Fucosylation Is a Promising Target for Cancer Diagnosis and Therapy
Abstract
:1. Introduction
2. Fucosylated Haptoglobin
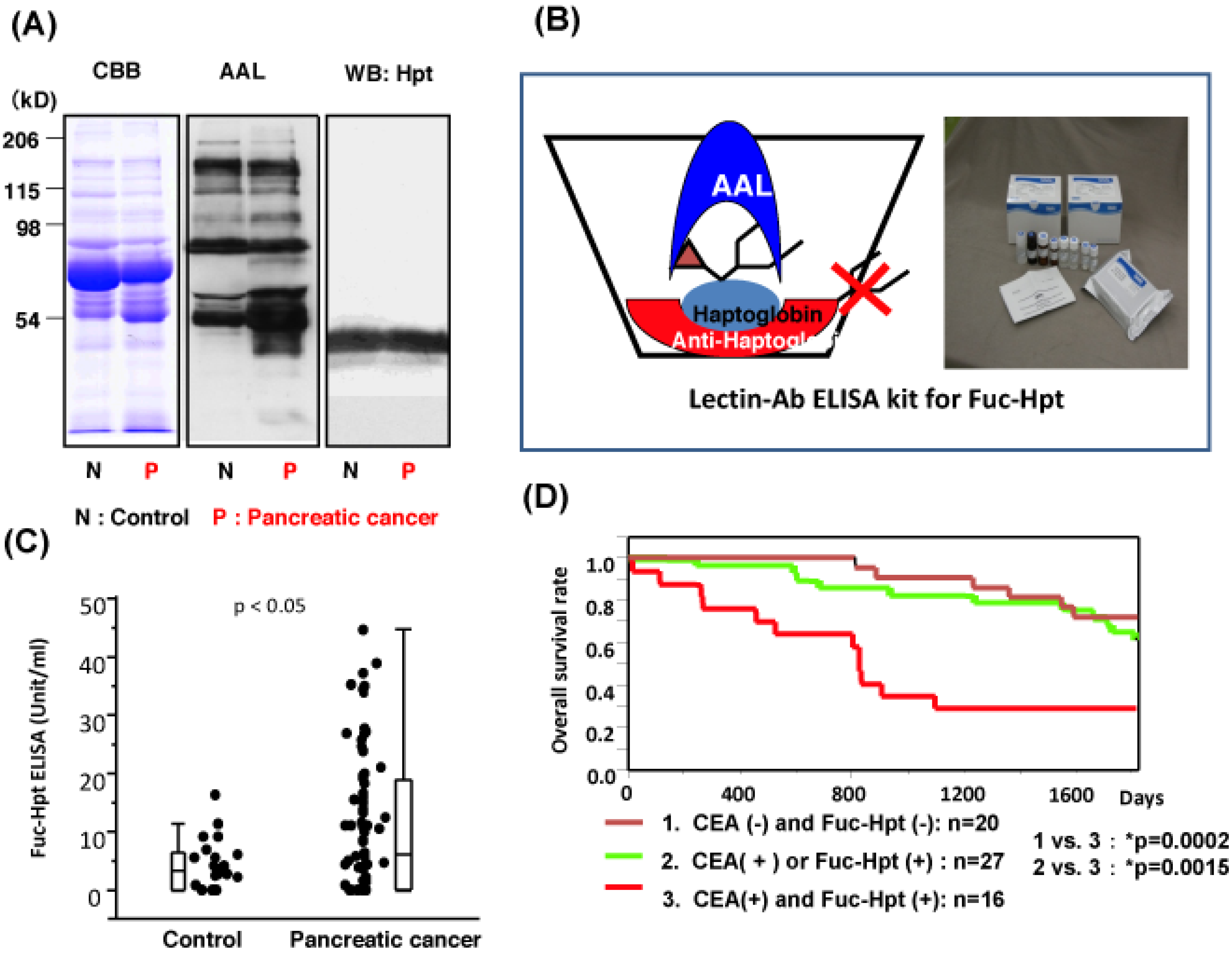
3. Development of Lectin-Antibody ELISA for Fuc-Hpt
4. How Are Fucosylated Proteins Produced in Cancer?
5. Biological Significance of Fucosylation Loss
6. Conclusions

Conflict of Interest
Acknowledgments
References
- Baumann, H.; Nudelman, E.; Watanabe, K.; Hakomori, S. Neutral fucolipids and fucogangliosides of rat hepatoma HTC and H35 cells, rat liver, and hepatocytes. Cancer Res. 1979, 39, 2637–2643. [Google Scholar]
- Simm, S.; Markowska, J.; Tomaszkiewicz, T.; Herwichowska, K.; Breborowicz, J. Monitoring the course of malignant neoplasms of the genital organs in women by means of determination of blood serum levels of fucose, sialic acid, CEA and AFP. Ginekol. Pol. 1979, 50, 1029–1036. [Google Scholar]
- Watanabe, A.; Taketa, K.; Kosaka, K. Microheterogeneity of rat alpha-fetoprotein. Ann. N. Y. Acad. Sci. 1975, 259, 95–108. [Google Scholar] [CrossRef]
- Breborowicz, J.; Mackiewicz, A.; Breborowicz, D. Microheterogeneity of alpha-fetoprotein in patient serum as demonstrated by lectin affino-electrophoresis. Scand. J. Immunol. 1981, 14, 15–20. [Google Scholar] [CrossRef]
- Taketa, K.; Izumi, M.; Ichikawa, E. Distinct molecular species of human alpha-fetoprotein due to differential affinities to lectins. Ann. N. Y. Acad. Sci. 1983, 417, 61–68. [Google Scholar] [CrossRef]
- Aoyagi, Y.; Suzuki, Y.; Igarashi, K.; Saitoh, A.; Oguro, M.; Yokota, T.; Mori, S.; Nomoto, M.; Isemura, M.; Asakura, H. The usefulness of simultaneous determinations of glucosaminylation and fucosylation indices of alpha-fetoprotein in the differential diagnosis of neoplastic diseases of the liver. Cancer 1991, 67, 2390–2394. [Google Scholar] [CrossRef]
- Taketa, K.; Endo, Y.; Sekiya, C.; Tanikawa, K.; Koji, T.; Taga, H.; Satomura, S.; Matsuura, S.; Kawai, T.; Hirai, H. A collaborative study for the evaluation of lectin-reactive alpha-fetoproteins in early detection of hepatocellular carcinoma. Cancer Res. 1993, 53, 5419–5423. [Google Scholar]
- Uozumi, N.; Yanagidani, S.; Miyoshi, E.; Ihara, Y.; Sakuma, T.; Gao, C.X.; Teshima, T.; Fujii, S.; Shiba, T.; Taniguchi, N. Purification and cDNA cloning of porcine brain GDP-L-Fuc: N-acetyl-β-D-glucosaminide α1-6 fucosyltransferase. J. Biol. Chem. 1996, 271, 27810–27817. [Google Scholar]
- Noda, K.; Miyoshi, E.; Uozumi, N.; Yanagidani, S.; Ikeda, Y.; Gao, C.X.; Suzuki, K.; Yoshihara, H.; Yoshikawa, M.; Kawano, K.; Hayashi, N.; Hori, M.; Taniguchi, N. Gene expression of α1-6 fucosyltransferase in human hepatoma tissues: A possible implication for increased fucosylation of α-fetoprotein. Hepatology 1998, 28, 944–952. [Google Scholar]
- Li, D.; Mallory, T.; Satomura, S. AFP-L3: A new generation of tumor marker for hepatocellular carcinoma. Clin. Chim. Acta 2001, 313, 15–19. [Google Scholar] [CrossRef]
- Kagebayashi, C.; Yamaguchi, I.; Akinaga, Y.; Kitano, H.; Yokoyama, K.; Satomura, M.; Kurosawa, T.; Watanabe, M.; Kawabata, T.; Chang, W.; et al. Automated immunoassay system for AFP-L3% using on chip electrokinetic reaction and separation by affinity electrophoresis. Anal. Biochem. 2009, 388, 306–311. [Google Scholar] [CrossRef]
- Wang, M.; Long, R.E.; Comunale, M.A.; Junaidi, O.; Marrero, J.; Di Bisceglie, A.M.; Block, T.M.; Mehta, A.S. Novel fucosylated biomarkers for the early detection of hepatocellular carcinoma. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 1914–1921. [Google Scholar] [CrossRef]
- Marrero, J.A.; Feng, Z.; Wang, Y.; Nguyen, M.H.; Befeler, A.S.; Roberts, L.R.; Reddy, K.R.; Harnois, D.; Llovet, J.M.; Normolle, D.; et al. Alpha-fetoprotein, Des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology 2009, 137, 110–118. [Google Scholar] [CrossRef]
- Gupta, S.; Bent, S.; Kohlwes, J. Test characteristics of alpha-fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C. A systematic review and critical analysis. Ann. Intern. Med. 2003, 139, 46–50. [Google Scholar]
- Shinkawa, T.; Nakamura, K.; Yamane, N.; Shoji-Hosaka, E.; Kanda, Y.; Sakurada, M.; Uchida, K.; Anazawa, H.; Satoh, M.; Yamasaki, M.; et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 2003, 278, 3466–3473. [Google Scholar]
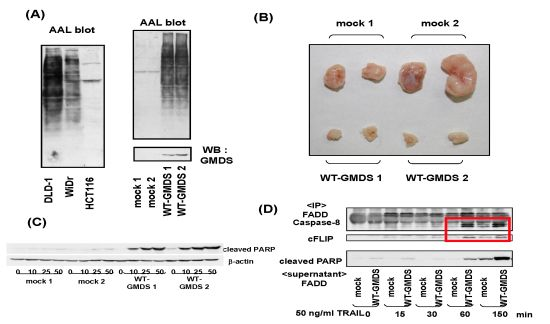
- Moriwaki, K.; Noda, K.; Furukawa, Y.; Ohshima, K.; Uchiyama, A.; Nakagawa, T.; Taniguchi, N.; Daigo, Y.; Nakamura, Y.; Hayashi, N.; Miyoshi, E. Deficiency of GMDS leads to escape from NK cell-mediated tumor surveillance through modulation of TRAIL signaling. Gastroenterology 2009, 137, 188–198. [Google Scholar] [CrossRef]
- Smith, P.L.; Myers, J.T.; Rogers, C.E.; Zhou, L.; Petryniak, B.; Becker, D.J.; Homeister, J.W.; Lowe, J.B. Conditional control of selectin ligand expression and global fucosylation events in mice with a targeted mutation at the FX locus. J. Cell Biol. 2002, 158, 801–815. [Google Scholar] [CrossRef]
- Thompson, S.; Dargan, E.; Turner, G.A. Increased fucosylation and other carbohydrate changes in haptoglobin in ovarian cancer. Cancer Lett. 1992, 66, 43–48. [Google Scholar] [CrossRef]
- Turner, G.A. Haptoglobin. A potential reporter molecule for glycosylation changes in disease. Adv. Exp. Med. Biol. 1995, 376, 231–238. [Google Scholar] [CrossRef]
- Miyoshi, E.; Nakano, M. Fucosylated haptoglobin is a novel marker for pancreatic cancer: Detailed analyses of oligosaccharide structures. Proteomics 2008, 8, 3257–3262. [Google Scholar] [CrossRef]
- Matsumura, K.; Higashida, K.; Ishida, H.; Hata, Y.; Yamamoto, K.; Shigeta, M.; Mizuno-Horikawa, Y.; Wang, X.; Miyoshi, E.; Gu, J.; et al. Carbohydrate-binding specificity of a fucose-specific lectin from aspergillus oryzae: A novel probe for core fucose. J. Biol. Chem. 2007, 282, 15700–15708. [Google Scholar]
- Okuyama, N.; Ide, Y.; Nakano, M.; Nakagawa, T.; Yamanaka, K.; Moriwaki, K.; Murata, K.; Ohigashi, H.; Yokoyama, S.; Eguchi, H.; et al. Fucosylated haptoglobin is a novel marker for pancreatic cancer: A detailed analysis of the oligosaccharide structure and a possible mechanism for fucosylation. Int. J. Cancer 2006, 118, 2803–2808. [Google Scholar]
- Takeda, Y.; Shinzaki, S.; Okudo, K.; Moriwaki, K.; Murata, K.; Miyoshi, E. Fucosylated haptoglobin is a novel type of cancer biomarker linked to the prognosis after an operation in colorectal cancer. Cancer 2011. [Google Scholar] [CrossRef]
- Park, S.Y.; Yoon, S.J.; Jeong, Y.T.; Kim, J.M.; Kim, J.Y.; Bernert, B.; Ullman, T.; Itzkowitz, S.H.; Kim, J.H.; Hakomori, S.I. N-glycosylation status of beta-haptoglobin in sera of patients with colon cancer, chronic inflammatory diseases and normal subjects. Int. J. Cancer 2010, 126, 142–155. [Google Scholar] [CrossRef]
- Zhang, S.; Shu, H.; Luo, K.; Kang, X.; Zhang, Y.; Lu, H.; Liu, Y. N-Linked glycan changes of serum haptoglobin beta chain in liver disease patients. Mol. Biosyst. 2011, 7, 1621–1628. [Google Scholar] [CrossRef]
- Zeng, X.; Hood, B.L.; Sun, M.; Conrads, T.P.; Day, R.S.; Weissfeld, J.L.; Siegfried, J.M.; Bigbee, W.L. Lung cancer serum biomarker discovery using glycoprotein capture and liquid chromatography mass spectrometry. J. Prot. Res. 2010, 9, 6440–6449. [Google Scholar] [CrossRef]
- Lin, Z.; Simeone, D.M.; Anderson, M.A.; Brand, R.E.; Xie, X.; Shedden, K.; Ruffin, M.T.; Lubman, D.M. Mass spectrometric assay for analysis of haptoglobin fucosylation in pancreatic cancer. J. Proteome Res. 2011, 10, 2602–2611. [Google Scholar] [CrossRef]
- Matsumoto, H.; Shinzaki, S.; Narisada, M.; Kawamoto, S.; Kuwamoto, K.; Moriwaki, K.; Kanke, F.; Satomura, S.; Kumada, T.; Miyoshi, E. Clinical application of a lectin-antibody ELISA to measure fucosylated haptoglobin in sera of patients with pancreatic cancer. Clin. Chem. Lab. Med. 2010, 48, 505–512. [Google Scholar] [CrossRef]
- Noda, K.; Miyoshi, E.; Gu, J.; Gao, C.X.; Nakahara, S.; Kitada, T.; Honke, K.; Suzuki, K.; Yoshihara, H.; Yoshikawa, K.; et al. Relationship between elevated FX expression and increased production of GDP-L-fucose, a common donor substrate for fucosylation in human hepatocellular carcinoma and hepatoma cell lines. Cancer Res. 2003, 63, 6282–6289. [Google Scholar]
- Nakagawa, T.; Uozumi, N.; Nakano, M.; Mizuno-Horikawa, Y.; Okuyama, N.; Taguchi, T.; Gu, J.; Kondo, A.; Taniguchi, N.; Miyoshi, E. Fucosylation of N-glycans regulates secretion of hepatic glycoproteins into bile ducts. J. Biol. Chem. 2006, 281, 29797–29806. [Google Scholar]
- Cheng, H.T.; Chang, Y.H.; Chen, Y.Y.; Lee, T.H.; Tai, D.I.; Lin, D.Y. AFP-L3 in chronic liver diseases with persistent elevation of alpha-fetoprotein. J. Chin. Med. Assoc. 2007, 70, 310–317. [Google Scholar] [CrossRef]
- Nakagawa, T.; Takeishi, S.; Kameyama, A.; Yagi, H.; Yoshioka, T.; Moriwaki, K.; Masuda, T.; Matsumoto, H.; Kato, K.; Narimatsu, H.; et al. Glycomic analyses of glycoproteins in bile and serum during rat hepatocarcinogenesis. J. Proteome Res. 2010, 9, 4888–4896. [Google Scholar]
- Narisada, M.; Kawamoto, S.; Kuwamoto, K.; Moriwaki, K.; Nakagawa, T.; Matsumoto, H.; Asahi, M.; Koyama, N.; Miyoshi, E. Identification of an inducible factor secreted by pancreatic cancer cell lines that stimulates the production of fucosylated haptoglobin in hepatoma cells. Biochem. Biophys. Res. Commun. 2008, 377, 792–796. [Google Scholar] [CrossRef]
- Nakagawa, T.; Moriwaki, K.; Terao, N.; Nakagawa, T.; Miyamoto, Y.; Kamada, Y.; Miyoshi, E. Analysis of polarized secretion of fucosylated alpha-fetoprotein in HepG2 cells. J. Proteome Res. 2012. submitted. [Google Scholar]
- Diehl, G.E.; Yue, H.H.; Hsieh, K.; Kuang, A.A.; Ho, M.; Morici, L.A.; Lenz, L.L.; Cado, D.; Riley, L.W.; Winoto, A. TRAIL-R as a negative regulator of innate immune cell responses. Immunity 2004, 21, 877–889. [Google Scholar] [CrossRef]
- Grosse-Wilde, A.; Voloshanenko, O.; Bailey, S.L.; Longton, G.M.; Schaefer, U.; Csernok, A.I.; Schutz, G.; Greiner, E.F.; Kemp, C.J.; Walczak, H. TRAIL-R deficiency in mice enhances lymph node metastasis without affecting primary tumor development. J. Clin. Invest. 2008, 118, 100–110. [Google Scholar] [CrossRef]
- Ashkenazi, A.; Herbst, R.S. To kill a tumor cell: the potential of proapoptotic receptor agonists. J. Clin. Invest. 2008, 118, 1979–1990. [Google Scholar] [CrossRef]
- Koschny, R.; Holland, H.; Sykora, J.; Haas, T.L.; Sprick, M.R.; Ganten, T.M.; Krupp, W.; Bauer, M.; Ahnert, P.; Meixensberger, J.; Walczak, H. Bortezomib sensitizes primary human astrocytoma cells of WHO grades I to IV for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis. Clin. Cancer Res. 2007, 13, 3403–3412. [Google Scholar]
- Nguyen, T.; Zhang, X.D.; Hersey, P. Relative resistance of fresh isolates of melanoma to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis. Clin. Cancer Res. 2001, 7, 966–973. [Google Scholar]
- Moriwaki, K.; Narisada, M.; Imai, T.; Shinzaki, S.; Miyoshi, E. The effect of epigenetic regulation of fucosylation on TRAIL-induced apoptosis. Glycoconj. J. 2010, 27, 649–659. [Google Scholar] [CrossRef]
- Moriwaki, K.; Shinzaki, S.; Miyoshi, E. GMDS deficiency renders colon cancer cells resistant to TRAIL receptor- and CD95-mediated apoptosis by inhibiting complex II formation. J. Biol. Chem. 2011, 286, 43123–43133. [Google Scholar]
- Wang, X.; Gu, J.; Ihara, H.; Miyoshi, E.; Honke, K.; Taniguchi, N. Core fucosylation regulates epidermal growth factor receptor-mediated intracellular signaling. J. Biol. Chem. 2006, 281, 2572–2577. [Google Scholar]
- Wang, X.; Inoue, S.; Gu, J.; Miyoshi, E.; Noda, K.; Li, W.; Mizuno-Horikawa, Y.; Nakano, M.; Asahi, M.; Takahashi, M.; et al. Dysregulation of TGF-beta1 receptor activation leads to abnormal lung development and emphysema-like phenotype in core fucose-deficient mice. Proc. Natl. Acad. Sci. USA 2005, 102, 15791–15796. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Miyoshi, E.; Moriwaki, K.; Terao, N.; Tan, C.-C.; Terao, M.; Nakagawa, T.; Matsumoto, H.; Shinzaki, S.; Kamada, Y. Fucosylation Is a Promising Target for Cancer Diagnosis and Therapy. Biomolecules 2012, 2, 34-45. https://doi.org/10.3390/biom2010034
Miyoshi E, Moriwaki K, Terao N, Tan C-C, Terao M, Nakagawa T, Matsumoto H, Shinzaki S, Kamada Y. Fucosylation Is a Promising Target for Cancer Diagnosis and Therapy. Biomolecules. 2012; 2(1):34-45. https://doi.org/10.3390/biom2010034
Chicago/Turabian StyleMiyoshi, Eiji, Kenta Moriwaki, Naoko Terao, Cheng-Cheng Tan, Mika Terao, Tsutomu Nakagawa, Hitoshi Matsumoto, Shinichiro Shinzaki, and Yoshihiro Kamada. 2012. "Fucosylation Is a Promising Target for Cancer Diagnosis and Therapy" Biomolecules 2, no. 1: 34-45. https://doi.org/10.3390/biom2010034