nichane, M.; Ribes, V.; Colas, A.; Riou, J.-F.F.; Pieler, T.; Dollé, P.; Bellefroid, E.J.; Umbhauer, M. Retinoic acid signalling is required for specification of pronephric cell fate. Dev. Biol. 2006, 299, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Heliot, C.; Desgrange, A.; Buisson, I.; Prunskaite-Hyyryläinen, R.; Shan, J.; Vainio, S.; Umbhauer, M.; Cereghini, S. HNF1B controls proximal-intermediate nephron segment identity in vertebrates by regulating Notch signalling components and Irx1/2. Development 2013, 140, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Pouilhe, M.; Gilardi-Hebenstreit, P.; Dinh, C.; Charnay, P. Direct regulation of vHnf1 by retinoic acid signaling and MAF-related factors in the neural tube. Dev. Biol. 2007, 309, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Power, S.; Cereghini, S. Positive regulation of the vHNF1 promoter by the orphan receptors COUP-TF1/Ear3 and COUP-TFII/Arp1. Mol. Cell. Biol. 1996, 16, 778–791. [Google Scholar]
- Demartis, A.; Maffei, M.; Vignali, R.; Barsacchi, G.; de Simone, V. Cloning and developmental expression of LFB3/HNF1 beta transcription factor in Xenopus laevis. Mech. Dev. 1994, 47, 19–28. [Google Scholar] [CrossRef]
- Pfeffer, P.L.; Gerster, T.; Lun, K.; Brand, M.; Busslinger, M. Characterization of three novel members of the zebrafish Pax2/5/8 family: Dependency of Pax5 and Pax8 expression on the Pax2.1 (noi) function. Development 1998, 125, 3063–3074. [Google Scholar] [PubMed]
- Toyama, R.; Kobayashi, M.; Tomita, T.; Dawid, I.B. Expression of LIM-domain binding protein (ldb) genes during zebrafish embryogenesis. Mech. Dev. 1998, 71, 197–200. [Google Scholar] [CrossRef]
- Gerlach, G.; Wingert, R. Zebrafish pronephros tubulogenesis and epithelial identity maintenance are reliant on the polarity proteins Prkc iota and zeta. Dev. Biol. 2014, 396, 183–200. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, L.L.; Grimaldi, M.; Kostun, Z.; Wingert, R.A.; Selleck, R.; Davidson, A.J. Wt1a, Foxc1a, and the Notch mediator Rbpj physically interact and regulate the formation of podocytes in zebrafish. Dev. Biol. 2011, 358, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Perner, B.; Englert, C.; Bollig, F. The Wilms tumor genes wt1a and wt1b control different steps during formation of the zebrafish pronephros. Dev. Biol. 2007, 309, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Bedell, V.M.; Person, A.D.; Larson, J.D.; McLoon, A.; Balciunas, D.; Clark, K.J.; Neff, K.I.; Nelson, K.E.; Bill, B.R.; Schimmenti, L.A.; et al. The lineage-specific gene ponzr1 is essential for zebrafish pronephric and pharyngeal arch development. Development 2012, 139, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, A.; Lun, K.; Brand, M.; Drummond, I.A. Zebrafish no isthmus reveals a role for pax2.1 in tubule differentiation and patterning events in the pronephric primordia. Development 2000, 127, 2089–2098. [Google Scholar] [PubMed]
- Wingert, R.A.; Davidson, A.J. Zebrafish nephrogenesis involves dynamic spatiotemporal expression changes in renal progenitors and essential signals from retinoic acid and irx3b. Dev. Dyn. 2011, 240, 2011–2027. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.W.; Przepiorski, A.; Ren, Q.; Yu, J.; Davidson, A.J. HNF1B is essential for nephron segmentation during Nephrogenesis. J. Am. Soc. Nephrol. 2012, 24, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.W.; Davidson, A.J. Hnf1beta and nephron segmentation. Pediatr. Nephrol. 2013, 29, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.N.; Wingert, R.A. Nephron proximal tubule patterning and corpuscles of Stannius formation are regulated by the sim1a transcription factor and retinoic acid in zebrafish. Dev. Biol. 2015, 399, 100–116. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cheng, C.N.; Verdun, V.A.; Wingert, R.A. Zebrafish nephrogenesis is regulated by interactions between retinoic acid, mecom, and Notch signaling. Dev. Biol. 2014, 386, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Grobstein, C. Inductive epitheliomesenchymal interaction in cultured organ rudiments of the mouse. Science 1953, 118, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Grobstein, C. Trans-filter induction of tubules in mouse metanephrogenic mesenchyme. Exp. Cell. Res. 1956, 10, 424–440. [Google Scholar] [CrossRef]
- Carroll, T.J.; Park, J.-S.S.; Hayashi, S.; Majumdar, A.; McMahon, A.P. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev. Cell 2005, 9, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Stark, K.; Vainio, S.; Vassileva, G.; McMahon, A.P. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 1994, 372, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Karner, C.M.; Das, A.; Ma, Z.; Self, M.; Chen, C.; Lum, L.; Oliver, G.; Carroll, T.J. Canonical Wnt9b signaling balances progenitor cell expansion and differentiation during kidney development. Development 2011, 138, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Nakai, S.; Sugitani, Y.; Sato, H.; Ito, S.; Miura, Y.; Ogawa, M.; Nishi, M.; Jishage, K.; Minowa, O.; Noda, T. Crucial roles of Brn1 in distal tubule formation and function in mouse kidney. Development 2003, 130, 4751–4759. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Kwan, K.-M.M.; Carroll, T.J.; McMahon, A.P.; Mendelsohn, C.L.; Behringer, R.R. Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development 2005, 132, 2809–2823. [Google Scholar] [CrossRef] [PubMed]
- Georgas, K.; Rumballe, B.; Valerius, T.; Chiu, H.; Thiagarajan, R.; Lesieur, E.; Aronow, B.; Brunskill, E.; Combes, A.; Tang, D.; et al. Analysis of early nephron patterning reveals a role for distal RV proliferation in fusion to the ureteric tip via a cap mesenchyme-derived connecting segment. Dev. Biol. 2009, 332, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Kreidberg, J.; Sariola, H.; Loring, J.; Maeda, M.; Pelletier, J.; Housman, D.; Jaenisch, R. WT-1 is required for early kidney development. Cell 1993, 74, 679–691. [Google Scholar] [CrossRef]
- Ryan, G.; Steele-Perkins, V.; Morris, J.; Rauscher, F.; Dressler, G. Repression of Pax-2 by WT1 during normal kidney development. Development 1995, 121, 867–875. [Google Scholar] [PubMed]
- Mugford, J.W.; Yu, J.; Kobayashi, A.; McMahon, A.P. High-resolution gene expression analysis of the developing mouse kidney defines novel cellular compartments within the nephron progenitor population. Dev. Biol. 2009, 333, 312–323. [Google Scholar] [CrossRef]
- Georgas, K.; Rumballe, B.; Wilkinson, L.; Chiu, H.S.; Lesieur, E.; Gilbert, T.; Little, M.H. Use of dual section mRNA in situ hybridisation/immunohistochemistry to clarify gene expression patterns during the early stages of nephron development in the embryo and in the mature nephron of the adult mouse kidney. Histochem. Cell Biol. 2008, 130, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-T.; Miner, J.; Lin, M.; Tansey, M.; Roth, K.; Kopan, R. γ-Secretase activity is dispensable for mesenchyme-to-epithelium transition but required for podocyte and proximal tubule formation in developing mouse kidney. Development 2003, 130, 5031–5042. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Pereira, F.A.; Beasley, D.; Zheng, H. Presenilins are required for the formation of comma- and S-shaped bodies during nephrogenesis. Development 2003, 130, 5019–5029. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-T.; Kim, M.; Valerius, M.T.; Surendran, K.; Schuster-Gossler, K.; Gossler, A.; McMahon, A.P.; Kopan, R. Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development 2007, 134, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Bonegio, R.G.; Beck, L.H.; Kahlon, R.K.; Lu, W.; Salant, D.J. The fate of Notch-deficient nephrogenic progenitor cells during metanephric kidney development. Kidney Int. 2011, 79, 1099–1112. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, S.; Boyle, S.; Zhu, Y.; Zhang, A.; Piwnica-Worms, D.R.; Ilagan, M.X.; Kopan, R. The extracellular domain of Notch2 increases its cell-surface abundance and ligand responsiveness during kidney development. Dev. Cell 2013, 25, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Massa, F.; Garbay, S.; Bouvier, R.; Sugitani, Y.; Noda, T.; Gubler, M.C.; Heidet, L.; Pontoglio, M.; Fischer, E. Hepatocyte nuclear factor 1β controls nephron tubular development. Development 2013, 40, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; Rookmaaker, M.B.; Kujala, P.; Ng, A.; Leushacke, M.; Snippert, H.; van de Wetering, M.; Tan, S.; van Es, J.H.; Huch, M.; et al. Lgr5(+ve) stem/progenitor cells contribute to nephron formation during kidney development. Cell Rep. 2012, 2, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Kinzel, B.; Pikiolek, M.; Orsini, V.; Sprunger, J.; Isken, A.; Zietzling, S.; Desplanches, M.; Dubost, V.; Breustedt, D.; Valdez, R.; et al. Functional roles of Lgr4 and Lgr5 in embryonic gut, kidney and skin development in mice. Dev. Biol. 2014, 390, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Hoyt, P.R.; Bartholomew, C.; Davis, A.J.; Yutzey, K.; Gamer, L.W.; Potter, S.S.; Ihle, J.N.; Mucenski, M.L. The Evi1 proto-oncogene is required at midgestation for neural, heart, and paraxial mesenchyme development. Mech. Dev. 1997, 65, 55–70. [Google Scholar] [CrossRef]
- Lindström, N.; Lawrence, M.; Burn, S.; Johansson, J.; Bakker, E.; Ridgway, R.; Chang, C.-H.; Karolak, M.; Oxburgh, L.; Headon, D.; et al. Integrated β-catenin, BMP, PTEN, and Notch signalling patterns the nephron. Elife 2015, 3, e04000. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Arraf, A.A.; Grinstein, M.; Yelin, R.; Schultheiss, T.M. Wnt signaling orients the proximal-distal axis of chick kidney nephrons. Development 2015, 142, 2686–2695. [Google Scholar] [CrossRef] [PubMed]
- Marlier, A.; Gilbert, T. Expression of retinoic acid-synthesizing and -metabolizing enzymes during nephrogenesis in the rat. Gene Expr. Patterns 2004, 5, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Dow, J.A.; Romero, M.F. Drosophila provides rapid modeling of renal development, function, and disease. Am. J. Physiol. Renal Physiol. 2010, 299, F1237–F1244. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desgrange, A.; Cereghini, S. Nephron Patterning: Lessons from Xenopus, Zebrafish, and Mouse Studies. Cells 2015, 4, 483-499. https://doi.org/10.3390/cells4030483
Desgrange A, Cereghini S. Nephron Patterning: Lessons from Xenopus, Zebrafish, and Mouse Studies. Cells. 2015; 4(3):483-499. https://doi.org/10.3390/cells4030483
Chicago/Turabian StyleDesgrange, Audrey, and Silvia Cereghini. 2015. "Nephron Patterning: Lessons from Xenopus, Zebrafish, and Mouse Studies" Cells 4, no. 3: 483-499. https://doi.org/10.3390/cells4030483




